Abstract
Subfossil midge remains were identified in surface sediment recovered from 88 lakes in the central Canadian Arctic. These lakes spanned five vegetation zones, with the southern-most lakes located in boreal forest and the northern-most lakes located in mid-Arctic tundra. The lakes in the calibration are characterized by ranges in depth, summer surface-water temperature (SSWT), average July air temperature (AJAT) and pH of 15.5 m, 10.60°C, 8.40°C and 3.69, respectively. Redundancy analysis (RDA) indicated that maximum depth, pH, AJAT, total nitrogen-unfiltered (TN-UF), Cl and Al capture a large and statistically significant fraction of the overall variance in the midge data. Inference models relating midge abundances and AJAT were developed using different approaches including: weighted averaging (WA), weighted averaging-partial least squares (WA-PLS) and partial least squares (PLS). A chironomid-based inference model, based on a two-component WA-PLS approach, provided robust performance statistics with a high coefficient of determination (r 2 = 0.77) and low root mean square error of prediction (RMSEP = 1.03°C) and low maximum bias. The use of a high-resolution gridded climate data set facilitated the development of the midge-based inference model for AJAT in a region with a paucity of meteorological stations and where previously only the development of a SSWT inference model was possible.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
It is increasingly recognized that high-latitude regions will be highly responsive and sensitive to future climate warming (Overpeck et al. 1997; Smol et al. 2005; IPCC 2007; Smol and Douglas 2007). In the Arctic, the increases in surface temperature and precipitation that are projected to occur as a result of global warming will not only affect ecosystems, soil carbon storage, surface hydrology and sea-ice extent, but could also further magnify the effects of global climate change due to feedback mechanisms (Oechel et al. 1993; Johannessen et al. 1995; Kattenberg et al. 1996; IPCC 2007). For example, changes in Arctic snow and ice cover extent could have dramatic effects on high-latitude albedo, further increasing temperatures (Bonan et al. 1992; Foley et al. 1994; MacDonald et al. 1998; Chapin et al. 2000).
Meteorological station data for the period 1961–1990 suggest that portions of the Arctic have warmed in recent decades (Chapman and Walsh 1995; Serreze et al. 2000; Moritz et al. 2002; Bonsal and Prowse 2003). However, temperature changes and vegetation response in recent decades have not been spatially uniform. For example, portions of the eastern Arctic, particularly the Foxe Basin, have experienced negligible warming and possibly cooling (Serreze et al. 2000; Saulnier-Talbot and Pienitz 2001; Ponader et al. 2002). In addition, multi-year to multi-decadal patterns of temperature and precipitation variability have been observed in instrumental climate records from the Arctic and adjacent regions (Serreze et al. 2000), whereas paleoclimatic records suggest that even lower frequency modes of variability operating on millennial timescales may be a persistent feature of the Arctic system (Szeicz and MacDonald 2001; Hu et al. 2003). These observations suggest that there are a number of modes of Arctic climate variability that function on varying time scales and can serve to accentuate or dampen the warming that might be anticipated due to global climate change. It is clear that a more detailed understanding of the temporal and spatial patterns of past and present Arctic climate change and the response of this ecosystem to this change is required before the accuracy of modeling scenarios of the future can be evaluated. Unfortunately, observational records of arctic climate and environmental changes are too short (generally <100 years) to document the full range of the periodicity and amplitude of modes of Arctic climate variability.
One means of providing such information is to produce quantitative paleoclimate records. However, paleoclimate records spanning more than a 100 years remain sparse for the Arctic, particularly in the central Canadian Arctic and the adjacent Arctic archipelago. As part of an effort to develop quantitative paleoclimate records for this region, we developed a modern calibration data set for the interpretation of down-core midge remains. Previous research reported the sensitivity and rapid response of paleolimnological proxies such as diatoms and chironomids to recent and long-term climate change (MacDonald et al. 1993; Douglas et al. 1994; Smol and Douglas 2007; Hu et al. 2006). However, until relatively recently (Gajewski et al. 2005; Barley et al. 2006; Walker and MacDonald 1995), only a few studies were undertaken that described chironomid distributions and the relationship between midges and the contemporaneous environment in the central Canadian Arctic. It is vital that we continue to refine our knowledge of chironomid ecology and biogeography to understand better the environmental optima and tolerances of specific midge taxa. Doing so will likely enable more meaningful interpretation of subfossil midge stratigraphies and will increase the robustness of midge-based quantitative paleoclimate reconstructions.
In this paper, we describe the distribution of midges in a suite of 88 lakes spanning boreal forest, forest tundra and tundra in the central Canadian Arctic, we identify environmental variables that account for a statistically significant amount of variance in midge distribution, and we develop a quantitative inference model for average July air temperature based on a high-resolution gridded air temperature dataset. The application of this model to fossil chironomid assemblages preserved in late-Quaternary lake sediment will provide estimates of past air-temperature variability in this region and may help identify the influence of warming phases upon Arctic environments and peoples in the past.
Study region
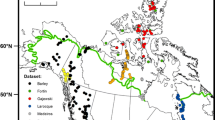
Sediment and water samples were collected from a suite of lakes crossing strong climate and vegetation boundaries in the central Canadian Arctic. The study area (Fig. 1) includes Victoria Island and adjacent continental Nunavut, extending south to the northern boreal forest ecotone, and spanning the Picea treeline, shrub tundra, and mid-Arctic tundra ecozones. The sites on Victoria Island are located on late-Wisconsin glacial deposits consisting primarily of till draped over pre-Quaternary carbonate bedrock (Sharpe 1993). The mid-Arctic ecozone on Victoria Island consists of undulating lowlands, extensive drumlin ridges, patterned soils and deep, continuous permafrost. The mainland portion of the transect, located in Canadian Shield’s Slave Province, is underlain by Archean granite, gneisses and metamorphosed sedimentary and volcanic rocks (Padgham and Fyson 1992). This portion of the study transect is located in the low Arctic ecozone, which is modified by permafrost processes, resulting in hummocky ground features.
