Abstract
Forest elephants Loxodonta cyclotis aggregate in large numbers in forest clearings. Whether they maintain contact as they move through the forest and are able to coordinate these aggregations, similar to the fission-fusion sociality of the well-studied savanna elephants Loxodonta africana, is currently unknown. Since sound attenuates faster in closed as compared to open habitats, the low-frequency rumble vocalizations of forest elephants may exhibit smaller detection ranges than measured for those of savanna elephants, which may restrict the ability of forest elephants to coordinate interactions between separated family units. Here, we modeled the attenuation of forest elephant rumbles using amplitude measurements of rumbles recorded in a rainforest in Gabon and estimated the distances at which elephants might be able to detect them under observed ambient sound conditions. Our results suggest an attenuation rate less than predictions of spherical spreading loss, suggesting that reflection of the sound waves within the forest results in constructive interference. Nevertheless, we found that forest elephant rumbles of average dominant frequency (31.07 Hz) under average ambient sound levels would not be detectable farther than 0.8 km. Moreover, for 50% of analyzed rumbles, the harmonic structure was completely attenuated at only 100 m. However, we estimated detection distances of up to 3.2 km for rumbles of average dominant frequency when ambient sound was at its lowest. Our findings suggest that long-distance communication to coordinate interactions among separated family units may be limited in forest elephants, with potentially important consequences for their social organization.
Significance statement
The challenges associated with the extent of, and variation in, detection distances of long-distance vocalizations used by animals to mediate interactions between separated group members has rarely been investigated. While it has been suggested that forest elephants exhibit a fission-fusion sociality similar to savanna elephants, our results indicate shorter detection distances for forest elephant rumbles, suggesting a limited ability to mediate interactions between separated family units. However, under optimal ambient sound conditions, detection distances increased considerably. The long detection distances estimated for savanna elephants may reflect the optimal conditions under which the playback experiments were conducted. On average, savanna elephants may be much more limited in communication distance. Further studies on the constraints and opportunities that the different environments impose on these species’ communication capability may be critical to understanding potential differences in the social complexity they express.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Animals in fission-fusion societies experience frequent spatial and temporal separation, which poses unique challenges for social interaction. Such socio-spatial fluidity may allow individuals to adapt to limitations in resource availability, while retaining the benefits of group living (Aureli et al. 2008). In a range of fission-fusion societies, long-distance vocalizations appear to play an important role in coordinating and initiating interactions between spatially separated associates (e.g., Charif et al. 2005; Spehar and Di Fiore 2013; Eckhardt et al. 2015). But the spatial extent at which vocal communication can achieve these functions depends on the distance over which a vocalization can be detected and interpreted (Marten and Marler 1977). Despite being a fundamental component of long-distance vocal communication, detection distances have been estimated for only a few species (e.g., Brenowitz 1982; Miller 2006; Todd 2007; Charlton et al. 2012), including the vocalization of two fission-fusion species, the bottlenose dolphins (Janik 2000) and African savanna elephants (Langbauer et al. 1991; Garstang et al. 1995; Larom et al. 1997; McComb et al. 2003).
The distance at which a vocal signal can be detected (hereafter “detection distance”) is determined by the interplay of several acoustic, physiological, and environmental factors, including (1) the signal’s source amplitude and (2) the rate at which the signal is attenuated as it propagates through the environment. When sound travels through a medium, its amplitude diminishes with distance. In idealized materials, signal amplitude is only reduced by the spreading of the wave from the source uniformly in all directions (i.e., spherical spreading). However, under natural conditions, additional attenuation results from scattering and absorption, depending on the interaction of environmental and acoustic features of the signal (i.e., excess attenuation). For example, in habitats with high vegetation density, high-frequency signals attenuate faster than they would in open habitats (Marten and Marler 1977). Moreover, sound waves usually do not propagate uniformly but are reflected at the forest canopy or off the ground (i.e., cylindrical spreading), leading to slower attenuation rates than expected from spherical spreading alone. (3) At the location of the listener, ambient sound can mask the acoustic signal and interfere with its perception. A listeners’ peripheral auditory system applies a filter bank to the acoustic signal reaching the eardrum, and detection requires a signal’s amplitude to exceed the ambient sound level to a degree that is defined by the bandwidth of these auditory filters (i.e., the critical ratio; Fletcher 1940).
