Abstract
Many animals produce long-distance acoustic signals to mediate a variety of social interactions, and the efficacy of transmission depends in part on environmental attenuation. Vocalizing from positions that optimize transmission is one key solution to minimizing attenuation, though few studies assess the magnitude of this effect in relation to receiver position. In this study, we assessed how transmission of high-frequency vocalizations produced by pinyon mice (Peromyscus truei) varied based on the position of senders and receivers. Pinyon mice are semi-arboreal rodents that produce sustained vocalizations to advertise to conspecifics. Synthesized signals derived from a population-sample-average of fundamental frequency, duration, and amplitude were broadcast and recorded at different heights (0, 1, and 2 m) and distances (1, 2, 4, and 8 m) in a full factorial design to mimic hypothetical senders and receivers. We also measured receiver hearing sensitivity using auditory brainstem responses (ABR) to quantify the audible distance (active space) of vocalizations at different heights. Vocalizations showed less attenuation when emitted and received from an elevation compared to the ground if the signal was received at least 4 m from the sender. Vocalizations emitted from a 1 m height had an approximately 3 times greater audible distance compared to the ground. Additionally, less attenuation occurred when both senders and receivers were elevated at the same height and when receivers were elevated, regardless of sender height. Our results highlight the importance of considering receiver position in animal communication, especially when senders produce highly directional signals.
Significance statement
Vocalizing animals often position themselves in locations that maximize sound transmission. However, the magnitude of this effect is not often quantified, especially in relation to the position of intended receivers. In this study, we combined acoustic recording, hearing experiments, and modelling of sound attenuation to quantify how sending and receiving vocalizations from trees impacts sound transmission in a semi-arboreal mouse. We found that vocalizations produced from 1 m above the ground could be heard by receivers at 3 times the distance compared to ground level. We also found that no matter the sender position, receivers benefitted from being at elevated positions. Finally, we found that the least attenuation occurred when senders and receivers were elevated at the same height. Our results highlight the importance of considering receiver position in animal communication, especially when senders produce highly directional signals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many animals produce long-distance acoustic signals to mediate a variety of social interactions (Ryan and Kime 2003). The efficacy of acoustic signal transmission depends on the environment (Marten and Marler 1977). Atmospheric absorption, ground attenuation, and deflection of sound by vegetation contribute to signal attenuation (Marten and Marler 1977; Waser and Brown 1986). Coping with environmental attenuation is therefore a major factor shaping the evolution of long-distance acoustic signaling (Römer and Lewald 1992; Boncoraglio and Saino 2007). Two key solutions to minimizing attenuation involve producing signals that are resistant to degradation (i.e. sensory drive or acoustic adaptation and associated receiver adaptations; Obrist et al. 1993; Ey and Fischer 2009; Römer 2020), or signaling at times and/or positions that optimize transmission (Waser and Waser 1977; Barker and Mennill 2009). Although studies on acoustic adaptation are numerous (e.g. Boncoraglio and Saino 2007; Goutte et al. 2018), comparatively fewer studies have explored the behavioral mechanisms used to minimize attenuation.
Animals can minimize signal degradation by advertising during times when climatic conditions are optimal for sound propagation (Wiley and Richards 1978; Hayes and Huntly 2005). For example, the dawn chorus of birds and primates and the evening chorus of insects and frogs are hypothesized to occur due to favorable atmospheric conditions; low temperatures and high relative humidity during these periods are presumed to reduce energy loss via absorption (Marten and Marler 1977; Wiley and Richards 1978). Similarly, animals may position themselves in a manner that increases signal propagation (Blumenrath and Dabelsteen 2004). Among taxa as diverse as crickets (Arak and Eiriksson 1992), treefrogs (Schwartz et al. 2015), and monkeys (Riondato et al. 2021), senders advertise from elevated positions to minimize attenuation from the ground and understory vegetation. Receivers may also benefit from adjusting their position relative to senders to improve signal detection. Indeed, playback of birdsong indicates that receivers benefit from selection of high perches to optimize signal reception (Dabelsteen et al. 1993; Holland et al. 1998; Mathevon et al. 2005). However, few studies have quantified the magnitude of signal efficacy sender and receiver position in tandem, especially in species that produce directional signals that characterize high-frequency vocalizations of many rodents (Richards and Wiley 1980; Fernández-Vargas et al. 2022).
Deer mice (genus Peromyscus) are a widespread and diverse group of rodents that exhibit a wide range of social behaviors, mating systems, and habitat use (Smartt 1978; Kalcounis-Rueppell et al. 2018a, c). Pinyon mice (P. truei) of the southwestern United States are among the most specialized species in the genus due to their reliance on pinyon (Pinus spp.) and juniper (Juniperus spp.) trees for food (Ribble and Samson 1987) and nest sites (Hall and Morrison 1997). Of particular importance, pinyon mice possess morphological adaptations thought to be suited for a semi-arboreal lifestyle, including long tails to facilitate balance (Horner 1954; Smartt and Lemen 1980; Kingsley et al. 2017; Hager and Hoekstra 2021) and larger brains to navigate spatially complex environments (Lemen 1980; Camargo et al. 2019; but see Mace and Eisenberg 1982). Similar to other congeners, pinyon mice produce a variety of vocalizations for social communication, including ca. 20 kHz sustained vocalizations (SVs) that function over long distances (> 1 m; Kalcounis-Rueppell et al. 2018b, c). SVs appear to function as general advertisement signals that help maintain territories (e.g. P. boylii; Petric and Kalcounis-Rueppell 2013) and/or spatial cohesion between mated pairs (P. californicus; Briggs and Kalcounis-Rueppell 2011). In pinyon mice, both sexes produce SVs in social isolation to advertise their presence to conspecifics, but females call more often (Kobrina et al. 2022a). Given that SVs operate over large spatial scales, quantifying how the geometry of senders and receivers influences signal transmission is fundamental to understanding the ecology and evolution of communication in rodents.