The study transect spanned five vegetation zones: mid-Arctic tundra, low Arctic tundra, forest-tundra and boreal forest. Boreal forest is distinguished by Picea mariana, P. glauca, Pinus banksiana and Abies balsamea (Ritchie 1984). The forest-tundra transition zone is characterized by P. mariana, P. glauca and Larix laricina (Sirois 1992). Low Arctic tundra is typified by shrub forms of Salix and Betula, as well as Alnus. The mid-Arctic tundra zone is characterized by nearly continuous cover of either dwarf shrub heath tundra, or cottongrass-dwarf shrub heath tundra (tussock tundra), consisting of Ericaceae, Empetraceae, Dryas and dwarf Salix. Cambridge Bay, Nunavut, located in the northern portion of the training set has a mean annual temperature of −14.9°C and a mean July temperature of 8.0°C. Yellowknife, located at the southern extent of the training set, has a mean annual temperature of −5.2°C and a mean July temperature of 16.5°C. Mean annual precipitation varies with latitude; Cambridge Bay receives 141 mm and Yellowknife receives 267 mm. We attempted to maximize the temperature gradient in the calibration set by selecting lakes located along a transect that crossed perpendicularly the mean July temperature isotherms in the region.
Methods
Field sampling
Sediment and water samples for 60 of the lakes, VI 1–60, were collected between August 6th, 2007 and August 11th, 2004. Sediment and water samples for the remaining lakes, TK 1–36, were collected between August 7th, 1996 and August 19th, 1996 (see Table 1 for lake coordinates). Replicate cores were recovered from the approximate centre of each lake using a Glew (1991) mini-corer deployed either from the pontoon of a Bell 206 helicopter (2004) or from an inflatable Zodiac (1996). The top (0–1) cm interval of each replicate sediment core was extruded immediately, stored in a Whirl-Pak® and kept cool and in the dark until shipped back to the lab. During surface sediment collection, physical variables including surface-water temperature, specific conductance, pH and depth were measured. Epilimnetic water samples were also collected 0.5 m below the water surface in pre-cleaned polyethylene bottles and immediately treated in the field following the protocols outlined in the Analytic Methods Manual (Environment Canada 1996a, b). Untreated water samples were collected in one 125-ml glass bottle to analyse total phosphorus and two 125-ml polyethylene bottles for major ions and trace metals analyses. Water samples were filtered for Chl a on a 4.7-cm-diameter GF/F filter, and total phosphorus and total nutrients on a 4.7-cm- and 0.45-cm-diameter cellulose-acetate filter, respectively. Each lake in the training set was characterized by a suite of 23 variables including: maximum depth, summer surface-water temperature (SSWT), average July air temperature (AJAT), specific conductivity, pH, dissolved organic carbon (DOC), dissolved inorganic carbon (DIC), total organic carbon (TOC), total nitrogen-unfiltered (TN-UF), total phosphorus-unfiltered (TP-UF), chlorophyll a (CHLA), Cl, SO4, Ca, Mg, K, SiO2, total Kjeldahl nitrogen (TKN), Al, Ba, Fe, Li and Sr. Trace metal analysis was based on the dissolved and extracted portion of the water samples. Water chemistry analyses were carried out by the National Laboratory for Environmental Testing (NLET) at the Canadian Centre for Inland Waters in Burlington, Canada. Lakes which did not have water chemistry or sediment samples available (TK 1–5, TK-35) were removed from the calibration set and not included in further analyses.
Laboratory analyses
Chironomid analysis followed standard procedures, as outlined by Walker (2001). A minimum of 2 ml (range: 2–10 ml) of sediment was deflocculated in an 8% KOH solution and heated at 30°C for 30 min. The sediment slurry was sieved through a 95-μm mesh and the material retained on the sieve was rinsed with distilled water and washed into a beaker. The resulting solution was sorted in a Bogorov plankton counting tray under a Wild 5× dissection microscope at 50× magnification, and subfossil chironomid remains were removed with forceps. The subfossil specimens were permanently mounted on slides in Permount or Entellan for identification. Identification of the mounted remains was typically made at 400× and was based predominantly on Simpson and Bode (1980), Cranston (1982), Oliver and Roussel (1983), Wiederholm (1983), Walker (1988), Larocque and Rolland (2006), Brooks et al. (2007) and an extensive reference collection of Arctic subfossil chironomid remains housed at the Department of Geography, The Ohio State University (USA). The calibration set discussed in this paper was independently analyzed by two of the authors (Porinchu and Rolland) and systematically harmonized based on the authors’ knowledge of Holarctic subfossil midge taxonomy. Increasingly, chironomid researchers have been dividing the subfossil remains of Heterotrissocladius spp. into finer taxonomic groupings, such as H. marcidus, H. subpilosus, H. maeaeri, and H. grimshawi (Brooks et al. 2007); however, we conservatively chose to merge all the Heterotrissocladius spp. into a single taxonomic entity. Diagnosis for Zalutschia sp. A is available in Brooks et al. (2007) where it is referred to as Zalutschia sp. B; diagnosis for Zalutschia sp. B is available in Barley et al. (2006). Appendix A depicts a number of morpho-types encountered in the sediment and grouped together as C. oliveri-type. At least 45 head capsules (mean = 67.5) were enumerated and identified from the surface sediment (0–1 cm) from each of the calibration lakes with the exception of VI-04, VI-21, VI-31 and VI-40 (40–45 head capsules) and TK-17 and VI-23 from which 38.5 and 39.5 head capsules were identified, respectively. A sum of 45–50 head capsules has been shown to provide relatively consistent estimates of SSWT (Heiri and Lotter 2001). Five lakes (VI-30, TK-22, TK-25, TK-26, TK-28) were removed from further analyses due to low head capsule recovery. One lake, TK-12, was removed due to poor subfossil specimen preservation.
Gridded climate data
In this study, the closest weather stations were Yellowknife (62°27′ N; 114°24′ W), Cambridge Bay (68°07′ N; 105°03′ W) and Kugluktuk (67°49′ N; 115°05′ W), however, only Cambridge Bay is situated on our transect. Because of the paucity of meteorological data, the use of regression to relate latitude and temperature will not provide meaningful estimates of air temperature. However, as already applied by Barley et al. (2006), the use of a high-resolution climate data set compiled by New et al. (2002) provided mean July air temperature for each site. New et al. (2002) used the climatic normal from 1961 to 1990 to develop a 10′ latitude/longitude data set of mean monthly surface climate over global land areas. A subsample of 8,633 points covering the area between 60°N–75°N and 100°W–120°W was extracted from this climate data set. These points, characterized by a latitude (x axis), longitude (y axis) and mean July air temperature for 1961–1990 (z axis) were used in ArcGIS to create a grid in which the sampled lakes were also plotted (Fig. 1). The temperature variations along this grid were represented as a color gradient surface made of 26 classes, each one representing a 0.5°C range. For each lake, a buffer was created to select the grid points that were within the limit of a radius of 8.25 km around the lake (Fig. 1). The radius size was carefully chosen to select at least two points for each lake. The selected grid points were then linked to their mean July air temperature and an average July air temperature for each buffer was calculated. These values were then related to their respective lakes.