Most of what we know about African elephant communication and social behavior derives from studies conducted on savanna elephants Loxodonta africana and little is known about the forest elephants Loxodonta cyclotis inhabiting the dense rainforests in Central Africa. Savanna elephants live in a multi-tiered, matrilineal, fission-fusion society that centers around stable family groups consisting of females with their dependent offspring (Wittemyer et al. 2005; Archie et al. 2006). Coordination of social interactions within their spatially, temporally, and socially complex environment appears to be facilitated in particular by “rumble” vocalizations. Rumbles are tonal calls with typically low fundamental frequencies and a rich harmonic structure (e.g., Poole et al. 1988), which can encode information about individual identity (McComb et al. 2003), sex (Baotic and Stoeger 2017), emotional state (Soltis et al. 2005, 2009), behavioral context (Poole 2011; Stoeger et al. 2012), and external events (Soltis et al. 2014). In savanna elephants, rumbles may function to exchange such socially relevant information over distances up to 1 km (McComb et al. 2003), but they appear to be able to detect each other’s rumbles over much larger distances. Savanna elephant rumbles have been estimated to be detectable over 4 km using playback experiments (Langbauer et al. 1991) and modeled to be potentially detectable at distances over 10 km (Garstang et al. 1995; Larom et al. 1997).
Forest environments stand in stark contrast to those of the savanna, with major differences in food and water availability, visibility, and environmental conditions, all of which may pose different constraints on the social and communication system of forest elephants (for an overview see Fishlock et al. 2015). Forest elephants may aggregate in large numbers in forest clearings, which provide access to mineral-rich water (Turkalo and Fay 2001). However, it is unknown whether these aggregations are coordinated, nor whether family units maintain contact as they move through the forest. In particular, since sound generally attenuates faster in closed as compared to open habitats (Morton 1975), the rumbles of forest elephants may exhibit smaller detection ranges than measured for savanna elephants, which may restrict their ability to coordinate and initiate interactions between separated family units.
Here, we modelled the attenuation of forest elephant rumbles, and the source amplitude of these calls, using amplitude measurements of rumbles recorded at different distances from calling elephants. Based on this, we calculated the probable detection distances of rumbles under observed ambient sound conditions. We predicted that forest elephant rumbles would have shorter detection distances compared to those of savanna elephants, with potentially important consequences for their social organization.
Methods
Acoustic recording
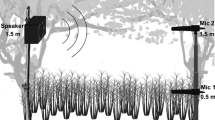
To determine the received amplitudes of rumbles at different distances from the callers, we installed an acoustic array consisting of nine custom-designed recording units (Calupca et al. 2000) around a forest clearing south of Ivindo National Park in northwestern Gabon (supplementary material 1 spectrograms and sound examples of analyzed rumbles). The habitat is a low-altitude mixed-species semi-evergreen rainforest. We acquired continuous 2 kHz 12-bit audio recordings on each unit from 23 September 2010 to 22 October 2010. Recorders were time-synchronized using a GPS signal such that we could use time of arrival to localize the source location of 98% of all recorded rumbles (Wrege et al. 2017). Calls were emitted at all directions and distances from each recorder. Source locations were calculated using the correlation sum location estimation (CSE) algorithm (Kurt Fristrup and Kathryn Cortopassi, pers. comm., used in Mennill et al. 2006), implemented in the Extensible Bioacoustics Tool XBAT (Figueroa and Robbins 2008). Uncertainty in the location estimate was within 6 m, given the specific array geometry and a speed of sound estimated to be 350 m/s given average temperature and humidity. Although recorders had similar acoustic sensitivities (see below), during the study, one unit recorded at consistently higher amplitude than any other did. Suspecting that this unit was either malfunctioning or placed in a peculiar location, it was omitted from all analyses.