In this study, we investigated if signaling and receiving from trees confers benefits for acoustic communication in pinyon mice. Specifically, if arboreality confers an advantage for acoustic signal transmission, then SVs produced and received at heights within trees should experience less attenuation than vocalizations produced and received at ground level. We broadcast and recorded synthesized vocalizations in pinyon mouse habitat at different heights and distances to test these predictions and contextualized our findings by incorporating receiver hearing sensitivities at ecologically relevant distances.
Materials and methods
Animals
Twenty pinyon mice (9 females (F), 11 males (M); average mass: F = 30.90 ± 3.92 g, M = 32.85 ± 3.11 g) were captured near Deadman Flat, 28 km north of Flagstaff, AZ, using Sherman live traps baited with peanut butter and oats. Mice were transferred to standard mouse cages and maintained in animal facilities at Northern Arizona University, Flagstaff, AZ, USA. Mice were housed individually in the vivarium, maintained on a 14:10 dark:light cycle (21 ± 2 °C), and provided rodent chow and water ad libitum. Mice were used for both acoustic recording and hearing experiments (below).
Acoustic recording
Individually-housed mice were placed in a semi-anechoic sound cubicle lined with acoustic foam and recorded over 3–7 days within their home cage. Calibrated microphones (1/4′′ Type 40BE, G.R.A.S.) connected to preamplifiers (Type 26CB, G.R.A.S.) placed 33.3 cm above the center of the cage were used to record mice. Microphone response was flat within ± 1.5 dB from 10 Hz—50 kHz, and pre-amplifier response was flat within ± 0.2 dB from 2 Hz—200 kHz. Microphones were connected to a National Instruments DAQ (USB 4431) sampling at 102.4 kHz to a desktop computer running MATLAB (v. 2018a).
Acoustic signal generation
To generate a signal for use in sound transmission experiments, all SV vocalizations (mean ± SD = 193 ± 427, range: 1–1310; n = 5 F, 3 M; see Kobrina et al. 2022a) were analyzed for average fundamental frequency (F0; kHz), average duration (s), and average amplitude (dB SPL re: 20 μPa). Frequency and duration measures were extracted using the automated parameter measurements function in Avisoft SASLab Pro (v. 4.2.27, Avisoft Bioacoustics, Germany; 1024-point Fast Fourier Transform, Hann window, 75% frame size, 94 Hz bandwidth, 47 Hz frequency resolution, 1.333 ms with 93.75% overlap temporal resolution). Amplitude measures from calibrated microphones were extracted using the Sound Pressure Level Calculator (Greene 2021) in MATLAB (v. 2018a). To correct amplitude values for the distance between the microphone and calling mouse (33.3 cm), we applied a standard equation that accounts for sound energy dissipation via spherical spreading (A.2 in Brumm and Zollinger 2011) to standardize playback SPL levels at 1 m (72.1 dB) for sound attenuation experiments (see below).
To avoid pseudo-replication (McGregor et al. 1992; Kroodsma et al. 2001), we calculated a species grand mean (F0 = 19.8 ± 1.5 kHz; duration = 0.14 ± 0.07 s; dB SPL = 81.6 ± 4 dB) from averaged parameters within individuals to synthesize a signal for use in sound transmission experiments using Avisoft SASLab Pro (48 kHz sampling rate, 16-bit resolution,.wav format). To improve signal to noise ratios, the file contained 50 replicates of the synthesized signal with 0.5 s silent gaps between each replicate.
Sound attenuation experiments
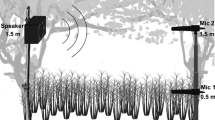
We conducted sound transmission experiments at Deadman Flat between March–May 2022 around dusk (18:00–22:00 h), coincident with times that mice become active. Twenty trees > 4 m (min. distance between trees 50 m) in height were selected randomly at the study site where mice were originally captured. At each site, we set a 4 m transect outward from the perimeter of the furthest live branch of the focal tree in a random direction. At 0 m, we placed a microphone (Sennheiser MKH 8060, 50 Hz – 25 kHz flat frequency response) 9 cm above the ground to mimic the height of a hypothetical mouse receiver. The microphone was connected to a field audio recorder (Sound Devices 702 T) to record stimuli at a sampling rate of 48 kHz and 16-bit resolution. We broadcast synthesized stimuli through a speaker (Ultrasonic Dynamic Speaker, ScanSpeak, Avisoft Bioacoustics, flat frequency response within ± 1.5 dB from 10–45 kHz) connected to an Avisoft Ultrasound Gate Player 416H connected to a laptop running Avisoft RECORDER (v. 5.2.14). We calibrated the amplitude of the playback file using a recorded 93.7 dB 1 kHz tone from a sound level calibrator (Brüel and Kjaer Type 4230) and input the resultant recording into Avisoft SASLab Pro to calibrate all subsequent recordings. We modeled the vertical position of senders (ground; 0 m, 1 m, 2 m) and receivers (ground; 0 m, 1 m, 2 m) in a full factorial design and recorded stimuli at 4 horizontal distances (1, 2, 4, and 8 m) per treatment. For example, to mimic a mouse calling from a 2 m height in a tree and a receiver at ground level, we mounted the speaker on a tripod 2 m above the ground and broadcast stimuli to a microphone placed on the ground iteratively at 1, 2, 4, and 8 m.
Prior to the analyses, all recordings were bandpass filtered between 18.73–20.73 kHz (Hamming window, 128 taps) in Avisoft SASLab Pro. The root mean square (rms) amplitude (20 ms averaging time) of each of the 50 replicates was then measured using automated detection in Avisoft SASLab Pro. We randomly selected a silent period between signal replicates to quantify the amplitude of background noise and subtracted noise values from signal amplitude values using a standard equation (equation A1 in Brumm and Zollinger 2011). Researchers were blind to treatment when attenuation data were analyzed to minimize observer bias.