Statistical analyses
Non-limnological variables such as vegetation type, latitude and longitude were removed from all subsequent analyses. Forms of indirect gradient analysis, principal components analysis (PCA) and detrended correspondence analysis (DCA), were implemented to assess whether the limnological or biological characteristics, respectively, of specific lakes in the training set should be considered outliers. Lakes were identified as outliers and removed from further analyses if their scores on the first two axes of a PCA of the environmental data and a DCA of midge data were greater than one standard deviation of the mean sample score for each respective axis (Birks 1995).
The ordination analyses (DCA, RDA and partial RDAs) were based on all taxa present in the training set lakes and the square-root transformed relative abundance data. A DCA of the midge relative abundance data, with rare taxa down-weighted and detrending-by-segments, was undertaken to identify the degree of compositional turnover in the midge distribution (Birks 1998). The length of the gradient in the distribution data was used to identify whether linear or unimodal approaches were suitable for direct gradient analyses. Linear approaches are appropriate when the gradient length captured by the assemblage data is short, i.e. <2 S.D. units. The gradient lengths of the DCA axis 1 and 2 were 2.13 and 1.95 S.D. units, respectively, indicating the use of RDA for constrained ordinations (Birks 1995). A series of RDAs constrained to individual environmental (predictor) variables (n = 23) with Monte Carlo permutations (499) was undertaken to identify those environmental variables that could account for a statistically significant amount of variance (P ≤ 0.05) in the midge assemblages. Of the remaining variables, those that were highly co-linear were removed one at a time until all variables had variance inflation factors (VIFs) < 10×. Forward selection, with Monte Carlo permutation tests (499 unrestricted permutations), was used to identify a minimal subset of the remaining environmental variables (n = 16) that could account for a statistically significant and large amount of variance present in the midge assemblage data. The amount of variance that AJAT or SSWT could account for, independent of the other variables identified in the forward selection procedure, was assessed using variance partitioning (Borcard et al. 1992), which was implemented through a series of partial RDAs.
Midge-based inference models for AJAT and SSWT were developed using a number of techniques including: weighted-averaging, weighted-averaging-partial least squares and partial least squares. The robustness of the transfer functions was evaluated through the use of jackknifing (Birks 1998). Model performance was assessed based on three criteria: (1) root mean square error of prediction (RMSEP), (2) maximum bias of the model, and (3) the minimum number of components (Birks 1998), incorporated in the model. Following Jones and Juggins (1995), Lotter et al. (1998) and Porinchu et al. (2002), samples were considered outliers and not included in the final model(s) if the absolute residual (predicted AJAT or SSWT—‘observed’ AJAT or SSWT) was greater than one standard deviation of AJAT or SSWT, respectively. The program C2 (Juggins 2003) was used for inference model development. A number of different statistical approaches such as weighted averaging (WA), weighted averaging-partial least squares (WA-PLS) and partial least squares (PLS) were used to model the relationship between AJAT/SSWT and midge abundances. Lakes with high absolute residuals, determined by a 1-component WA-PLS model, were removed from the AJAT and SSWT inference models prior to calculation of the performance statistics.
Results
Screening of the environmental data (PCA) and midge assemblage data (DCA) indicated that TK-24, which is characterized by unusual water chemistry and midge community composition, is an outlier. The midge community in this lake is atypical with high relative abundances of Procladius, Corynoneura and Cricotopus. Additionally, the water chemistry of this lake is unusual with high trace-metal concentrations (Sr, Ba), conductivity, DOC and TN-UF (see Table 1 for a summary of the values of major environmental variables).
The biogeographical distribution (arranged by latitude) and relative abundances of the major midge taxa are illustrated in Figs. 2 and 3. Lakes on Victoria Island are characterized by chironomid communities dominated by Tanytarsus spp., Chironomus anthracinus-type, Corynocera oliveri-type, Psectrocladius sordidellus-type and, surprisingly, Dicrotendipes, which is generally considered a thermophilous taxon. Chironomid communities found in the northern mainland Arctic tundra zone are distinguished by relatively high abundances of Limnophyes and Zalutschia sp. A and the near absence of Corynocera oliveri-type and Micropsectra. The southern mainland Arctic tundra sites are notable for their high proportion of Corynocera ambigua-type, Heterotrissocladius, Micropsectra, Zalutschia sp. B and the near absence of Chironomus anthracinus-type. The forest-tundra zone is distinguished by Heterotanytarsus, whereas taxa such as Microtendipes, Polypedilum and Dicrotendipes, typically considered warm thermophiles, are most abundant and commonly found in the lakes located in boreal forest.
The results of the RDA restricted to individual predictor variables (n = 23) indicated that DOC and SiO2 did not explain a statistically significant amount of variance in the midge assemblages. Of the remaining variables, DIC, Ca, Mg and TKN had high variance inflation factors (VIFs) and were removed sequentially until the VIFs of all remaining variables were below 10× (n = 16). As indicated by Fig. 3, SSWT co-varied with AJAT, and was therefore removed in order to keep AJAT in the analysis as an explanatory variable. The eigenvalues of axis 1 and 2 of a RDA limited to the remaining 15 variables (maximum depth, AJAT, specific conductivity, pH, TOC, TN-UF, TP-UF, CHLA, Cl, SO4, K, Al, Ba, Fe, Li and Sr), were 0.226 and 0.045, respectively (see Table 2). These 15 predictor variables explained 40.6% of the variance present in the chironomid abundance data. The forward selection procedure identified six variables which could capture a large and statistically significant fraction of the overall variance present in the midge data: maximum depth, pH, AJAT, TN-UF, Cl and Al. A RDA constrained to these six variables accounted for 75.8% of the variance captured by the full suite of 15 predictor variables. The eigenvalues for the first two RDA axes constrained to the six forward selected variables were 18.4 and 4.5, respectively. These two axes captured 22.9% of the variance in the midge abundance data and 77.6% of the variance in the taxa-environment relationship. The forward selected variables summarized the relationship between midge distributions and the measured environmental variables well, and therefore, were used to construct the RDA bi-plots (Fig. 4).