To obtain absolute amplitude values, each recording system (microphone combined with recording components) was characterized in an anechoic chamber with reference to a Brüel-Kjaer 4190 instrumentation microphone using Audio Precisions® APx-520 audio analyzer. There was very little variation in the frequency response and overall gain among recording units, allowing use of the same calibration values across the different recorders. Because the recorder microphone sensitivity decreased with decreasing frequency below 50 Hz, we obtained calibration values at 2 Hz intervals from 24 to 70 Hz. We recognize that these calibration values are only approximate, because limitations in the size of the anechoic chamber meant that we were recording in the near field (less than one wavelength), which can produce some anomalies (e.g., Bradbury and Vehrencamp 2011). Since we were unable to obtain reliable calibration values for frequencies below 25 Hz, we restricted all call and ambient sound measurements to frequencies 25 Hz and above.
Acoustic measurements of rumbles
All measurements were made on spectrograms generated in Raven Pro Sound Analysis Software® (version 1.5) using a Hann window with an FFT size of 1024 samples and 95% overlap, resulting in a frequency and time resolution of 0.98 Hz and 25.5 ms. Because there were few elephants within the array during the day, we included only calls produced at night, between 1800 h and 0600 h the next day. This also helped to control environmental factors such as temperature and humidity. Calls were omitted from analysis if they overlapped another elephant call, were coincident with some other acoustic signal, or if the distance to source was less than two wavelengths of the measured frequency (to eliminate possible near-field effects on measurements).
We focused measurements on the dominant (highest amplitude) frequency contour, which in forest elephants is usually the second harmonic, or “F1” (Thompson 2009), and hereafter, we refer to this contour interchangeably as the “call” or rumble. For each call on each recorder, we drew a measurement rectangle such that the upper and lower boundaries exactly enclosed all frequency modulation in the call contour and extended along the temporal axis from the start to the end of the rumble. We constructed an identical measurement rectangle nearby, enclosing the same frequency bounds, to measure the background sound level. These were generally located within 1 s of the call rectangle. For each rectangle, we measured amplitude with the “inband power” tool in Raven®, which returns amplitude values in decibel units independent of spectrogram parameter settings. We converted these power measurements to pascals and calculated the received amplitude of calls by subtracting the inband power of the corresponding “background sound” rectangle. Because attenuation is assumed to be frequency-dependent (Marten and Marler 1977), we also calculated the center frequency of the call (referred to as frequency hereafter) as Fcenter = Fmin + ((Fmax − Fmin) / 2) with Fmin and Fmax indicating the maximum and minimum values of the frequency range of the measurement rectangles.
Attenuation of rumbles
Dominant frequency
To examine the attenuation of the dominant frequency of rumbles, we focused on calls that only traveled through the forest environment. We excluded all calls that traversed the clearing before reaching a recording unit given the location of the caller. We did so because impedance differences at boundaries can strongly affect sound propagation (Bradbury and Vehrencamp 2011). Center frequency of recorded rumbles ranged from 17 to 61 Hz. Although we excluded calls below 25 Hz to allow for the calibration of received amplitude measurements (see above), the resulting dataset included 80% of the initial frequency range and did not considerably differ in mean or 10th and 90th percentile for the center frequency. Because adult forest elephants predominantly produce rumbles with dominant frequencies below 41 Hz (Thompson 2009), the majority of recorded rumbles were likely produced by adult forest elephants (supplementary material 2 provides histograms of center frequencies). To model the attenuation of call amplitude over distance, we used a generalized linear mixed model (GLMM, Baayen 2008) (Table 1, details of model design and evaluation of model assumptions are presented in supplementary material 3).