Hearing sensitivity
Auditory brainstem responses (ABRs) (n = 9 F, 8 M; a subsample of mice from the acoustic recording experiment) were conducted in a semi-anechoic chamber (ETS Lindgren SD-1; internal dimensions 91.4 cm × 91.4 cm × 91.4 cm) lined with acoustic foam. We administered ketamine/dexmedetomidine (75/0.5 mg/kg) intraperitoneally to anesthetize animals. Occasionally, we injected an additional dose of ketamine (< 0.01 mL) 15 min after the initial dose to maintain an anesthetic plane. Anesthetized animals were transferred to a gel heating pad (32 ± 5 °C) to maintain a stable body temperature for the duration of the experiment. Monaural (randomly assigned ear; right n = 10, left n = 7) ABR measurements were obtained by placing three subdermal needle electrodes (27 gauge, 12 mm; Rochester Electro-Medical Inc., Lutz, FL, USA) on the mastoid of ear receiving the stimulus (reference), the vertex of the skull (active channel), and in the dorsum close to the base of the tail (ground). Electrodes were connected to a head stage (RA4LI, Tucker Davis Technologies (TDT), Alachua, FL, USA) and a preamplifier (RA4RA, TDT) attached to a processor (RZ6, TDT) via a fiber optic cable.
We generated and presented test stimuli, and collected responses using SigGenRZ and BioSigRZ (v. 5.7.0, TDT), a TDT Multi I/O processor RZ6, and a PC. ABR experiments were controlled by a PC Windows computer running an Optibit interface on a TDT driver using BioSigRZ. The stimuli were generated by the RZ6 and played through a speaker (MF1, TDT) located 10 cm away from the pinna of the pinyon mouse at 0-degree azimuth. Digitized data were recorded to the RZ6 processor through the RA4LI preamplifier. Stimuli consisted of clicks (0.1 ms square wave pulse of alternating polarity, obtained from the TDT root click file) and 5-ms single-channel cosine-squared gated tone bursts at frequencies of 4, 8, 16, 20, 24, 32, and 42 kHz (Cos2 (10%—90%) gating type, obtained from the TDT root tone file) presented 21 times per second for a total set of 512 repetitions. Test frequencies were selected to examine hearing abilities of both low and high-frequency communication signals previously recorded in P. truei (Kobrina et al. 2021) and typical of rodent audiograms (Dent et al. 2018). However, in this study, we only use sensitivities derived from the 20 kHz stimulus that was most similar to the F0 of SVs (19.8 kHz; above). The click stimulus has more spectral energy below 10 kHz and usually elicits a more robust ABR response than pure tones. Each stimulus was presented at descending levels starting at 90 dB until a threshold was reached. Step sizes were 10–15 dB at suprathreshold levels, and then decreased to 5 dB bracketing the threshold. The system was calibrated prior to each experiment using a microphone (Brüel and Kjær, Type 2670, Nærum, Denmark) connected to a microphone supply (Brüel and Kjær, Type 5935 L, Nærum, Denmark).
Attenuation statistical analyses
To assess how SVs were affected by distance and height, we used a generalized linear mixed model (GLMM) with the lme4 package in R studio, v. 3.3.3 (Bates et al. 2015; R Core Team 2017). The GLMM included the log-transformed synthesized stimuli SPL as the response variable and microphone distance (1 m, 2 m, 4 m, 8 m), speaker height (0 m, 1 m, 2 m), and microphone height (0 m, 1 m, 2 m) as main effects, and all 2- and 3-way interactions. ANOVA and posthoc Tukey models revealed background noise was variable among sites (F1,19 = 3.29, p < 0.0001). Therefore, we included site as a random effect in the GLMM to control for background noise variation. We verified assumptions of normality and homoscedasticity by visual inspection of q-q and residual plots. Finally, we computed η2 and confidence intervals (CI) as effect sizes for significant results in the effectsize package for R (Ben-Shachar et al. 2020).
ABR statistical analyses
We used the visual detection method (Green et al. 2019; Kobrina et al. 2021, 2022b) to determine the lowest stimulus level (dB) per stimulus that evoked an ABR response. Thresholds were operationally defined as the dB level halfway (2.5 dB) between the last detectable ABR response and next lowest stimulus level (see Kobrina et al. 2022b for details). A two-way repeated-measures mixed ANOVA was used to determine whether hearing sensitivity varied across stimuli and between ears (lsr package in R; Navarro 2013). Paired Tukey’s t-test post hoc analyses were conducted to assess significance. We computed η2 and confidence intervals (CI) as measures of effect sizes for significant results (effectsize package in R; Ben-Shachar et al. 2020).
Audible distance estimation
Finally, we assessed the audible distance of pinyon mouse SVs produced from the ground (height = 0 m) and from a tree (height = 1 m). To do so, we first calculated sound attenuation via spherical spreading of SV amplitude at 0 m and 1 m heights, using the attenuation function in the Seewave package (Sueur et al. 2008). All factors held equal, sound is expected to attenuate 6 dB for every doubling of distance (Brumm and Zollinger 2011). We then modelled excess attenuation for our observed amplitude values at each height by fitting a logarithmic decay model in R at 1 m, 2 m, 4 m, and 8 m distances, and extrapolated amplitude values beyond 8 m using the line of best fit equation. Finally, to estimate the audible distance of pinyon mice SVs, we used the line of best fit equation to calculate the distance at which sound attenuation intersected receiver hearing sensitivity (41.7 dB at 20 kHz; see Results) measured from ABRs.
