Abstract
Seabirds are prominent biovectors whose guano and other wastes are an important source of nutrients that can eutrophy terrestrial and aquatic environments surrounding their breeding and nesting habitats. When these ornithogenically derived nutrients are introduced to waterbodies, they alter aquatic conditions, resulting in shifts in benthic invertebrate communities. In this paleolimnological study, we examined subfossil Chironomidae (non-biting midge) assemblages to assess the impacts of changes in the colony size of the Leach’s Storm-Petrel in three ponds on Baccalieu Island (Newfoundland and Labrador, Canada) over the past ~ 1700 years. Our results indicate that chironomids tracked the growth of the storm-petrel colony (determined by five additional paleolimnological proxies) starting in the early-1800s, and the decline of the colony in the 1980s. Given the shallow nature of the study ponds, assemblage changes likely occurred due to a combination of fluctuations in pH, metal concentrations, and bottom-water oxygen. In the ponds influenced by storm-petrels, we observed a poorly described form of degradation in subfossil chironomids that we attribute to chitinolytic processes mediated by bacteria and/or fungi that thrive on organic matter in productive aquatic systems. This study provides complementary proxy data regarding bottom-water habitats for use alongside other established paleolimnological methods to determine the long-term population dynamics of seabirds.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Biovectors are organisms that transport and concentrate large amounts of nutrients, metals, and contaminants across ecosystems via their feeding and nesting behaviours (Blais et al. 2007). Seabirds are important biovectors as they are typically gregarious, upper-trophic organisms that primarily feed in the ocean, transporting and concentrating marine-derived nutrients to terrestrial ecosystems where they congregate to nest and breed (Otero et al. 2018). Because of the protein-rich diet of seabirds, their waste products tend to be high in nitrogen (N) and phosphorus (P) (Otero et al. 2018). Further, because seabirds are globally distributed in large numbers, they are an important source of nutrient cycling, excreting an estimated 3800 gigagrams nitrogen per year (Gg N y-1) and 631 gigagrams phosphorus per year (Gg P y-1) onto terrestrial environments globally (Otero et al. 2018). This is equivalent to approximately 3% of deposited reactive N from fertilisers globally (Ngatia et al. 2019) and 18% of atmospheric P deposited through mineral aerosols and global flux (Peñuelas et al. 2013).
The Leach’s Storm-Petrel (Hydrobates leucorhous Vieillot, 1818; hereafter referred to as storm-petrels) is the most abundant seabird nesting in Eastern Canada (Hedd et al. 2006). Storm-petrels form large congregations that release large amounts of nutrients into their nesting environment through their wastes in the forms of feces, carcasses, regurgitated food, eggshells, and feathers. Because the storm-petrel is an upper-trophic-level predator that nests in burrows (Pollet et al. 2020), their nutrient-rich and acidic wastes fertilise nearby aquatic and terrestrial environments of the islands they inhabit (Duda et al. 2020a, b). In 2016, the storm-petrel was reclassified as “Vulnerable” by the International Union for Conservation of Nature (IUCN) Red List (Birdlife International 2018). This reclassification was based on global declines since the 1980s and indicates the Leach’s Storm-petrel is at risk of extinction without mediation.
A recent paleolimnological study by Duda et al. (2020a) conducted on ponds influenced by the world’s largest storm-petrel colony on Baccalieu Island determined the long-term colony dynamics of storm-petrels over the past ~ 1700 years and suggested that the movement of storm-petrels amongst colonies may partly explain the declines in the storm-petrel colony size. However, anthropogenic impacts likely contributed to their declines, including mercury contamination (Bond and Diamond 2009), light pollution (Cabrera-Cruz et al. 2018), and collisions with offshore infrastructure (Burke et al. 2012). Non-anthropogenic sources may also influence the storm-petrel population size, including climate-related changes to marine ecosystems such as the Atlantic Multidecadal Oscillation (Edwards et al. 2013), long-term predator-prey relationships (Buren et al. 2015), or natural population dynamics (Duda et al. 2020a). Regardless of the driver(s) of population decline, an understanding of long-term population dynamics is required before best practices regarding management and conservation can be implemented.
In this study, we provide a detailed description of changes in chironomid assemblages to better understand the effects of the varying colony size of storm-petrels on bottom-water environments on Baccalieu Island. Paleolimnological studies have been used to understand seabird population dynamics (Hargan et al. 2019; Duda et al. 2020a), successfully validating existing monitoring data or compensating for the lack of long-term monitoring data (Smol 2019). In paleolimnology, multi-proxy studies are typically required to effectively estimate complex population dynamics of species as no single indicator can indiscriminately track population changes. Chironomid assemblages are well-established and reliable indicators used to track population dynamics as they respond to limnological variables affected by seabird inputs like pH (Porinchu and MacDonald 2003) and oxygen (Quinlan and Smol 2001a). However, the use of chironomids has not been fully developed to reconstruct population dynamics of seabirds, with only a few studies conducted in the High Arctic (Michelutti et al. 2009; Stewart et al. 2013; Luoto et al. 2014).
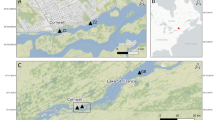
Located off the east coast of Newfoundland (Canada), Baccalieu Island (48°08′ N, 52°48′ W; Fig. 1) is the site of the largest colony of Leach’s Storm-Petrels in the Northwest Atlantic (Montevecchi and Tuck 1987; Wilhelm et al. 2020), and supports 48–59% of the global Leach’s Storm-Petrel population (Duda et al. 2020a). Baccalieu is a small (~ 6 km2 surface area) island located ~ 64 km north of St. John’s and is part of the Eastern Hyper-Oceanic Ecoregion, which is categorized by a rugged topography of mainly grass, heath, krummholz trees (e.g. Black Spruce (Picea mariana), Balsam Fir (Abies balsamea)), and shrubs (e.g. Paper Birch (Betula papyrifera) and Alder (Alnus spp.)) (Montevecchi and Tuck 1987; Wilhelm et al. 2020). Although smaller populations of other seabirds also nest on Baccalieu Island, they predominately nest on the cliff edge on the periphery of the island for easier access to the ocean (Montevecchi and Tuck 1987; Wilhelm et al. 2020; Duda et al. 2020a). Therefore, the nutrient inputs to the ponds located in the central parts of the island and the changes observed in the sedimentary record are associated chiefly to the storm-petrels that nest in high density on the inland of the island (Duda et al. 2020a).