(a) Redundancy analysis (RDA) of taxa. The arrows represent the vectors of the six environmental variables explaining the distribution of chironomids in the 77 lakes. The numbers refer to the taxon id# presented in Table 6. (b) RDA analysis of the 77 lakes classified by vegetation
The canonical coefficients, intra-set correlations and approximate t-tests indicate that maximum depth, TN-UF and pH are strongly correlated with RDA axis 1, and that AJAT and pH are strongly correlated with RDA axis 2 (Table 3). Although depth does account for the largest amount of variance in this dataset, the primary purpose in developing this training set is to assess the relationship between midge distributions and SSWT/AJAT, therefore, we focus on these variables for the remaining analyses. The ratio of λ1 to λ2 in a RDA restricted to AJAT as the sole explanatory variable is low (0.245); however, this low ratio is due to the large amount of variance captured on RDA axis 2 by AJAT (Fig. 4). Partial RDAs indicate that AJAT captures 3.6% of the variance in the midge dataset, independent of the effects of the other forward selected variables (Table 4).
The RDA bi-plots primarily separate the deep, nitrogen-limited, southern mainland arctic tundra sites from the shallow, nutrient-rich Victoria Island and northern mainland arctic tundra sites (Fig. 4). In addition, the majority of boreal forest and forest-tundra lakes load high on the AJAT axis, distinguishing them from the majority of the arctic tundra sites. Midge taxa associated with the deep, nitrogen-limited lakes include: Abiskomyia, Sergentia, Stictochironomus, Protanypus and Ablabesmyia. Taxa associated with the well-buffered, nutrient-rich lakes include: Cladotanytarsus mancus-group, Dicrotendipes and Cladopelma. RDA axis 2, which primarily captures AJAT, indicates taxa such as Microtendipes, Polypedilum, Pagastiella and Stempellina/Zavrelia are associated with the warmest calibration set lakes, whereas taxa such as Pseudodiamesa, Hydrobaenus/Oliveridia, Sergentia and Stictochironomus are most abundant in the coldest lakes.
The inference model developed for AJAT consisted of 50 taxa and was based on 77 lakes, whereas the inference model developed for SSWT consisted of 50 taxa and was based on 75 lakes. Totals of 11 and 13 lakes were removed from the AJAT and SSWT inference models, respectively, due to high residuals. The performance statistics for the various inference models are presented in Table 5. A 2-component WA-PLS model provides the most robust performance statistics for AJAT, with an r 2jack = 0.77, RMSEP = 1.03°C and a maximum bias of 1.37°C. The beta coefficients used in the 2-component WA-PLS inference model for AJAT are presented in Table 6. Plots of the cross-validated inferred AJAT against observed AJAT and the residuals from the 2-component WA-PLS inference model are depicted in Fig. 5, with no trend apparent in the residuals (negative trend r 2 = 0.22, P < 0.0001). The best model, (Birks 1998), for SSWT, based on a 1-component WA-PLS approach, provides an r 2jack = 0.75, an RMSEP = 1.39°C and maximum bias of 2.33°C (Table 7).
Discussion
The development and expansion of training sets in the western Arctic (Barley et al. 2006) and the eastern Arctic (Larocque et al. 2006) has further focused attention on the identification and distribution of Corynocera species in the circum-Arctic region. Barley et al. (2006) discuss the difficulty associated with consistently identifying C. oliveri-type, which is characterized by a raised mentum, a diagnostic characteristic shared by Tanytarsus lugens-type, and the presence of a large surface tooth on the mandible. Barley et al. (2006) describe the occurrence of chironomid subfossils possessing C. oliveri-type mandibles in the western Arctic, but note that there are a number of types each with distinct mentum morphologies. Our results are in agreement with the Barley et al. (2006) assessment; there appear to be a number of Corynocera oliveri morpho-types in our training set. These Corynocera oliveri morpho-types all possess a large surface tooth on the mandible and a raised mentum, but each morpho-type has a distinct median tooth complex (see Appendix 1). The biogeography of Corynocera is also interesting. For example, the limited presence of C. ambigua-type in the Canadian Arctic archipelago has been used to support the suggestion that the modern distribution of Corynocera ambigua-type in the Canadian Arctic reflects the existence of a Beringian refugium for this taxon (Barley et al. 2006). However, in our training set, C. ambigua-type is present in 10 of the 30 lakes we sampled on Victoria Island and, in addition, it comprises over 20% of the chironomid community in six of the lakes. These results suggest that the biogeographical history of this taxon in the Canadian Arctic may be more complex than previously thought.
Surprisingly, Dicrotendipes was also present at high levels (30–40%) in three lakes on Victoria Island. Dicrotendipes is generally considered a thermophilous taxon and is more commonly associated with sites located south of treeline (Oliver and Roussel 1983; Walker and MacDonald 1995; Porinchu and Cwynar 2000; Larocque et al. 2006). The Victoria Island lakes in which Dicrotendipes is most abundant are between 0.8 and 1.2 m deep; it is likely that depth and amount of littoral habitat are important determinants of Dicrotendipes abundances. These results could also suggest changes in the distribution of this taxon due to recent regional warming on Victoria Island. A strong negative correlation exists between Dicrotendipes and C. oliveri-type in the northern training set lakes, suggesting a possible inter-specific link between the abundances of these two taxa. Also of note is the bi-modal distribution of Sergentia, with high relative abundances in both the northern and southern portion of the training set. A similar pattern has been reported from northern Quebec and Labrador and may be due to the presence of two distinct Sergentia types (Larocque et al. 2006). However, in our dataset the high abundance of Sergentia in the southern boreal forest lakes appears to be a function of lake depth, with high abundances of Sergentia occurring in the two deepest lakes in the training set.