Results
Sound attenuation
Attenuation of synthesized stimuli were influenced by sender (speaker) height (β = -19.859, df = 1, 663, p < 0.0001, η2 = 0.36; CI [0.31, 0.41]), receiver (microphone) height (β = -24.483, df = 1, 663, p < 0.0001, η2 = 0.43; CI [0.38, 0.48]), receiver (microphone) distance (β = -4.230, df = 1, 663, p < 0.0001; η2 = 0.22; CI [0.17, 0.27]), and all 2- and 3-way interactions (Table 1). SVs produced and/or received from the ground were more attenuated than above ground. At all distances beyond 1 m, attenuation was greater when both the sender and receiver were on the ground than at any elevation (Fig. 1). On average, trials where both the sender and receiver were elevated 1 m experienced the least attenuation (Fig. 1). When the sender was on the ground, the 1 m receiver height trials experienced the least attenuation at longer distances (4 m and 8 m; Fig. 1). The interaction between speaker and microphone height was the strongest effect in our model (β = 20.97, df = 1.363, p < 0.0001; η2 = 0.50; CI [0.45, 0.54]), indicating amplitude was loudest when the sender and receiver height was matched (Fig. S1). When heights were mismatched, amplitude was optimized if the receiver height was within 1 m of the sender height (Fig. S1). Site, as a random effect, accounted for a relatively large amount of variation (σ2 = 2.83, SD = 1.68).
Attenuation of pinyon mice sustained vocalization (SVs) amplitude (SPL) vary based on the position of the sender and receiver. Heat map shows relative amplitude values of SVs from relatively quiet (yellow hue) to relatively loud (red hue), produced from a speaker at 0 m, 1 m, and 2 m heights (“sender height” in gray), and recorded from a microphone height at 0 m, 1 m, and 2 m heights (“receiver height” secondary y-axis) at distances of 1 m, 2 m, 4 m, and 8 m. Amplitude values were interpolated between distances
Hearing sensitivity
In general, pinyon mice were able to detect all stimuli. ANOVA revealed a significant main effect of stimulus (F7,96 = 69.76, p < 0.001, η2 = 0.82; CI [0.75, 0.86]) and a non-significant main effect of ear (F1,96 = 0.07, p = 0.79). Paired Tukey’s post-hoc analyses indicated that pinyon mice had lower thresholds (i.e. were more sensitive) to click stimuli than to 4, 8, 20, 24, 32, and 42 kHz tones (p < 0.004). Pinyon mice were most sensitive to 16 kHz tones than to all other frequencies (p < 0.02) with the exception of 8 kHz (p > 0.05). Mice were least sensitive to 32 and 42 kHz tones than to all other frequencies (p < 0.001). Hearing sensitivity for 20 kHz tones were 41.67 ± 6.69 dB and were not significantly different from 4, 8, or 24 kHz tones (p > 0.05; Fig. 2).
Audible distance estimation
We assessed the audible distance of SVs on the ground and in a tree by integrating our ABR results with sound attenuation models based on spherical spreading and excess attenuation. Attenuation curves indicated stimuli produced from the ground experienced greater excess attenuation relative to spherical spreading (Fig. 3). From this model, the audible distance, or active space, of SVs was 4.35 m (Fig. 3); beyond this distance, amplitude fell below the estimated 41.67 dB hearing threshold of pinyon mice. In contrast, stimuli produced from trees at 1 m height was predominantly affected by spherical spreading and had little excess attenuation. Under this scenario, the audible distance of SVs was 3 times farther than the ground (12.4 m; Fig. 3).
Attenuation curves based on spherical spreading (open circles) and excess attenuation (black circles) of synthesized pinyon mouse stimuli emitted and received on the ground (left panel) vs. emitted and received at 1 m height (right panel). Horizontal dashed lines represent the hearing sensitivity (41.67 dB) of pinyon mice at 20 kHz. Vertical dashed lines and the corresponding value represent the maximum audible distance (m) of vocalizations
Discussion
Our findings indicate both senders and receivers of acoustic signals benefit from positioning themselves at elevated heights. Vocalizations emitted and received at 1 m off the ground had an active space that was approximately 3 times larger compared to on the ground, in part due to ground and vegetation effects that increase acoustic attenuation, especially at high frequencies (Brenowitz 1986; Marten and Marler 1977). We discuss our findings in relation to the ecology and evolution of acoustic communication in animals in general and rodents in particular.
Our results concur with studies in other taxa that exploit elevated heights to minimize attenuation and thereby extend the communication range of acoustic signals (Arak and Eiriksson 1992; Mathevon et al 2005; Schwartz et al. 2015; Riondato et al. 2021). Benefits to elevated heights include increasing the probability of detection, reducing search costs associated with localizing mates and/or competitors, and/or increasing the number of receivers (Richards and Wiley 1980; Ryan and Kime 2003). In some birds, both senders and receivers exploit elevated perches to optimize production and reception (Dabelsteen et al. 1993). For example, in Rufous-and-white wrens (Thryophilus rufalbus) and Blackcaps (Sylvia atricapilla), certain perch locations extend communication range, especially if senders and receivers are at the same height (Holland et al. 1998; Mathevon et al 2005). Similarly, our results indicate that both senders and receivers benefit at elevated heights, particularly when each party is on a similar plane. However, benefits wane at higher heights (2 m), likely because most attenuation is caused by ground effects.