Locations of the study ponds. The inset map of Newfoundland highlights the study sites with a red square box. On Baccalieu Island, A is the highly-influenced Lunin Pond, B is the highly-influenced Brister Pond, and C is the moderately-influenced Gull Pond. Approximately 4 km west of Baccalieu Island is the mainland reference pond, identified as D
The first population survey on Baccalieu Island in 1984 estimated 3.36 million breeding pairs of Leach’s Storm-Petrels (Sklepkovych and Montevecchi 1989). In order to protect this important seabird nesting ground, the island was designated as an ecological reserve in 1995 (Parks and Natural Areas Division 1995). Despite the protection of Baccalieu Island, a subsequent ornithological survey conducted in 2013 recorded a ~ 40% decline in the storm-petrel colony to 1.95 million pairs (Wilhelm et al. 2020). To examine when the decline began and to identify its potential drivers, Duda et al. (2020a) reconstructed the dynamics of the storm-petrel colony on Baccalieu Island over the past ~ 1700 years. Synchronous shifts in several paleolimnological proxies (including diatoms, δ15N, ornithogenically introduced metal concentrations, sedimentary chlorophyll a, and cholesterol) corroborated the surveys indicating declines in the storm-petrel colony on Baccalieu Island since the 1980s, but also revealed that the colony was much smaller in the past. In fact, the colony size was estimated to be the highest in the 1980s compared to the last two millennia. The authors also described a smaller peak in the storm-petrel colony ca. 500 CE (Duda et al. 2020a).
Here we tracked changes in chironomid assemblages over the past ~ 1700 years from ponds on Baccalieu Island using the same dated sediment cores analysed by Duda et al. (2020a, b). We examined three ponds on Baccalieu Island (Brister, Lunin, and Gull ponds) and a mainland reference pond to contrast varying degrees of storm-petrel influence (Fig. 1). The relative storm-petrel inputs into each pond were determined by measuring the area of each watershed occupied by storm-petrel nesting grounds (Duda et al. 2020a) to a priori categorize Lunin and Brister ponds as highly-influenced ponds, and Gull Pond as a moderately influenced pond. To identify regional impacts such as climate, we contrasted our results to a mainland reference pond located ~ 4 km west of Baccalieu Island on mainland Newfoundland that is unimpacted by storm-petrel-derived inputs. This reference pond was selected based on its close proximity to Baccalieu Island, and the similarity in physical features, such as elevation, depth, and surface area (Table 1). As presented in Duda et al. (2020a), Lunin and Brister ponds were considered hypereutrophic, with elevated production-related variables (total dissolved phosphorus (TP), total dissolved nitrogen (TN), chlorophyll a), and were nitrogen limited (TN: TP < 20; Guildford and Hecky 2000), compared to the mainland reference pond (Table 1). All study ponds were considered shallow (range = 1.5–6 m maximum depth) and acidic (pH range = 4.0–5.6; mean = 4.6) (Table 1).
Our data complement ornithological survey data from 1984 and 2013, and the Duda et al. (2020a, b) paleolimnological studies by providing a more holistic examination of the effects of the colony dynamics of storm-petrels on benthic aquatic environments on Baccalieu Island. Specifically, this study examined responses of chironomid assemblages to past changes in the storm-petrel colony size and their nutrient inputs in shallow, acidic, temperate ponds.
Methods
Field methods
The sediment cores from Lunin Pond (core length: 39 cm), Brister Pond (28 cm), Gull Pond (31 cm), and the mainland reference pond (11 cm) were collected between 13 and 17 September 2017, as detailed in Duda et al. (2020a). Sediment cores were retrieved using a high-resolution push corer specifically developed for retrieving sediment from shallow water environments (Glew and Smol 2016). Cores were retrieved from the deepest point of the pond (as determined by a handheld depth sounder) as this area typically archives the most complete sediment profile representing overall limnological changes (Smol 2008). Next, the sediment cores were sectioned on-site at 0.5 cm intervals using a Glew (1989) extruder. The sediments were then transported from the field sites to the Paleoecological Environmental Assessment and Research Laboratory (PEARL) at Queen’s University, where they were freeze-dried.
Laboratory methods
A detailed description of our sediment dating procedures is available in Duda et al. (2020a). Briefly, we used 210Pb gamma spectrometry to estimate the age of recent sediments using a constant rate of supply (CRS) model (Appleby and Oldfield 1978). Also, we used accelerator mass spectrometry (AMS) radiocarbon dating on a terrestrial stem fragment retrieved from the 38–38.5 cm interval sediment core from Lunin Pond to estimate the basal age of our longest core. The 14C date of the sample was then calibrated using the IntCal13 calibration curve, and the remaining dates were extrapolated using a smooth spline in the package CLAM v.2.3.2 (Blaauw and Christen 2011) in R v.3.6.0 (RStudio Team 2015). We extrapolated the basal age of the sediment cores from Brister and Gull ponds using a second-order polynomial regression from the 210Pb curve, and therefore these dates should be viewed with caution.