The majority of calibration sets that have been developed in the Arctic and sub-Arctic regions, to date, have identified depth and various measures of temperature (AJAT, SSWT) and lake productivity (O2 concentration, total P, sediment organic content and Chl a) as being strongly correlated with midge distribution (e.g. Barley et al. 2006; Brodersen and Anderson 2002; Brooks et al. 2001; Larocque et al. 2001; Olander et al. 1999; Quinlan et al. 1998). Because the relationship between midges, depth, temperature and productivity has been discussed in great detail in the paleolimnological literature, it will not be repeated here. The identification of pH as a predictor variable, while not commonly reported, is not unexpected. Researchers have identified the impacts of natural and anthropogenic acidification using midge remains (Johnson and McNeil 1988; Brodin 1990; Velle et al. 2005) and a number of lake-pH classification schemes have been developed that rely on midge community composition (Johnson et al. 1990; Halvorsen et al. 2001; Bitusik et al. 2006). In addition, Barley et al. (2006) found that pH accounted for a statistically significant amount of variance in midge community composition in their calibration set. Owing to variations in underlying geology, our dataset spans a large pH gradient (3.69), with Victoria Island sites well buffered due to the presence of carbonate marine and glacial deposits, whereas most of the northern mainland sites are poorly buffered due to their location on the Canadian Shield. Therefore, it is not surprising that pH has a statistically significant relationship with midge distributions. The midge-based AJAT model compares favorably to previously developed inference models from the Canadian Arctic (see Table 7). The AJAT inference model presented in this paper, based on 77 lakes, has the lowest RMSEPjack and maximum bias relative to other regional training sets (Barley et al. 2006; Francis et al. 2006; Larocque et al. 2006). The range of AJAT captured by this model is less than the previously published models, which accounts for the slightly greater, but comparable value of ‘RMSEP as a percentage of the AJAT range.’ The SSWT, based on 75 lakes, was less robust than the AJAT inference model; however, it captured a larger SWT range and it had the lowest RMSEP and maximum bias relative to the existing SWT inference models (Walker et al. 1997; Francis et al. 2006; Larocque et al. 2006).
A number of studies in northern temperate and sub-arctic regions that used calibration sets and multivariate statistical techniques have demonstrated that mean summer air temperature and/or summer surface-water temperature have a statistically significant relationship with the distribution of chironomids. This has led to the development of many chironomid-based inference models for temperature (Lotter et al. 1997; Walker et al. 1997; Olander et al. 1999; Larocque et al. 2001, 2006; Brooks and Birks 2001; Barley et al. 2006; Francis et al. 2006). Researchers have applied these models to sub-fossil chironomid assemblages preserved in lake sediments to reconstruct quantitatively changes in past surface-water temperature in Atlantic Canada, the northeastern United States and the western United States during the late-glacial and Holocene (Walker et al. 1991; Levesque et al. 1993, 1997; Cwynar and Levesque 1995; Potito et al. 2006) and reconstruct past air temperature in Arctic Canada and western Europe during the late-glacial and the early Holocene (Lotter et al. 1999; Brooks and Birks 2001; Heiri and Lotter 2005; Velle et al. 2005; Rolland et al. in press). In some cases, intra- and inter-regional comparison of model performance through the merging or the expansion of datasets has been attempted (Porinchu et al. 2007; Lotter et al. 1999; Barley et al. 2006). With the inclusion of the lakes in this calibration set, four major training sets, incorporating over 300 lakes, exist for the circum-Canadian Arctic. Therefore, it should be possible to begin systematically assessing the larger-scale distributions and biogeographic relationships of arctic midge taxa and midge communities. Such a project should be pursued.
Increasingly, midge training sets are being used to develop quantitative inference models for air temperature, rather than mean summer/July surface water temperature, the variable most commonly used in earlier calibration studies (Walker et al. 1991; Olander et al. 1997; Lotter et al. 1998). From an ecological standpoint, there are a number of reasons midge community composition may be influenced by ambient air temperature. For example, most midges possess limited dispersal ability and, therefore, thermal conditions during adult emergence can greatly alter the reproductive success (fitness) of specific taxa, which in turn may influence midge community composition (Armitage et al. 1995). The value of using air temperature is two-fold: gridded climate data of sufficient spatial resolution will provide more representative estimates of site-specific thermal conditions than spot measurements of surface-water temperature, and the interpretation of air temperature is more straightforward because surface-water temperature is a function of multiple variables including lake depth, elevation/latitude and air temperature. However, the relationship between SSWT and midge communities is more direct and immediate. Water temperature can directly affect chironomid communities by influencing the rate of egg and larval development and the timing of eclosion (Armitage et al. 1995).
The performance of AJAT or SSWT inference models will be influenced greatly by the range of lake depths present in the training set. Lake depth, which influences lake water temperature, has been shown to strongly influence midge distributions in the circum-Arctic region (Olander et al. 1999; Porinchu and Cwynar, 2000; Larocque et al. 2001, Barley et al. 2006). Application of these models to down-core assemblages, therefore, should take into account the depth of the lake for which the reconstruction is being developed. For example, it may be more appropriate to remove deep lakes (i.e. >10 m) from an inference model if the model is being applied to chironomid stratigraphies recovered from shallow, Arctic ponds. Additionally, it is imperative that training sets incorporating sediment collected in the 21st century use the most recent climate normal period (1971–2000). The Arctic has been dramatically warming in recent decades. Calibrating subfossil midge distributions with the 1961–1990 Climate Normal will likely systematically underestimate Holocene reconstructions, although trends that are identified will still be meaningful.
Global climate models project significant increases in surface temperatures by 2100 AD. Although this change represents a global average, it is clear that differing responses will occur at regional and sub-regional scales. The recent Arctic Climate Impact Assessment (ACIA 2005) and IPCC report (IPCC 2007) indicate that the Arctic has, and will continue to warm over the next century. Recent rates of circum-Arctic climate and environmental change make it clear that improving our understanding of the temporal and spatial patterns of Arctic climate change in the past and the response of Arctic systems, particularly vegetation, to climatic change will be critical to assess the accuracy of future modeling scenarios. For example, the spatial and temporal imprint of the Holocene Thermal Event (HTM), a period of prolonged warming in the Arctic, may provide useful comparative data and potential analogues for anticipated future warming. In addition, a number of abrupt climate perturbations, such as the 9.2 k and 8.2 k events, have been well documented in Greenland ice cores (Alley et al. 1997) and the eastern Canadian Arctic archipelago (Miller et al. 2005), yet little evidence of these events has been discovered in the central Canadian Arctic (Seppa et al. 2003). Application of the quantitative midge-based inference model developed in this paper to subfossil midge assemblages extracted from Holocene sediment cores recovered from the central Canadian Arctic will provide the first quantitative estimates of the thermal conditions that existed during this period. When this approach is combined with pollen analyses, it will improve our understanding of vegetation response to climate change in this region.