Although a growing body of literature exists on acoustic communication in mice (Kalcounis-Rueppell et al. 2018c; Rieger and Marler 2018; Fernández-Vargas et al. 2022; Kobrina et al. 2022a, b), few studies are contextualized ecologically. In closely related California mice (P. californicus), SVs appear to facilitate spatial contact and territorial defense between monogamous pairs (Briggs and Kalcounis-Rueppell 2011). Our estimate of audible distance of SVs at ground level (4.35 m) concur with values reported in P. californicus (3.12 m; Timonin et al. 2018) that occupies denser vegetation, adding support to their utility over long distances. In polygynous to promiscuous brush mice (P. boylii; Ribble and Stanley 1998; Kalcounis-Rueppell and Spoon 2009), SVs are implicated in territorial advertisement, especially among females (Petric and Kalcounis-Rueppell 2013). Similar to brush mice, pinyon mice are promiscuous (Ribble and Stanley 1998) and SVs are produced primarily by females (Kobrina et al. 2022a). Given their large home range sizes (0.4—1.6 ha; Ribble and Stanley 1998), female SVs likely facilitate advertisement of their position to neighbors throughout the year and roaming males during the mating season (Emlen and Oring 1977). Our findings suggest that elevated advertisement and reception increases the probability of signal efficacy to mediate intra- and inter-sexual interactions. Notably, varying levels of arboreality are reported in different species of Peromyscus (e.g. P. boylii, P. californicus, P. leucopus, P. maniculatus; Meserve 1977; Harney and Dueser 1987; Kalcounis-Rüppell and Millar 2002) that use SVs in similar long-distance contexts (Kalcounis-Rueppell et al. 2006; Petric and Kalcounis-Rueppell 2013; Rieger and Marler 2018). While the origins of arboreal behavior may be due to food availability (Holbrook 1978) and/or interspecific competition (Stah 1980), the communicative benefits realized from the reduction of acoustic attenuation at ground level may help maintain arboreality. Comparative studies that assess the geometry of vocal behavior across this diverse genus (Kalcounis- Rueppell et al. 2018a, c) would provide important insight and resolution.
The tendency for higher frequencies (≥ 20 kHz) that characterize SVs to be highly directional and attenuate rapidly in the environment requires novel adaptations in both senders and receivers to increase signal efficacy (Richards and Wiley 1980). In bats, active control (Yovel et al. 2011) of high-amplitude echolocation calls (Jakobsen et al. 2013) produced by unique laryngeal mechanisms (Metzner and Schuller 2010) are accompanied by morphologically diverse (Leiser-Miller and Santana 2020) and positionally dynamic (Müller et al. 2017) pinnae to facilitate reception of high frequencies. Peromyscus similarly have unique laryngeal anatomy to produce SVs (Fernández-Vargas et al. 2022), but mechanisms of reception are less studied (Dice and Barto 1952; Ralls 1967; Capshaw et al. 2022). In pinyon mice, large, conspicuous pinnae are hypothesized to facilitate detection of low-frequency predator cues (Hoffmeister 1951, 1981), not higher frequency conspecific vocalizations. Indeed, broader and larger pinnae are associated with lower frequency echolocation calls in bats (Obrist et al. 1993; Huihua et al. 2003). Furthermore, we did not find secondary peaks in pinyon mouse audiograms as is expected when pinnae morphology is involved in high frequency hearing (Heffner et al. 2001). Although more studies are needed on rodent hearing, such patterns further implicate the importance of studying relative positions of senders and receivers.
Our inferences come with minor caveats. Playback of synthesized signals highlight theoretical benefits gained by senders and receivers if they attended to spatial locations when producing or evaluating signals. Although pinyon mice are agile climbers and commonly observed in trees (Bailey 1931; Horner 1954), assessment of realized benefits will require simultaneous tracking and vocal recording of animals in three-dimensional space. In addition, we focused our study on quantifying signal attenuation of a single note of SVs, which represents only one aspect of signal degradation. Pinyon mouse SVs are typically composed of multiple rapidly repeated notes (Kobrina et al. 2021) that likely serve to facilitate detection and recognition (Bradbury and Vehrencamp 2011). Studies that integrate other measures of degradation (e.g. distortion of temporal and frequency patterns; Mathevon et al. 2005) can help clarify the robustness of our findings. Finally, our estimates of signal active space are conservative because electrophysiological methods of hearing sensitivity (e.g. ABRs) are typically less sensitive (10–30 dB) than behavioral paradigms (Heffner and Heffner 2003; Dent et al. 2018). Thus, the ecological validity of the spatial extent of acoustic interactions may be greater than reported herein.
From a basic perspective, our study extends the taxonomic scope of positional advertisement and contributes to our understanding of behavioral mechanisms used to facilitate animal communication. From an applied perspective, consideration of the environment where signaling behaviors occur is critical. For example, pinyon mice rely on pinyon-juniper woodlands (Hall and Morrison 1997) that are receding in their southern ranges due to drought (Clifford et al. 2011; Redmond et al. 2012; Whipple et al. 2019). Given the importance of elevated heights for sound propagation, reductions in tree densities may not only negatively impact food (Hall and Morrison 1997) and nest sites but indirectly influence demographic rates that rely on behaviors reliant on acoustic communication. Integrating signaling and sensory ecology will be key to understanding species-specific resilience (Srivathsa et al. 2019) in response to environmental change.
Data availability
All datasets generated and analyzed during this study are included in the supplementary information files of this published article.