Chironomid subsamples were prepared following the laboratory techniques described in Walker (2001). Briefly, 80 ml of 5% KOH was added to 0.1 g to 4.9 g (dry weight) of each sample, and the solution was heated at 80 °C for 30 min to deflocculate the sediments and digest excess organic matter. Next, we passed each sample through a 100-µm sieve, washed it with deionised water, and backwashed the sievings into sample pots. Several drops of 95% ethanol were added to each sample for preservation. We performed chironomid counts along four transverses per tray on the grooved Perspex (Bogorov) sorter; one pass focusing on the surface of the liquid and one pass focusing on the bottom of the tray. The passes were repeated after mixing the entire subsample and allowing it to settle. For all ponds on Baccalieu Island, at least 50 chironomid head capsules were picked and identified from each interval (Quinlan and Smol 2001b). For the reference pond, there were very low counts, and therefore 50 head capsules could not be reached for every interval. As such, all other intervals, except 10.75 cm, were combined with their adjacent sample (e.g. 1.0–1.5 cm combined with 1.5–2.0 cm, 2.0–2.5 cm combined with 2.5–3.0 cm, etc.). All chironomid head capsules were picked using fine forceps with a Nikon SMZ645 stereoscopic zoom microscope at 25–40 times magnification, placed on a glass coverslip with their ventral sides facing upward, and mounted on a microscope slide using Entellan®. The chironomid head capsules were identified at 100–400 times magnification under a Leica DM2500 optical microscope, using standard identification manuals and the taxonomy from Cranston (2010) and Anderson et al. (2013). All chironomids were identified to the genus level and photographed. We counted complete head capsules or head capsules with more than half a mentum as one, and capsules with half a mentum as half. Head capsules with less than half a mentum were omitted. Similar to earlier works (e.g. Heiri and Lotter 2003; Urrutia et al. 2010; Williams et al. 2012; Thienpont et al. 2015), the following taxa were combined due to their similar morphology and/or poor preservation: Corynoneura/Thienemaniella spp., Cricotopus/Orthocladius spp., Limnophyes/Paralimnophyes spp., and Smittia/Parasmittia spp. The combined taxa also share similar ecological characteristics. Members of the tribe Tanytarsini were also grouped due to poor preservation of identifying features (Brooks et al. 1997). It should be noted that the genus-level grouping of these chironomid taxa could have led to the potential loss of pertinent ecological information that could have been achieved with species-level identification or groupings. The taxonomic identification of chironomids is also continually improving, and recent work has begun to distinguish between the groups (Brooks et al. 2007). A complete list of chironomids from each pond is available in the electronic supplementary material (ESM1 Tables 1–4).
Data analysis
Chironomid assemblage data were expressed as relative abundances. The diversity of chironomids was calculated using Hill’s N2, which is a measure of the effective number of species, while downweighting the rare taxa (Hill 1973).
Stratigraphic diagrams of chironomid assemblages were constructed for each site using the program C2 (Juggins 2007). Included in each stratigraphy are chironomid taxa that appear in at least one sample depth with ≥ 10% relative abundance. Major assemblage zones were identified using a constrained incremental sum of squares (CONISS) (Grimm 1987; Juggins 2020) on all observed taxa in R Studio v.3.6.0 (RStudio Team 2015), and were assessed using a broken stick-model (Bennett 1996).
A chironomid-based volume-weighted hypolimnetic oxygen (VWHO) reconstruction was attempted with the chironomid assemblages from all the ponds using a training (also known as calibration) set from Quinlan and Smol (2001a). However, due to the high dissimilarity indices and negative reconstructed oxygen values, the chironomid-based VWHO was omitted from further interpretation. The high dissimilarity indices could be explained by differences in morphology of the ponds examined and those reported in Quinlan and Smol (2001a). Unlike the lakes used in the training set by Quinlan and Smol (2001a), the ponds in this study are shallow and acidic (Table 1). Instead, qualitative interpretations based on the known ecological optima of key taxa were used for interpretations.
In each figure, we summed the storm-petrel colony trends as interpreted by each sediment record; we provide a summary of all measured proxies in the site as a Z-score. An increase in Z-score can therefore be interpreted as an increase in the number of seabirds. The summary for Lunin Pond includes δ15N, ornithogenic diatoms, Chironomus spp., Cd and Zn concentrations, sedimentary chlorophyll-a, and cholesterol (Duda et al. 2020a). The summaries for Brister and Gull Pond include the same proxies, without cholesterol as it was not measured for those sites. As the summary line is relative to site-specific changes in the ornithogenic proxies, it was omitted from the reference pond.
Chironomid degradation
When analysing chironomid head capsules from the ponds, we observed what we believe was a hitherto undescribed form of degradation in some chironomid head capsules. The degradation was unlike typical degradation observed in chironomid head capsules, such as the loss of parts of the head capsule or its identifying features (Velle et al. 2005). To explore whether seabird-derived nutrients enhanced the level of degradation, we quantified the percent degradation of chironomid head capsules by surface area (Fig. 2). As it would be unrealistic and time consuming to measure degradation of every head capsule, we measured the amount of degradation on 20% of randomly selected chironomids per sample from all ponds as representative sample degradation. Given the low number of chironomids retrieved in the mainland reference pond, all picked head capsules were analysed. The percent surface area degradation for each chironomid head capsule was determined using ImageJ, a Java-based image processing program (Abràmoff et al. 2004). The full visible areas and degraded areas of each chironomid head capsule were manually demarcated in the program (Fig. 2). The use of statistics was omitted from the analyses due to the complexity of the dataset, such as temporal autocorrelation and non-parametric distribution.
Results
Lunin Pond
A total of 1137.5 chironomid head capsules from 22 genera were identified in the 39-cm-long sediment core retrieved from Lunin Pond (Fig. 3; ESM1 Table 1). The pond was dominated by the subfamily Chironominae (71.4% relative abundance) followed by Orthocladiinae (28.3%). The majority of chironomids consist of the genus Microtendipes spp. and the tribe Tanytarsini consist of Microtendipes spp. (33.1%) and Tanytarsini (19.6%) (Fig. 3). Head capsule concentration ranged from 113 to 179 head capsules per gram dry weight (Fig. 3). Below, we describe overall trends based on the zones identified using a full chironomid assemblage CONISS (Grimm 1987). The CONISS-derived zones are demarcated in brackets.
Stratigraphic diagram of the relative abundances (%) of chironomid taxa from Lunin Pond. Green shaded areas demarcate periods of increased storm-petrel abundance (Duda et al. 2020a). Darker green shading represents the larger, modern storm-petrel colony and lighter green represents the smaller, earlier colony. Dotted lines demarcate chironomid-inferred zones. Only chironomid taxa that appeared in at least one sample depth with a relative abundance of ≥ 10% were included in the stratigraphic diagram. Dates in italics are extrapolated and should therefore be interpreted with caution
Zone L2 (0–7.25 cm; ~ 1990–2017 CE)
From the late-1900s until present, when the storm-petrel colony was at its largest size in the last ~ 1700 years (Duda et al. 2020a), the head capsule concentration ranged from 172 to 1193 head capsules per gram dry weight (Fig. 3). Microtendipes spp. was the most abundant genus (39.1% relative abundance), followed by Chironomus spp. (22.3%) (Fig. 3). In this zone, Hill’s N2 diversity was lower (mean = 4.2) than in Zone L1 (Fig. 3).