References
ACIA (2005) Arctic climate impact assessment. Cambridge University Press, Cambridge, UK
Alley RB, Mayewski PA, Sowers T, Stuiver M, Taylor KC, Clark PU (1997) Holocene climatic instability: a prominent, widespread event 8200 yr ago. Geology 25:483–486. doi:10.1130/0091-7613(1997)025<0483:HCIAPW>2.3.CO;2
Armitage PD, Cranston PS, Pinder LCV (1995) The chironomidae: the biology and ecology of non-biting midges. Chapman & Hall, London
Barley EM, Walker IR, Kurek J, Cwynar LC, Mathewes RW, Gajewski K et al (2006) A northwest North American training set: distribution of freshwater midges in relation to air temperature and lake depth. J Paleolimnol 36:295–314. doi:10.1007/s10933-006-0014-6
Birks HJB (1995) Quantitative paleoenvironmental reconstructions. In: Maddy D, Brew JS (eds) Statistical modelling of quaternary science data. Quaternary Research Association, London, pp 161–254
Birks HJB (1998) Frey DG & Deevey ES review #1—numerical tools in palaeolimnology—progress, potentialities, and problems. J Paleolimnol 20:307–332. doi:10.1023/A:1008038808690
Bitusik P, Svitok M, Kolosta P, Hubkova M (2006) Classification of the Tatra Mountain lakes (Slovakia) using chironomids (Diptera, Chironomidae). Biologia 61:S191–S201. doi:10.2478/s11756-006-0131-8
Bonan GB, Pollard D, Thompson SL (1992) Effects of Boreal forest vegetation on global climate. Nature 359:716–718. doi:10.1038/359716a0
Bonsal BR, Prowse TD (2003) Trends and variability in spring and autumn 0 degrees C-isotherm dates over Canada. Clim Change 57:341–358. doi:10.1023/A:1022810531237
Borcard D, Legendre P, Drapeau P (1992) Partialling out the spatial component of ecological variation. Ecology 73:1045–1055. doi:10.2307/1940179
Brodin YW (1990) Midge fauna development in acidified lakes in Northern Europe. Philos Trans R Soc Lond B Biol Sci 327:295–298. doi:10.1098/rstb.1990.0065
Brodersen KP, Anderson NJ (2002) Distribution of chironomids (Diptera) in low arctic West Greenland lakes: trophic conditions, temperature and environmental reconstruction. Freshw Biol 47:1137–1157
Brooks SJ, Birks HJB (2001) Chironomid-inferred air temperatures from Lateglacial and Holocene sites in north-west Europe: progress and problems. Quat Sci Rev 20:1723–1741. doi:10.1016/S0277-3791(01)00038-5
Brooks SJ, Bennion H, Birks HJB (2001) Tracing lake trophic history with a chironomid-total phosphorus inference model. Freshw Biol 46:513–533
Brooks SJ, Langdon PG, Heiri O (2007) The identification and use of palaeoarctic chironomidae larvae in palaeoecology. Quaternary Research Association, London
Chapin FS, McGuire AD, Randerson J, Pielke R, Baldocchi D, Hobbie SE et al (2000) Arctic and boreal ecosystems of western North America as components of the climate system. Glob Change Biol 6:211–223. doi:10.1046/j.1365-2486.2000.06022.x
Chapman WL, Walsh JE (1995) Recent variations of sea ice and air temperatures in high latitudes. Bull Am Meteorol Soc 74:33–47. doi:10.1175/1520-0477(1993)074<0033:RVOSIA>2.0.CO;2
Cranston PS (1982) A key to the larvae of the British Orthocladiinae (Chironomidae). Sci Publ Freshw Biol Assoc 45:1–152
Cwynar LC, Levesque AJ (1995) Chironomid evidence for late-glacial climatic reversals in Maine. Quat Res 43:405–413
Douglas MSV, Smol JP, Blake W (1994) Marked post-18th century environmental change in high-arctic ecosystems. Science 266:416–419
Environment Canada (1996a) Manual of analytic methods. Volume 1: major ions and nutrients. The National Laboratory of Environmental Testing, Canada Centre for Inland Waters, Burlington, Canada
Environment Canada (1996b) Manual of analytic methods. Volume 2: trace Metals. The National Laboratory of Environmental Testing, Canada Centre for Inland Waters, Burlington, Canada
Foley JA, Kutzbach JE, Coe MT, Levis S (1994) Feedbacks between climate and Boreal forests during the Holocene epoch. Nature 371:52–54. doi:10.1038/371052a0
Francis DR, Wolfe AP, Walker IR, Miller GH (2006) Interglacial and Holocene temperature reconstructions based on midge remains in sediments of two lakes from Baffin island, Nunavut, Arctic Canada. Palaeogeogr Palaeoclimatol Palaeoecol 236:107–124. doi:10.1016/j.palaeo.2006.01.005
Gajewski K, Bouchard G, Wilson SE, Kurek J, Cwynar LC (2005) Distribution of Chironomidae (Insecta: Diptera) head capsules in recent sediments of Canadian Arctic lakes. Hydrobiologia 549:131–143. doi:10.1007/s10750-005-5444-z
Glew J (1991) Miniature gravity corer for recovering short sediment cores. J Paleolimnol 5:285–287. doi:10.1007/BF00200351
Halvorsen GA, Heneberry JH, Snucins E (2001) Sublittoral chironomids as indicators of acidity (Diptera: Chironomidae). Water Air Soil Pollut 130:1385–1390. doi:10.1023/A:1013975905893
Heiri O, Lotter AF (2001) Effect of low count sums on quantitative environmental reconstructions: an example using subfossil chironomids. J Paleolimnol 26:343–350. doi:10.1023/A:1017568913302
Heiri O, Lotter AF (2005) Holocene and Lateglacial summer temperature reconstruction in the Swiss Alps based on fossil assemblages of aquatic organisms: a review. Boreas 34:506–516. doi:10.1080/03009480500231229
Hu FS, Kaufman D, Yoneji S, Nelson D, Shemesh A, Huang Y et al (2003) Cyclic variation and solar forcing of Holocene climate in the Alaskan subarctic. Science 301:1890–1893. doi:10.1126/science.1088568
Hu FS, Nelson DM, Clarke GH, Ruhland KM, Huang YS, Kaufman DS et al (2006) Abrupt climatic events during the last glacial-interglacial transition in Alaska. Geophys Res Lett:33 doi:10.1029/2006GLO27261