References
Arak A, Eiriksson T (1992) Choice of singing sites by male bushcrickets (Tettigonia viridissima) in relation to signal propagation. Behav Ecol Sociobiol 30:365–372. https://doi.org/10.1007/BF00176170
Bailey V (1931) Mammals of New Mexico. United States Department of Agriculture, Bureau of Biological Survey, Washington
Barker NKS, Mennill DJ (2009) Song perch height in Rufous-and-White Wrens: Does behaviour enhance effective communication in a tropical forest? Ethology 115:897–904. https://doi.org/10.1111/j.1439-0310.2009.01674.x
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48. https://doi.org/10.18637/jss.v067.i01
Ben-Shachar MS, Lüdecke D, Makowski D (2020) effectsize: Estimation of effect size indices and standardized parameters. J Open Source Softw 5:2815
Blumenrath S, Dabelsteen T (2004) Degradation of Great Tit (Parus Major) song before and after foliation: Implications for vocal communication in a deciduous forest. Behaviour 141:935–958. https://doi.org/10.1163/1568539042360152
Boncoraglio G, Saino N (2007) Habitat structure and the evolution of bird song: A meta-analysis of the evidence for the acoustic adaptation hypothesis. Funct Ecol 21:134–142. https://doi.org/10.1111/j.1365-2435.2006.01207.x
Bradbury JW, Vehrencamp SL (2011) Principles of animal communication, 2nd edn. Sinauer Associates, Sunderland, Massachusetts
Brenowitz EA (1986) Environmental influences on acoustic and electric animal communication. Brain Behav and Evol 28:32–42. https://doi.org/10.1159/000118690
Briggs JR, Kalcounis-Rueppell MC (2011) Similar acoustic structure and behavioural context of vocalizations produced by male and female California mice in the wild. Anim Behav 82:1263–1273. https://doi.org/10.1016/j.anbehav.2011.09.003
Brumm H, Zollinger SA (2011) The evolution of the Lombard effect: 100 years of psychoacoustic research. Behaviour 148:1173–1198. https://doi.org/10.1163/000579511X605759
Camargo NF, Machado LF, Mendonça AF, Vieira EM (2019) Cranial shape predicts arboreal activity of Sigmodontinae rodents. J Zool 308:128–138. https://doi.org/10.1111/jzo.12659
Capshaw G, Vicencio-Jimenez S, Screven LA, Burke K, Weinberg MM, Lauer AM (2022) Physiological evidence for delayed age-related hearing loss in two long-lived rodent species (Peromyscus leucopus and P. californicus). J Assoc Res Otolaryngol 23:617–631. https://doi.org/10.1007/s10162-022-00860-4
Clifford MJ, Cobb NS, Buenemann M (2011) Long-Term Tree Cover Dynamics in a Pinyon-Juniper Woodland: Climate-Change-Type Drought Resets Successional Clock. Ecosyst 14:949–962. https://doi.org/10.1007/s10021-011-9458-2
Dabelsteen T, Pedersen SB, Larsen ON (1993) Habitat-induced degradation of sound signals: quantifying the effects of communication sounds and bird location on blur ratio, excess attenuation and signal-to- noise ratio. J Acoust Soc Am 93:2206–2220. https://doi.org/10.1121/1.406682
Dent ML, Screven LA, Kobrina A (2018) Hearing in rodents. In: Dent M, Fay R, Popper A (eds) Rodent Bioacoustics. Springer, Cham, pp 71–105. https://doi.org/10.1007/978-3-319-92495-3_4
Dice LR, Barto E (1952) Ability of mice of the genus Peromyscus to hear ultrasonic sounds. Science 116:110–111. https://doi.org/10.1126/science.116.3005.110
Emlen T, Oring LW (1977) Ecology, sexual selection, and the evolution of mating systems. Science 197:215–223. https://doi.org/10.1126/science.327542
Ey E, Fischer J (2009) The “acoustic adaptation hypothesis”—a review of the evidence from birds, anurans and mammals. Bioacoustics 19:21–48. https://doi.org/10.1080/09524622.2009.9753613
Fernández-Vargas M, Riede T, Pasch B (2022) Mechanisms and constraints underlying acoustic variation in rodents. Anim Behav 184:135–147. https://doi.org/10.1016/j.anbehav.2021.07.011
Goutte S, Dubois A, Howard SD, Márquez R, Rowley JJL, Dehling JM, Grandcolas P, Xiong RC, Legendre F (2018) How the environment shapes animal signals: a test of the acoustic adaptation hypothesis in frogs. J Evol Biol 31:148–158. https://doi.org/10.1111/jeb.13210
Green DM, Scolman T, Pasch B (2019) A broad filter between call frequency and peripheral auditory sensitivity in northern grasshopper mice (Onychomys leucogaster). J Comp Physiol A 205:481–489. https://doi.org/10.1007/s00359-019-01338-0
Greene C (2021) MATLAB Central File Exchange: Sound Pressure Level Calculator. MathWorks. https://www.mathworks.com/matlabcentral/fileexchange/35876-sound-pressure-level-calculator
Hager ER, Hoekstra HE (2021) Tail length evolution in deer mice: Linking morphology, behavior, and function. Integr Comp Biol 61:385–397. https://doi.org/10.1093/icb/icab030
Hall LS, Morrison ML (1997) Den and relocation site characteristics and home ranges of Peromyscus truei in the White Mountains of California. Great Basin Nat 57:124–130
Harney BA, Dueser RD (1987) Vertical stratification of activity of two Peromyscus species: an experimental analysis. Ecology 68:1084–1091. https://doi.org/10.2307/1938380
Hayes AR, Huntly NJ (2005) Effects of wind on the behavior and call transmission of pikas (Ochotona princeps). J Mammal 86:974–981. https://doi.org/10.1644/1545-1542(2005)86[974:EOWOTB]2.0.CO;2
Heffner HE, Heffner RS (2003) Audition. In: Davis SF (ed) Handbook of research methods in experimental psychology. Blackwell, Boston, MA, pp 413–440. https://doi.org/10.1002/9780470756973.ch19
Heffner RS, Koay G, Heffner HE (2001) Audiograms of five species of rodents: implications for the evolution of hearing and the perception of pitch. Hear Res 157:138–152. https://doi.org/10.1016/S0378-5955(01)00298-2