Zone L1 (7.25–38.25 cm; ~ 350 CE–1990 CE)
Before the late-1990s, when the storm-petrel colony size was smaller than in Zone L2 and there was less nutrient input in Lunin Pond (Duda et al. 2020a), the head capsule concentration ranged from 131 to 236 head capsules per gram dry weight (Fig. 3). Microtendipes spp. was the most abundant genus (31.6% relative abundance). There was a notable decline in Chironomus spp., and an increase in Brillia spp., Cricotopus/Orthocladius spp., Dicrotendipes spp., and Heterotrissocladius spp. (Fig. 3). The relative abundance of Tanytarsini was similar between Zones L1 and L2 (Fig. 3). In this zone, Hill’s N2 diversity was at its highest (mean = 6.2) (Fig. 3).
Brister Pond
A total of 874 chironomid head capsules were identified from 20 chironomid genera in the 28-cm long sediment core retrieved from Brister Pond (Fig. 4; ESM1 Table 2). The profile was dominated by the subfamily Chironominae (74.0% relative abundance) followed by Orthocladiinae (26.0%). The majority of chironomids consisted of Microtendipes spp. (29.1%) and Tanytarsini (22.8%) (Fig. 4).
Stratigraphic diagram of the relative abundances (%) of chironomid taxa from Brister Pond. Green shaded areas demarcate periods of increased storm-petrel abundance (Duda et al. 2020a). Dotted lines demarcate chironomid-inferred zones. Only chironomid taxa that appeared in at least one sample depth with a relative abundance of ≥ 10% were included in the stratigraphic diagram. Dates in italics are extrapolated and should therefore be interpreted with caution
Zone B2 (0–17.25 cm; ~ 1730–2017 CE)
When the storm-petrel colony was large (Duda et al. 2020a), the head capsule concentration was lower in Zone B2, ranging from 109 to 257 head capsules per gram dry weight (Fig. 4). Microtendipes spp. was the most abundant genus (32.4% relative abundance) (Fig. 4). The relative abundances of most taxa were relatively stable, with the exception of Glyptotendipes spp., which decreased down the core and became absent below 12.25 cm (Fig. 4). Hill’s N2 diversity was lower in this zone compared to Zone B1, with an average of 5.6 (Fig. 4).
Zone B1 (17.25–26.25 cm; ~ 1380–1730 CE)
When the storm-petrel colony size was smaller, based on colony dynamics determined by Duda et al. (2020a), the head capsule concentration ranged from 114 to 403 head capsules per gram dry weight and was generally higher than Zone B2 (Fig. 4). Head capsule concentrations increased and peaked at 20.25 cm before declining thereafter. Microtendipes spp. was also the most abundant genus (22.4% relative abundance) (Fig. 4). Tanytarsini had a consistent relative abundance in Zones B1 and B2, but there was an increase in Cricotopus/Orthocladius spp. and Heterotrissocladius spp. (Fig. 4). Conversely, Chironomus spp. and Glyptotendipes spp. were mostly absent (Fig. 4). In this zone, Hill’s N2 diversity was stable and high, with an average of 6.8 (Fig. 4).
Gull Pond
A total of 883 chironomid head capsules from 17 chironomid genera were identified in the 31-cm long sediment core retrieved from Gull Pond (Fig. 5; ESM1 Table 3). The pond was dominated by the subfamily Chironominae (70.9% relative abundance) and Orthocladiinae (29.1%) (Fig. 5). The majority of chironomids consisted of Tanytarsini (31.0%) and Microtendipes spp. (27.2%) (Fig. 5).
Stratigraphic diagram of the relative abundances (%) of chironomid taxa from Gull Pond. Green shaded areas demarcate periods of increased storm-petrel abundance (Duda et al. 2020a). Dotted lines demarcate chironomid-inferred zones. Only chironomid taxa that appeared in at least one sample depth with a relative abundance of ≥ 10% were included in the stratigraphic diagram. Dates in italics are extrapolated and should therefore be interpreted with caution
Zone G2 (0–7.25 cm; ~ 1920–2017 CE)
When the storm-petrel colony was large after the early-1900s (Duda et al. 2020a), the head capsule concentration was highest in Zone G2, ranging from 235 to 360 head capsules per gram dry weight, where it peaked at 4.25 cm (Fig. 5). Microtendipes spp. was the most abundant genus (44.2% relative abundance), followed by Tanytarsini (29.5%) (Fig. 5). Despite having a low relative abundance of < 10%, Glyptotendipes spp. was included in the stratigraphy due to its increasing abundance in Zone G2 (Fig. 5). The average Hill’s N2 diversity was 3.3 (Fig. 5).
Zone G1 (7.25–30.25 cm; ~ 930–1920 CE)
When the storm-petrel colony size was smaller (Duda et al. 2020a), the head capsule concentration ranged from 73 to 277 head capsules per gram dry weight, the head capsule concentration was lower in Zone G1 compared to Zone G2 (Fig. 5). There was an abundance of Tanytarsini (31.6% relative abundance), which maintained consistent relative abundances in Zones G1 and G2 (Fig. 5). In comparison to Zone G2, the relative abundance of Microtendipes spp. was lower in Zone G1, while that of Cricotopus/Orthocladius spp., Heterotrissocladius spp., and Dicrotendipes spp. was higher (Fig. 5). Glyptotendipes spp. was rarely observed (Fig. 5). The Hill’s N2 diversity was highest in this zone compared to the rest of the core, with an average of 4.8 (Fig. 5).
Mainland Reference Pond
A total of 244.5 chironomid head capsules from 17 chironomid genera were identified in the 11-cm long sediment core retrieved from the mainland reference pond (Fig. 6; ESM1 Table 5). The pond was dominated by the subfamily Chironominae (63.6% relative abundance) and Orthocladiinae (36.8%) (Fig. 6). The majority of chironomids consisted of Tanytarsini (17.4%) and Cricotopus/Orthocladius spp. (15.3%) (Fig. 6). 1.33 g to 4.91 g (dry weight) of sediment was digested per sample in the mainland reference pond. However, head capsule concentrations were very low, ranging from 1 to 39.8 head capsules per gram (dry weight) (Fig. 6).