IPCC (2007) Working Group I Report (WGI): Climate Change 2007: the physical science basis. Cambridge University Press, Cambridge, UK
Johannessen OM, Miles M, Bjorgo E (1995) The Arctic’s shrinking sea-ice. Nature 376:126–127. doi:10.1038/376126a0
Johnson MG, McNeil OC (1988) Fossil midge associations in relation to trophic and acidic state of the Turkey Lakes. Can J Fish Aquat Sci 45:136–144. doi:10.1139/f88-278
Johnson MG, Kelso JRM, McNeil OC, Morton WB (1990) Fossil midge associations and the historical status of fish in acidified lakes. J Paleolimnol 3:113–127. doi:10.1007/BF00414066
Jones VJ, Juggins S (1995) The construction of a diatom-based chlorophyll a transfer function and its application at three lakes on Signy Island (maritime Antarctic) subject to differing degrees of nutrient enrichment. Freshw Biol 34:433–445. doi:10.1111/j.1365-2427.1995.tb00901.x
Juggins S (2003) Program C2 data analysis. Version 1.4.2. University of Newcastle, Newcastle UK
Kattenberg A et al (1996) Climate models––Projections of future climate. Climate Change 1995. In Houghton et al (eds) The science of climate change. Cambridge University Press, pp 285–357
Larocque I, Rolland N (2006) A visual guide to sub-fossil chironomids from Québec to Ellesmere Island. Rapport R-900. Institut National de la Recherche Scientifique, Québec
Larocque I, Hall RI, Grahn E (2001) Chironomids as indicators of climate change: a 100-lake training set from a subarctic region of northern Sweden (Lapland). J Paleolimnol 26:307–322. doi:10.1023/A:1017524101783
Larocque I, Pienitz R, Rolland N (2006) Factors influencing the distribution of chironomids in lakes distributed along a latitudinal gradient in northwestern Quebec, Canada. Can J Fish Aquat Sci 63:1286–1297. doi:10.1139/F06-020
Levesque AJ, Mayle FE, Walker IR, Cwynar LC (1993) A previously unrecognized Late-Glacial cold event in Eastern North. Am Nat 361:623–626
Levesque AJ, Cwynar LC, Walker IR (1997) Exceptionally steep north south gradients in lake temperatures during the last deglaciation. Nature 385:423–426. doi:10.1038/385423a0
Lotter AF, Birks HJB, Hofmann W, Marchetto A (1997) Modern diatom, cladocera, chironomid, and chrysophyte cyst assemblages as quantitative indicators for the reconstruction of past environmental conditions in the Alps.1. Climate. J Paleolimnol 18:395–420
Lotter AF, Birks HJB, Hofmann W, Marchetto A (1998) Modern diatom, cladocera, chironomid, and chrysophyte cyst assemblages as quantitative indicators for the reconstruction of past environmental conditions in the Alps. II. Nutrients. J Paleolimnol 19:443–463. doi:10.1023/A:1007994206432
Lotter AF, Walker IR, Brooks SJ, Hofmann W (1999) An intercontinental comparison of chironomid palaeotemperature inference models: Europe vs North America. Quat Sci Rev 18:717–735. doi:10.1016/S0277-3791(98)00044-4
MacDonald GM, Edwards TWD, Moser KA, Pienitz R, Smol JP (1993) Rapid response of treeline vegetation and lakes to past climate warming. Nature 361:243–246
MacDonald GM, Szeicz JM, Claricoates J, Dale KA (1998) Response of the central Canadian treeline to recent climatic changes. Ann Assoc Am Geogr 88:183–208. doi:10.1111/1467-8306.00090
Miller GH, Wolfe AP, Briner JP, Sauer PE, Nesje A (2005) Holocene glaciation and climate evolution of Baffin Island Arctic Canada. Quat Sci Rev 24:1703–1721. doi:10.1016/j.quascirev.2004.06.021
Moritz RE, Bitz CM, Steig EJ (2002) Dynamics of recent climate change in the Arctic. Science 297:1497–1502. doi:10.1126/science.1076522
New M, Lister D, Hulme M, Makin I (2002) A high-resolution data set of surface climate over global land areas. Clim Res 21:1–25. doi:10.3354/cr021001
Oechel WC, Hastings SJ, Vourlitis G, Jenkins M, Riechers G, Grulke N (1993) Recent change of Arctic Tundra ecosystems from a net carbon-dioxide sink to a source. Nature 361:520–523. doi:10.1038/361520a0
Olander H, Korhola A, Blom T (1997) Surface sediment Chironomidae (Insecta: Diptera) distributions along an ecotonal transect in subarctic Fennoscandia: developing a tool for palaeotemperature reconstructions. J Paleolimnol 18:45–59
Olander H, Birks HJB, Korhola A, Blom T (1999) An expanded calibration model for inferring lakewater and air temperatures from fossil chironomid assemblages in northern Fennoscandia. Holocene 9:279–294. doi:10.1191/095968399677918040
Oliver DR, Roussel ME (1983) The insects and arachnids of Canada, Part II: the genera of larval midges of Canada-Diptera: Chironomidae. Agric Can Publ 1746:1–263
Overpeck J, Hughen K, Hardy D, Bradley R, Case R, Douglas M et al (1997) Arctic environmental change of the last four centuries. Science 278:1251–1256. doi:10.1126/science.278.5341.1251
Padgham WA, Fyson WK (1992) The Slave Province—a distinct Archean Craton. Can J Earth Sci 29:2072–2086
Ponader K, Pienitz R, Vincent W, Gajewski K (2002) Limnological conditions in a subarctic lake (northern Quebec, Canada) during the late Holocene: analyses based on fossil diatoms. J Paleolimnol 27:353–366. doi:10.1023/A:1016033028144
Porinchu DF, Cwynar LC (2000) The distribution of freshwater Chironomidae (Insecta : Diptera) across treeline near the lower Lena River, northeast Siberia, Russia. Arct Antarct Alp Res 32:429–437. doi:10.2307/1552392
Porinchu DF, MacDonald GM, Bloom AM, Moser KA (2002) The modern distribution of chironomid sub-fossils (Insecta: Diptera) in the Sierra Nevada, California: potential for paleoclimatic reconstructions. J Paleolimnol 28:355–375. doi:10.1023/A:1021658612325