Hoffmeister DF (1951) A taxonomic and evolutionary study of the piñon mouse, Peromyscus truei. University of Illinois Press, Urbana. https://doi.org/10.5962/bhl.title.50289
Hoffmeister DF (1981) Peromyscus truei. Mamm Species 161:1–5
Holbrook S (1978) Habitat relationships and coexistence of four sympatric species of Peromyscus in northwestern New Mexico. J Mammal 59:18–26
Holland J, Dabelsteen T, Pedersen SB, Larsen ON (1998) Degradation of song in the wren Troglodytes troglodytes: Implications for information transfer and ranging. J Acoust Soc Am 103:2154–2166. https://doi.org/10.1121/1.421361
Horner E (1954) Arboreal adaptations of Peromyscus, with special reference to use of the tail. University of Michigan, Ann Arbor, MI
Huihua Z, Shuyi Z, Mingxue Z, Jiang Z (2003) Correlations between call frequency and ear length in bats belonging to the families Rhinolophidae and Hipposideridae. J Zool 259:189–195. https://doi.org/10.1017/S0952836902003199
Jakobsen L, Brinkløv S, Surlykke A (2013) Intensity and directionality of bat echolocation signals. Front Physiol 4:89. https://doi.org/10.3389/fphys.2013.00089
Kalcounis-Rueppell MC, Spoon TR (2009) Peromyscus boylii. Mamm Species 838:1–14
Kalcounis-Rueppell MC, Metheny JD, Vonhof MJ (2006) Production of ultrasonic vocalizations by Peromyscus mice in the wild. Front Zool 3:3. https://doi.org/10.1186/1742-9994-3-3
Kalcounis-Rueppell MC, Petric R, Marler CA (2018) The bold, silent type: predictors of ultrasonic vocalizations in the genus Peromyscus. Front Ecol Evol 6:198. https://doi.org/10.3389/fevo.2018.00198
Kalcounis-Rueppell MC, Pultorak JD, Blake BH, Marler CA (2018) Ultrasonic vocalizations of young mice in the genus Peromyscus. Handb Behav Neurosci 25:149–156. https://doi.org/10.1016/B978-0-12-809600-0.00014-7
Kalcounis-Rueppell MC, Pultorak JD, Marler CA (2018) Ultrasonic vocalizations of mice in the genus Peromyscus. Handb Behav Neurosci 25:227–235. https://doi.org/10.1016/B978-0-12-809600-0.00014-7
Kalcounis-Rüppell MC, Millar JS (2002) Partitioning of space, food, and time by syntopic Peromyscus boylii and P. californicus. J Mammal 83:614–625. https://doi.org/10.1644/15451542(2002)083%3c0614:POSFAT%3e2.0.CO;2
Kingsley EP, Kozak KM, Pfeifer SP, Yang DS, Hoekstra HE (2017) The ultimate and proximate mechanisms driving the evolution of long tails in forest deer mice. Evol 71:261–273. https://doi.org/10.1111/evo.13150
Kobrina A, Hidau MK, Riede T, Guthrie O, Pasch B (2021) Age-related and noise-induced hearing loss alters grasshopper mouse (Onychomys) vocalizations. Hear Res 404:108210. https://doi.org/10.1016/j.heares.2021.108210
Kobrina A, Letowt ME, Pasch B (2022) The influence of social context on pinyon mouse (Peromyscus truei) vocalizations. J Mammal 103:275–286. https://doi.org/10.1093/jmammal/gyab127
Kobrina A, Letowt ME, Pasch B (2022) Vocal repertoire and auditory sensitivity of white-throated woodrats (Neotoma albigula). J Comp Psychol 137:116–128. https://doi.org/10.1037/com0000330
Kroodsma DE, Byers BE, Goodale E, Johnson S, Liu WC (2001) Pseudoreplication in playback experiments, revisited a decade later. Anim Behav 61:1029–1033. https://doi.org/10.1006/anbe.2000.1676
Leiser-Miller LB, Santana SE (2020) Morphological diversity in the sensory system of phyllostomid bats: Implications for acoustic and dietary ecology. Funct Ecol 34:1416–1427. https://doi.org/10.1111/1365-2435.13561
Lemen C (1980) Relationship between relative brain size and climbing ability in Peromyscus. J Mammal 61:360–364. https://doi.org/10.2307/1380068
Mace GM, Eisenberg JF (1982) Competition, niche specialization and the evolution of brain size in the genus Peromyscus. Biol J Linn Soc 17:243–257. https://doi.org/10.1111/j.1095-8312.1982.tb02019.x
Marten K, Marler P (1977) Sound transmission and its significance for animal vocalization. Behav Ecol Sociobiol 2:271–290. https://doi.org/10.1007/BF00299740
Mathevon N, Dabelsteen T, Blumenrath SH (2005) Are high perches in the blackcap Sylvia atricapilla song or listening posts? A sound transmission study. J Acoust Soc Am 117:442–449. https://doi.org/10.1121/1.1828805
McGregor PK, Catchpole CK, Dabelsteen T et al (1992) Playback and studies of animal communication. Springer, Boston. https://doi.org/10.1007/978-1-4757-6203-7_1
Meserve PL (1977) Three-dimensional home ranges of cricetid rodents. J Mammal 58:549–558. https://doi.org/10.2307/1380003
Metzner W, Schuller G (2010) Vocal control in echolocating bats. Handb Behav Neurosci 19:403–415. https://doi.org/10.1016/B978-0-12-374593-4.00037-1
Müller R, Gupta AK, Zhu H, Pannala M, Gillani US, Fu Y, Caspers P, Buck JR (2017) Dynamic substrate for the encoding sensory information in bat biosonar. Phys Rev Lett 118:158102. https://doi.org/10.1103/PhysRevLett.118.158102
Navarro D (2013) Learning statistics with R. Lulu Press Inc, Morrisville, North Carolina. https://learningstatisticswithr.com
Obrist MK, Fenton MB, Eger JL, Schlegel PA (1993) What ears do for bats: a comparative study of pinna sound pressure transformation in Chiroptera. J Exp Biol 180:119–152. https://doi.org/10.1242/jeb.180.1.119
Petric R, Kalcounis-Rüppell MC (2013) Female and male adult brush mice (Peromyscus boylii) use ultrasonic vocalizations in the wild. Behaviour 150:1747–1766. https://doi.org/10.1163/1568539X-00003118
R Core Team (2017) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org
Ralls K (1967) Auditory sensitivity in mice: Peromyscus and Mus musculus. Anim Behav 15:123–128. https://doi.org/10.1016/S0003-3472(67)80022-8
Redmond M, Forcella F, Barger N (2012) Declines in pinyon pine cone production associated with regional warming. Ecosphere 3:120. https://doi.org/10.1890/ES12-00306.1