Stratigraphic diagram of the relative abundances (%) of chironomid taxa from the mainland reference pond. White bars refer to sample depths with < 50 chironomid head capsules and therefore must be interpreted with caution. All chironomids were included in the stratigraphic diagram. Dates in italics are extrapolated and should therefore be interpreted with caution
Chironomid degradation
We described the observed degradation in many of the chironomid head capsules recovered from Baccalieu Island as a radial hollowing of chitin, with extended branching patterns (Fig. 7). Across all the study ponds, the average degradation of all taxa was 1.4% ± 1.3 SD (Figs. 2, 3, 4, 5). Overall, taxa that were highly degraded included Heterotrissocladius spp., Microtendipes spp., Dicrotendipes spp., and Cricotopus/Orthocladius spp.
When there was a larger storm-petrel colony after the 1800s (Duda et al. 2020a), Gull Pond had the highest total degradation (average degradation = 2.9%; Fig. 5), followed by Lunin Pond (1.5%; Fig. 3) and Brister Pond (1.5%; Fig. 4). The average degradation was greater in the presence of a larger storm-petrel colony in all the ponds, except Lunin Pond. Across Lunin, Brister and Gull ponds, the chironomids with high average degradation when there was a larger storm-petrel colony were Heterotrissocladius spp., Microtendipes spp., and Dicrotendipes spp.
When there was a smaller storm-petrel colony before the 1800s, Lunin Pond had the highest total degradation (average degradation = 2.7%; Fig. 3), followed by Gull Pond (1.5%; Fig. 5), Brister Pond (0.9%; Fig. 4) and the mainland reference pond (0.4%; Fig. 6). Across all the ponds, the chironomids with high average degradation when there was a smaller storm-petrel colony are Heterotrissocladius spp., Microtendipes spp., and Cricotopus/Orthocladius spp.
Discussion
Chironomid assemblages
Across all the study sites, we observed shifts in the chironomid assemblages that corresponded to the timing of changes in the storm-petrel colony on Baccalieu Island determined by Duda et al. (2020a). As such, chironomid assemblages predominantly tracked changes related to fluctuations in storm-petrel-derived nutrient inputs over the past ~ 1700 years and suggest that, alongside other proxies, chironomids can be an effective proxy to reconstruct past seabird colonies. These avian inputs increased the overall primary production of ponds on Baccalieu Island (Duda et al. 2020a) (Table 1), and likely decreased bottom-water oxygen and pH within the ponds. The changes in chironomid assemblages were also consistent with the degree of storm-petrel inputs into the ponds, with the highly-influenced Lunin and Brister ponds experiencing greater changes in the assemblage than the moderately influenced Gull Pond during similar timing. Despite the potential effects of recent climate on the ponds (Engels et al. 2020), it is unlikely that climate had a notable impact on our study systems relative to the seabird colony due to the synchronicity of trends with other paleolimnological proxies (Duda et al. 2020a, b), and the overwhelming impact of the large storm-petrel population on the island.
In the highly influenced Brister and Lunin ponds, the chironomid assemblages were dominated by taxa associated with low oxygen and acidic pH optima in the presence of a large storm-petrel colony on the island (Duda et al. 2020a). For example, we measured a 20% and 10% increase in Chironomus spp. in Brister and Lunin ponds, respectively, after the early-1900s, when there was a large storm-petrel colony on the island (Figs. 2, 3). Chironomus spp. are associated with periods of anoxia and acidity (Porinchu and MacDonald 2003) as they possess a greater concentration of haemoglobin that increases their buffering capacity (Jernelöv et al. 1981). Longer periods of low bottom-water oxygen concentrations can be linked to eutrophication (Foley et al. 2012), which we associate with the deposition and decomposition of storm-petrel-derived inputs into the ponds. The acidic storm-petrel guano (pH = 5.86 ± 0.66 SD; Duda et al. 2020a) would result in a decline in the pH of the ponds.
In Brister and Gull ponds, we observed a 4% increase in Glyptotendipes spp. after the early-1900s when the storm-petrel colony size was large (Figs. 3, 4). Similar to Chironomus spp., Glyptotendipes spp. are capable of thriving in low bottom-water oxygen concentrations (Quinlan and Smol 2001a). The increase in these taxa could indicate declining bottom-water oxygen levels linked to an increase in storm-petrel colony on Baccalieu Island after the mid-1900s.
In Lunin, Brister, and Gull ponds, we observed a synchronous decrease in Cricotopus/Orthocladius spp. and Heterotrissocladius spp. (Lunin Pond by 6% relative abundance, and by 8%, respectively; Brister Pond: 7% and 7%; Gull Pond: 7% and 9%) after the early-1900s when there was a large storm-petrel colony on Baccalieu Island (Figs. 3, 4, 5). Cricotopus/Orthocladius spp. are generally intolerant of pollution, rarely surviving low dissolved oxygen conditions below 6 mg L−1 (Arimoro et al. 2007) and pH beyond the range of 5.8–6.4 (Boggero et al. 2006). Heterotrissocladius spp. are acid-tolerant and typically associated with well-oxygenated, oligotrophic conditions (Porinchu and MacDonald 2003; Nazarova et al. 2017). The decrease in these taxa suggest declines in pH and benthic oxygen driven by seabird-derived eutrophication of these ponds from a larger storm-petrel colony.
In the mainland reference pond, Cricotopus/Orthocladius spp. and Heterotrissocladius spp. were present (Fig. 6), suggesting well-oxygenated conditions attributed to low lake productivity (Arimoro et al. 2007; Porinchu and MacDonald 2003). However, due to its low chironomid counts, the ecological interpretations of the mainland reference pond were made with caution. We associate the low chironomid counts in the mainland reference pond to unproductive systems that limit chironomid production, as has also been noted by Stewart et al. (2013) in similarly unproductive ponds.