Porinchu DF, Potito AP, MacDonald GM, Bloom AM (2007a) Subfossil chironomids as indicators of recent climate change in Sierra Nevada, California, lakes. Arct Antarct Alp Res 39:286–296. doi:10.1657/1523-0430(2007)39[286:SCAIOR]2.0.CO;2
Porinchu DF, Moser KA, Munroe J (2007b) Development of a midge-based summer surface water temperature inference model for the Great Basin of the Western United States. Arct Antarct Alp Res 39:566–577. doi:10.1657/1523-0430(07-033)[PORINCHU]2.0.CO;2
Potito AP, Porinchu DF, MacDonald GM, Moser KA (2006) A late Quaternary chironomid-inferred temperature record from the Sierra Nevada, California, with connections to northeast Pacific sea surface temperatures. Quat Res 66:356–363. doi:10.1016/j.yqres.2006.05.005
Quinlan R, Smol JP, Hall RI (1998) Quantitative inferences of past hypolimnetic anoxia in south-central Ontario lakes using fossil midges (Diptera: Chironomidae). Can J Fish Aquat Sci 55:587–596. doi:10.1139/cjfas-55-3-587
Ritchie JC (1984) Past and present vegetation of the far northwest of Canada. University of Toronto Press, Toronto, 251 pp
Rolland N, Larocque I, Francus P, Pienitz R, Laperrière L Holocene climate inferred from biological (Diptera: Chironomidae) analyses in a Southampton Island (Nunavut, Canada) lake. Holocene (in press)
Saulnier-Talbot E, Pienitz R (2001) Postglacial isolation of a coastal basin near Kuujjuaraapik-Whapmagoostui, Hudsonie: a diatom biostratigraphical investigation. Geogr Phys Quat 55:63–74
Seppa H, Cwynar LC, MacDonald GM (2003) Post-glacial vegetation reconstruction and a possible 8200 cal. yr BP event from the low arctic of continental Nunavut, Canada. J Quat Sci 18:621–629
Serreze MC, Walsh JE, Chapin FS, Osterkamp T, Dyurgerov M, Romanovsky V et al (2000) Observational evidence of recent change in the northern high-latitude environment. Clim Change 46:159–207. doi:10.1023/A:1005504031923
Sharpe DR (1993) Surficial geology, Cambridge Bay, District of Franklin, Northwest Territories, 1825A. Geological Survey of Canada
Simpson KW, Bode RW (1980) Common larvae of Chironomidae (Diptera) from New York State streams and rivers with particular reference to the fauna of artificial substrates. Bull N Y State Mus 439:1–105
Sirois L (1992) The Transition between boreal forest and tundra. In: Shugart H, Leemans R, Bonan G (eds) A systems analysis of the Global Boreal forest. Cambridge University Press, Cambridge, pp 196–215
Smol JP, Douglas MSV (2007) Crossing the final ecological threshold in high Arctic ponds. Proc Natl Acad Sci USA 104:12395–12397. doi:10.1073/pnas.0702777104
Smol JP, Wolfe AP, Birks HJB, Douglas MSV, Jones VJ, Korhola A et al (2005) Climate-driven regime shifts in the biological communities of arctic lakes. Proc Natl Acad Sci USA 102:4397–4402. doi:10.1073/pnas.0500245102
Szeicz JM, MacDonald GM (2001) Montane climate and vegetation dynamics in easternmost Beringia during the Late Quaternary. Quat Sci Rev 20:247–257. doi:10.1016/S0277-3791(00)00119-0
Velle G, Brooks SJ, Birks HJB, Willassen E (2005) Chironomids as a tool for inferring Holocene climate: an assessment based on six sites in southern Scandinavia. Quat Sci Rev 24:1429–1462. doi:10.1016/j.quascirev.2004.10.010
Walker IR (1988) Late-Quaternary Paleoecology of Chironomidae (Diptera: Insecta) from Lake Sediments in British Columbia, PhD dissertation, Simon Fraser University, Burnaby, Canada, 204 pp
Walker IR (2001) Midges: Chironomidae and related Diptera. In: Smol JP, Birks HJB, Last WM (eds) Tracking environmental change using lake sediments. vol 4: zoological indicators. Kluwer Academic Publishers, Dordrecht, pp 43–66
Walker IR, MacDonald GM (1995) Distributions of Chironomidae (Insecta, Diptera) and other fresh-water midges respect to treeline, northwest-territories, Canada. Arct Alp Res 27:258–263. doi:10.2307/1551956
Walker IR, Mott RJ, Smol JP (1991) Allerod-Younger Dryas lake temperatures from midge fossils in Atlantic Canada. Science 253:1010–1012. doi:10.1126/science.253.5023.1010
Walker IR, Levesque AJ, Cwynar LC, Lotter AF (1997) An expanded surface-water palaeotemperature inference model for use with fossil midges from eastern Canada. J Paleolimnol 18:165–178. doi:10.1023/A:1007997602935
Wiederholm T (ed) (1983) Chironomidae of the Holarctic region. Keys and diagnoses. Part I––Larvae. Entomol Scand Suppl 19:1–457
Acknowledgements
This work was funded by a NSF Paleoclimate award (ATM-0442177) to D.F.P and K.A.M. We are grateful to NSF, VECO, the Polar Continental Shelf Project (PCSP) and the Nunavut Research Institute (NRI) for field and logistical support. We are also grateful to Glen MacDonald and two anonymous reviewers for detailed, constructive criticism of this paper. We thank Derek Muir, Xiaowa Wang and their colleagues at the water chemistry lab in the National Laboratory for Environmental Testing (NLET), Water Science and Technology Directorate of Environment Canada (Burlington, ON) for water chemistry analyses. We also thank Ken Clogg-Wright and Glen MacDonald for their help in the field and Lori Miller and Russ Brandenburg for sediment processing.
Author information
Authors and Affiliations
Corresponding author
Additional information
David Porinchu and Nicolas Rolland contributed equally to the work.
Rights and permissions
About this article
Cite this article
Porinchu, D., Rolland, N. & Moser, K. Development of a chironomid-based air temperature inference model for the central Canadian Arctic. J Paleolimnol 41, 349–368 (2009). https://doi.org/10.1007/s10933-008-9233-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10933-008-9233-3