Ribble DO, Samson FB (1987) Microhabitat associations of small mammals in southeastern Colorado, with special emphasis on Peromyscus (Rodentia). Southwest Nat 32:291–303. https://doi.org/10.2307/3671446
Ribble DO, Stanley S (1998) Home ranges and social organization of syntopic Peromyscus boylii and P. truei. J Mammal 79:932–941. https://doi.org/10.2307/1383101
Richards DG, Wiley RH (1980) Reverberations and amplitude fluctuations in the propagation of sound in a forest: implications for animal communication. Am Nat 115:381–399. https://doi.org/10.1086/283568
Rieger NS, Marler CA (2018) The function of ultrasonic vocalizations during territorial defence by pair-bonded male and female California mice. Anim Behav 135:97–108. https://doi.org/10.1016/j.anbehav.2017.11.008
Riondato I, Gamba M, Tan CL, Niu K, Narins PM, Yang Y, Giacoma C (2021) Allometric escape and acoustic signal features facilitate high-frequency communication in an endemic Chinese primate. J Comp Physiol A 207:327–336. https://doi.org/10.1007/s00359-021-01465-7
Römer H (2020) Insect acoustic communication: The role of transmission channel and the sensory system and brain of receivers. Funct Ecol 34:310–321. https://doi.org/10.1111/1365-2435.13321
Römer H, Lewald J (1992) High-frequency sound transmission in natural habitats: implications for the evolution of insect acoustic communication. Behav Ecol Sociobiol 29:437–444. https://doi.org/10.1007/BF00170174
Ryan MJ, Kime NM (2003) Selection on long-distance acoustic signals. In: Simmons AM, Fay RR, Popper AN (eds) Acoustic Communication. Springer, New York, pp 225–274
Schwartz JJ, Hunce R, Lentine B, Powers K (2015) Calling site choice and its impact on call degradation and call attractiveness in the gray treefrog, Hyla versicolor. Behav Ecol Sociobiol 70:1–19. https://doi.org/10.1007/s00265-015-2016-8
Sikes RS (2016) Guidelines of the American Society of Mammalogists for the use of wild mammals in research and education. J Mammal 97:663–688. https://doi.org/10.1093/jmammal/gyw078
Smartt RA (1978) A comparison of ecological and morphological overlap in a Peromyscus community. Ecology 59:216–220. https://doi.org/10.2307/1936365
Smartt RA, Lemen C (1980) Intrapopulational morphological variation as a predictor of feeding behavior in deermice. Am Nat 116:891–894
Srivathsa A, Tietje W, Rolland V, Polyakov A, Oli MK (2019) Climatic drivers of pinyon mouse Peromyscus truei population dynamics in a resource-restricted environment. Popul Ecol 61:122–131. https://doi.org/10.1002/1438-390X.1006
Stah CD (1980) Vertical nesting distribution of two species of Peromyscus under experimental conditions. J Mammal 61:141–143
Sueur J, Aubin T, Simonis C (2008) Seewave, a free modular tool for sound analysis and synthesis. Bioacoustics 18:213–226. https://doi.org/10.1080/09524622.2008.9753600
Timonin M, Kalcounis-Rueppell M, Marler CA (2018) Testosterone pulses at the nest site modify ultrasonic vocalization types in a monogamous and territorial mouse. Ethology 124:804–815. https://doi.org/10.1111/eth.12812
Waser PM, Brown CH (1986) Habitat acoustics and primate communication. Am J Primatol 10:135–154. https://doi.org/10.1002/ajp.1350100205
Waser PM, Waser MS (1977) Experimental studies of primate vocalization: Specializations for long-distance propagation. Z Tierpsychol 43:239–263. https://doi.org/10.1111/j.1439-0310.1977.tb00073.x
Whipple AV, Cobb NS, Gehring CA, Mopper S, Flores-Rentería L, Whitham TG (2019) Long-term studies reveal differential responses to climate change for trees under soil- or herbivore-related stress. Front Plant Sci 10:132. https://doi.org/10.3389/fpls.2019.00132
Wiley RH, Richards DG (1978) Physical constraints on acoustic communication in the atmosphere: Implications for the evolution of animal vocalizations. Behav Ecol Sociobiol 3:69–94. https://doi.org/10.1007/BF00300047
Yovel Y, Falk B, Moss CF, Ulanovsky N (2011) Active control of acoustic field-of-view in a biosonar system. PLoS Biol 9:e1001150. https://doi.org/10.1371/journal.pbio.1001150
Acknowledgements
We thank E. Roden and D. Hendershott for assistance with trapping and recording mice. We thank two anonymous reviewers for their insightful comments that greatly improved the manuscript.
Funding
The study was funded by the American Society of Mammalogists Grants-in-Aid (RB), a Jean Schuler Research Mini-Grant from Northern Arizona University (RB), and the National Science Foundation IOS # 1755429 (BP).
Author information
Authors and Affiliations
Contributions
RB and BP conceived the study, obtained the funding, and wrote the initial draft of the manuscript. RB, BP, and AK conducted the fieldwork. AK conducted the hearing experiments. SMM and all authors analyzed the data, and revised, edited, and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
All procedures were approved by Northern Arizona University’s Institutional Animal Care and Use Committee (19–006). Animals were captured with permits from the Arizona Game and Fish Department (SP403209). All research followed the guidelines for ethical treatment of animals established by the American Society of Mammalogists (Sikes 2016).
Competing interests
The authors declare no competing interests.
Additional information
Communicated by: E. Korpimäki.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Brzozowski, R., Kobrina, A., Mahoney, S.M. et al. Advertising and receiving from heights increases transmission of vocalizations in semi-arboreal mice. Behav Ecol Sociobiol 77, 83 (2023). https://doi.org/10.1007/s00265-023-03352-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-023-03352-4