When there was a larger storm-petrel colony on Baccalieu Island, the shifts in chironomid taxa from the storm-petrel-influenced ponds suggest changes in aquatic conditions linked to ornithogenically induced eutrophication. Chironomids likely responded indirectly to environmental factors associated to changes in the storm-petrel colony size, such as declining pH due to the acidic feces of storm-petrels. For example, Jernelöv et al. (1981) showed that the Chironomus spp. are more competitive under acidic conditions due to the high buffering capacity of their haemolymph.
The CONISS breaks based on changes in chironomid assemblages were delayed in timing compared to those derived from diatoms in Duda et al. (2020a) (Figs. 2, 3, 4). Diatom taxa, like Asterionella ralfsii var. americana and Fragilaria exigua, are associated with low pH environments linked to storm-petrel-derived inputs (Duda et al. 2020a). The delayed responses in chironomid assemblages are likely associated with the subsequent changes in bottom-water oxygen compared to the rapid changes typically recorded in diatom assemblages linked to direct changes in water chemistry parameters by storm-petrel inputs, such as nutrients and pH. Moreover, the smaller peak in the storm-petrel colony at ~ 500 CE (Duda et al. 2020a) was not detected by the chironomid assemblages as the aquatic changes from this earlier storm-petrel colony was likely not large enough to be detected by the bottom-water chironomid assemblages. Chironomid assemblages responded slower to the changes in aquatic conditions when there was a larger storm-petrel colony on the island. Conversely, chironomid assemblages responded more rapidly when there was a smaller storm-petrel colony on the island, evident from the shift in chironomid assemblages that tracked declines in the storm-petrel colony after the 1980s.
Chironomid degradation
We present evidence of elevated levels of chironomid degradation in the storm-petrel-impacted ponds (average degradation = 1.9% ± 1.2 SD; Figs. 2, 3, 4) compared to the mainland reference pond (0.4% ± 1.0 SD; Fig. 6), as well as elevated levels of chironomid degradation in the presence of a larger storm-petrel colony across all the seabird-impacted ponds (1.1% vs 0.04%). We associate these trends in chironomid degradation to the hypereutrophic and acidic aquatic conditions resulting from storm-petrel colonisation.
In aquatic environments, bacteria and fungi are capable of degrading chitin through chitinolysis (i.e. depolymerisation of chitosan) or deacetylation (i.e. removal of an acetyl group from a molecule) (Gooday 1990a). Productive aquatic systems, such as those on Baccalieu Island, can support large populations of bacteria and fungi as they can colonise and degrade the increased accumulation of organic matter linked to eutrophication (Wurzbacher et al. 2010). Such chitin degradation may occur at the sediment/water-interface and/or post-burial, but this requires further research. This may explain the greater degradation observed in the storm-petrel-impacted ponds compared to the mainland reference pond.
Chitinolytic bacteria, like Serratia, Chromobacterium, Pseudomonas, and Flavobacterium, have been identified as mediators of chitin degradation in aquatic systems (Gooday 1990b; Wurzbacher et al. 2010). Importantly, highly productive aquatic systems, such as the ponds in this study, support the growth of bacteria, as the organic matter from eutrophication is colonised and degraded by bacteria (Wurzbacher et al. 2010).
Fungi can also degrade chitin. Fungi that exist in lentic environments (Wurzbacher et al. 2010) are important chitinoclastic agents (Beir and Bertilsson 2013) capable of degrading natural polymers, such as arthropod skeletons (Wurzbacher et al. 2010). Moreover, dense fungal colonies have also been observed on chitinous zooplankton carapaces (Wurzbacher et al. 2010). Given the low pH of Lunin, Brister and Gull ponds (Table 1), and the abundance of fungal spores and hyphae in these ponds (Duda et al. 2020b), it is possible that fungi were favoured over bacteria in the degradation of chironomid head capsules (Wurzbacher et al. 2010).
The literature pertaining to subfossil chironomid head capsule degradation is limited and requires further study to resolve the source of the observed degradation. Although rare, the understanding of chironomid degradation is important as it provides insights into the changes in the composition of subfossil chironomid head capsules and other chitinous materials, which may affect the interpretation of ecological data in paleolimnology. Fortunately, in this study, the taxonomic features of chironomids were sufficiently well preserved, such that head capsules could still be identified to the genus level.
Conclusions
Long-term data, such as those provided by this study, are required to develop effective management strategies for the numerous in-decline seabird taxa. Paleolimnological approaches can provide insights on the population dynamics of seabirds, but to be truly effective, multiple corroborative proxies are necessary to ensure the most accurate interpretation of past environments and to determine which proxies are best suited to reconstruct populations. In this study, our chironomid data provided a qualitative reconstruction of changes of bottom-water habitats linked to the long-term dynamics of storm-petrels. These data complement ornithological survey data from 1984 (Sklepkovych and Montevecchi 1989) to 2013 (Willhelm et al. 2020), and the paleolimnological research conducted by Duda et al. (2020a, b) by providing a holistic examination of the effects of storm-petrels population dynamics on benthic aquatic environments. Long-term perspectives are critical for the development of more effective and informed conservation strategies (Birks 2012). Such data are especially important to establish the baseline population dynamics of seabirds, particularly in light of significant fluctuations in seabird populations that are predicted to occur in response to the effects of climate warming and other anthropogenic stressors (Jenouvrier 2013).
References
Abràmoff MD, Magalhães PJ, Ram SJ (2004) Image processing with ImageJ. Biophotonics Int 11:36–42
Appleby RG, Oldfield F (1978) The calculation of lead-210 dates assuming a constant rate of supply of unsupported 210Pb to the sediment. Catena 5:1–8
Arimoro FO, Ikomi RB, Iwegbue CMA (2007) Water quality changes in relation to Diptera community patterns and diversity measured at an organic effluent impacted stream in the Niger Delta, Nigeria. Ecol Indic 7:541–552
Bennett KD (1996) Determination of the number of zones in a biostratigraphical sequence. New Phytol 132:155–170
BirdLife International (2018) Hydrobates leucorhous. The IUCN red list of threatened species 2018: e.T132438298A132438484
Birks HJB (2012) Ecological palaeoecology and conservation biology: controversies, challenges, and compromises. Int J Biodivers Sci Ecosyst Serv Manag 8:292–304
Blais JM, Macdonald RW, Mackay D, Webster E, Harvey C, Smol JP (2007) Biologically mediated transport of contaminants to aquatic systems. Environ Sci Technol 41:1075–1084
Blaauw M, Christen JA (2011) Flexible paleoclimate age-depth models using an autoregressive gamma process. Bayesian Anal 6:457–474
Boggero A, Füreder L, Lencioni V, Simcic T, Thaler B, Ferrarese U, Lotter AF, Ettinger R (2006) Littoral chironomid communities of Alpine lakes in relation to environmental factors. Hydrobiologia 562:145–165
Bond AL, Diamond AW (2009) Mercury concentrations in seabird tissues from Machias Seal Island, New Brunswick, Canada. Sci Total Environ 407:4340–4347
Brooks SJ, Lowe JJ, Mayle FE (1997) The Late Devensian Lateglacial palaeoenvironmental record from Whitrig Bog, SE Scotland. 2. Chironomidae (Insecta: Diptera). Boreas 26:297–308
Buren AD, Koen-Alonso M, Pepin P, Mowbray F, Nakashima B, Stenson G, Ollerhead N, Montevecchi WA (2015) Bottom-up regulation of capelin, a keystone forage species. PLoS ONE 9:e87589
Burke CM, Montevecchi WA, Wiese FK (2012) Inadequate environmental monitoring around offshore oil and gas platforms on the Grand Bank of Eastern Canada: are risks to marine birds known? J Environ Manag 104:121–126
Cabrera-Cruz SA, Smolinsky JA, Buler JJ (2018) Light pollution is greatest within migration passage areas for nocturnally-migrating birds around the world. Sci Rep 8:3261
Carpenter SR, Cole JJ, Kitchell JF, Pace ML (1998) Impact of dissolved organic carbon, phosphorus, and grazing on phytoplankton biomass and production in experimental lakes. Limnol Oceanogr 43:73–80
Cranston PS (2010) Chiro Key. http://chirokey.skullisland.info/references/. Accessed 30 Oct 2019
Duda MP, Robertson GJ, Lim JE, Kissinger JA, Eickmeyer DC, Grooms C, Kimpe LE, Montevecchi WA, Michelutti N, Blais JM, Smol JP (2020a) Striking centennial-scale changes in the population size of a threatened seabird. Proc R Soc B 287:20192234
Duda MP, Glew JR, Michelutti N, Robertson GJ, Montevecchi WA, Kissinger JA, Eickmeyer DC, Blais JM, Smol JP (2020b) Long-term changes in terrestrial vegetation driven by shifts in a colonial seabird population. Ecosystems 23:1643–1656
Edwards M, Beaugrand G, Helaouët P, Alheit J, Coombs S (2013) Marine ecosystem response to the Atlantic Multidecadal Oscillation. PLoS ONE 8:e57212
Engels S, Medeiros AS, Axford Y, Brooks SJ, Heiri O, Luoto TP, Nazarova L, Porinchu DF, Quinlan R, Self AE (2020) Temperature change as a driver of spatial patterns and long-term trends in chironomid (Insecta: Diptera) diversity. Glob Change Biol 26:1155–1169
Foley B, Jones ID, Maberly SC, Rippey B (2012) Long-term changes in oxygen depletion in a small temperate lake: effects of climate change and eutrophication. Freshw Biol 57:278–289
Glew JR (1989) A new trigger mechanism for sediment samplers. J Paleolimnol 2:241–243
Glew JR, Smol JP (2016) A push corer developed for retrieving high-resolution sediment cores from shallow waters. J Paleolimnol 56:67–71
Gooday GW (1990a) Physiology of microbial degradation of chitin and chitosan. Biodegradation 1:279–312
Gooday GW (1990b) The ecology of chitin degradation. Advances in microbial ecology, vol 11. Springer, Boston, pp 387–430
Grimm EC (1987) CONISS: a FORTRAN 77 program for stratigraphically constrained cluster analysis by the method of incremental sum of squares. Comput Geosci 13:13–35
Guildford SJ, Hecky RE (2000) Total nitrogen, total phosphorus, and nutrient limitation in lakes and oceans: Is there a common relationship? Limnol Oceanogr 45:1213–1223
Hargan KE, Gilchrist HG, Clyde NMT, Iverson SA, Forbes MR, Kimpe LE, Mallory ML, Michelutti N, Smol JP, Blais JM (2019) Multicentury perspective assessing the sustainability of the historical harvest of seaducks. Proc Natl Acad Sci 116:8425–8430
Hedd A, Montevecchi WA (2006) Diet and trophic position of Leach’s storm-petrel Oceanodrama leucorhoa during breeding and moult, inferred from stable isotope analysis of feathers. Mar Ecol Prog Ser 322:291–301
Heiri O, Lotter AF (2003) 9000 years of chironomid assemblage dynamics in an Alpine lake: long-term trends, sensitivity to disturbance, and resilience of the fauna. J Paleolimnol 30:273–289
Hill MO (1973) Diversity and evenness: a unifying notation and its consequences. Ecology 54:427–432
Jernelöv A, Nagell B, Svenson A (1981) Adaptation to an acid environment in Chironomus riparius (Diptera, Chironomidae) from Smoking Hills, NWT, Canada. Ecography 4:116–119
Jenouvrier S (2013) Impacts of climate change on avian populations. Glob Change Biol 19:2036–2057
Juggins S (2007) C2: Software for ecological and palaeoecological data analysis and visualisation (user guide version 1.5). Newcastle University, Newcastle upon Tyne, p 77
Juggins S (2020). rioja: Analysis of Quaternary Science Data. R package version 0.9-26. http://cran.r-project.org/package=rioja
Luoto TP, Brooks SJ, Salonen V-P (2014) Ecological responses to climate change in a bird-impacted High Arctic pond (Nordaustlandet, Svalbard). J Paleolimnol 51:87–97
Michelutti N, Keatley BE, Brimble S, Blais JM, Liu H, Douglas MSV, Mallory ML, Macdonald RW, Smol JP (2009) Seabird-driven shifts in Arctic pond ecosystems. Proc R Soc B 276:591–596
Montevecchi WA, Tuck LM (1987) Newfoundland birds: exploitation. Study, conservation. Nuttall Ornithological Club, Cambridge, p 273
Nazarova LB, Self AE, Brooks SJ, Solovieva N, Syrykh LS, Dauvalter VA (2017) Chironomid fauna of the lakes from the Pechora River basin (East of European part of Russian Arctic): ecology and reconstruction of recent ecological changes in the region. Contemp Probl Ecol 10:350–362
Ngatia L, Grace IJM, Moriasi D, Taylor R (2019) Nitrogen and phosphorus eutrophication in marine ecosystems. In: Fouzia HB (eds) Monitoring of marine pollution, pp 1–17
Otero XL, De La Peña-Lastra S, Pérez-Alberti A, Ferreira TO, Huerta-Diaz MA (2018) Seabird colonies as important global drivers in the nitrogen and phosphorus cycles. Nat Commun 9:246
Parks and Natural Areas Division (1995) Department of environment and conservation. Baccalieu Island ecological reserve management plan and regulations. Government of Newfoundland and Labrador, Canada, pp 1–31
Peñuelas J, Poulter B, Sardans J, Ciais P, van der Velde M, Bopp L, Boucher O, Godderis Y, Hinsinger P, Llusia J, Nardin E, Vicca S, Obersteiner M, Janssens IA (2013) Human-induced nitrogen–phosphorus imbalances alter natural and managed ecosystems across the globe. Nat Commun 4:2934
Pollet IL, Bond AL, Hedd A, Huntington CE, Butler RG, Mauck R (2020) Leach's Storm-Petrel (Oceanodroma leucorhoa), version 1.0. In: Billerman SM, Keeney BK, Rodewald PG, Schulenberg TS (eds) The birds of North America. Cornell Lab of Ornithology, Ithaca. https://doi.org/10.2173/bow.lcspet.01
Porinchu DF, MacDonald GM (2003) The use and application of freshwater midges (Chironomidae: Insecta: Diptera) in geographical research. Prog Phys Geogr 27:378–422
Quinlan R, Smol JP (2001a) Chironomid-based inference models for estimating end-of-summer hypolimnetic oxygen from south-central Ontario shield lakes. Freshw Biol 46:1529–1551
Quinlan R, Smol JP (2001b) Setting minimum head capsule abundance and taxa deletion criteria in chironomid-based inference models. J Paleolimnol 26:327–342
RStudio Team (2015) RStudio: Integrated Development for R. RStudio, Inc., Boston, MA. http://www.rstudio.com/. Accessed 30 Oct 2019
Sklepkovych BO, Montevecchi WA (1989) The world’s largest known nesting colony of Leach’s Storm-Petrels on Baccalieu Island, Newfoundland. Am Birds 43:38–42
Smol JP (2008) Pollution of lakes and rivers: a paleoenvironmental perspective. Wiley-Blackwell publishing, Oxford, p 396
Smol JP (2019) Under the radar: long-term perspectives on ecological changes in lakes. Proc R Soc B 286:20190834
Stewart EM, Michelutti N, Blais JM, Mallory ML, Douglas MSV, Smol JP (2013) Contrasting the effects of climatic, nutrient, and oxygen dynamics on subfossil chironomid assemblages: a paleolimnological experiment from eutrophic High Arctic ponds. J Paleolimnol 49:205–219
Thienpont JR, Steele C, Vermaire JC, Pisaric MFJ, Kokelj SV, Smol JP (2015) Synchronous changes in chironomid assemblages in two Arctic delta lake ecosystems after a major saltwater intrusion event. J Paleolimnol 53:177–189
Urrutia R, Araneda A, Torres L, Cruces F, Vivero C, Torrejón F, Barra R, Fagel N, Scharf B (2010) Late Holocene environmental changes inferred from diatom, chironomid, and pollen assemblages in an Andean lake in Central Chile, Lake Laja (36 °S). Hydrobiologia 648:207–225
Velle G, Brooks SJ, Birks HJB, Willassen E (2005) Chironomids as a tool for inferring Holocene climate: as assessment based on six sites in southern Scandinavia. Quat Sci Rev 24:1429–1462
Walker IR (2001) Midges: Chironomidae and related Diptera. In: Smol JP, Birks HJB, Last WM (eds) Tracking environmental change using lake sediments: zoological indicators. Kluwer Academic Publishers, Dordrecht, pp 43–66
Wilhelm SI, Hedd A, Robertson GJ, Mailhiot J, Regular PM, Ryan PC, Elliot RD (2020) The world’s largest breeding colony of Leach’s Storm-petrel Hydrobates leucorhous has declined. Bird Conserv Int 30:40–57
Williams JJ, Brooks SJ, Gosling WD (2012) Response of chironomids to late Pleistocene and Holocene environmental change in the eastern Bolivian Andes. J Paleolimnol 48:485–501
Wurzbacher CM, Bärlocher F, Grossart H-P (2010) Fungi in lake ecosystems. Aquat Microb Ecol 59:125–149
Acknowledgements
We thank Dr. Olivier Heiri for his insights on chironomid degradation. We also thank D. Fifield, A. Hedd, G, Robertson, P. Ryan and S. Wilhelm at Environment and Climate Change Canada (ECCC) in St. John’s, Newfoundland, for their assistance preparing for fieldwork, and C. Grooms for assistance with the fieldwork. We also thank B. Montevecchi of Memorial University of Newfoundland, St. John’s, Newfoundland and Labrador for his insights on this project. We acknowledge the Land Management Division, Newfoundland and Labrador Department of Fisheries and Land Resources as the managing agency for the Baccalieu Island Ecological Reserve. This study was funded by the Natural Sciences and Engineering Research Council (NESRC) of Canada (Grant No. RGPIN-2017-04548) awarded to JPS. We also thank the two journal reviewers and editors for their constructive comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lim, J.E., Duda, M.P. & Smol, J.P. Chironomid assemblage changes and chitin degradation in response to ~ 1700-years of seabird population fluctuations at the world’s largest colony of Leach’s Storm-Petrels (Atlantic Canada). J Paleolimnol 65, 429–443 (2021). https://doi.org/10.1007/s10933-021-00181-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10933-021-00181-1