Harmonic structure
To examine the attenuation of the information-rich harmonic structure of rumbles, we used calls that originated in the clearing and were recorded on an acoustic unit at the edge of the clearing. Each call’s spectrogram was inspected for presence of call energy above the second harmonic (i.e., > F1) and classed as “present” or “absent.” Because this analysis did not require measuring received amplitude and hence calibration, we included calls with dominant frequency below 25 Hz. We used logistic regression to model the loss of harmonic structure over distance, controlling for source amplitude (Table 1).
Source amplitude
To estimate source amplitude, we used two approaches. (1) Based on the results of the forest attenuation model, we used Eq. (1) to calculate the predicted amplitudes A of rumbles of different frequencies F, with distance to the caller D set to 1 m.
Edist, Efreq, and Int correspond to the estimates for the predictor variables distance and frequency, as well as the intercept term, derived from the attenuation model. Int + Efreq * F is the frequency-specific received amplitude at average distance from the source. Frequency and distance were log and z-transformed. (2) We independently estimated source amplitude using calls that originated within the clearing and were recorded on the edge of the clearing (N = 157 with center frequency 25 Hz or above), thus having a transmission path unobstructed by forest vegetation. Assuming that spherical spreading was the only source of attenuation, source amplitudes (dB) could be estimated using Eq. (2):
where D is the distance (m) from recorder to the caller. We used a linear model to investigate the relationship between estimated source amplitude and the dominant frequency of rumbles (Table 1).
Masking and detection distance
Determining at what amplitude ambient sound masks a signal requires an understanding of the auditory perception of signals against masking ambient sound. Few data are available on auditory filter bandwidths and critical ratios for large terrestrial mammals with low-frequency calls (Fay 1988), and none for elephants. Since masking occurs in a narrow band of frequencies surrounding the signal’s frequency (Fletcher 1940), we measured ambient sound in bands surrounding the average and maximum center frequency (31.07 and 60.56 Hz, respectively) of rumbles recorded in this study. Assuming that elephants have evolved auditory filters to match their vocal communication system, we measured ambient sound levels within frequency bands with a bandwidth of 13 Hz, corresponding to the average frequency modulation of the dominant frequency of rumbles analyzed in this study. In line with the general approach to measure noise in 1/3 octave bands surrounding the signal when the critical bandwidth is unknown (Erbe et al. 2016), the resulting frequency bands approximately correspond to the 4th and 7th 1/3 octave bands. Using Fletcher’s (1940) equal-power-assumption, we calculated a critical ratio for these frequency bands as CR = 10*log(13 Hz), resulting in detection threshold of 11.14 dB above the ambient sound level. Given that critical ratios decrease with decreasing frequency (Moore 1995), this threshold seems reasonable in comparison to previous estimates for other terrestrial mammals for slightly higher signal frequencies (Fay 1988).
We measured ambient sound levels within the two frequency bands at our study site at 20-min intervals at all eight recorder locations throughout the 25-day study. To gain a broader understanding of ambient sound conditions across the distribution range of forest elephants, we also measured ambient sound levels on randomly selected days at 1-h intervals at two other forest locations: 55 km from the main study location in a similar forest environment (“site A”; March–September 2010) and 390 km away in Loango National Park on the coast of Gabon (“site B,” July–September 2007 and January–April 2008).
We generated 1-s-long measurement rectangles in Raven®, which we visually inspected and repositioned if they overlapped irrelevant sounds (e.g., rain drops falling onto the recorder housing, elephant rumbles, and anthropogenic noise). If we were unable to reposition the rectangles within 5 s of the original location, the sample was removed. The sound amplitude in each rectangle was measured with the inband power tool. We investigated the effects of site and frequency band, while controlling for season and time of day, using a linear model (Table 1). To examine the diel pattern of ambient sound levels, we calculated hourly averages at each site for the average and high frequency bands.
Using coefficients and their standard errors from the attenuation model, we estimated the detection distances for calls for different dominant frequencies and ambient sound levels by solving the model Eq. (1) to derive the distance D. We calculated the distance at which A corresponds to the ambient sound level, i.e., at which a rumble would be fully immersed in the ambient sound (in the following referred to as the maximum detection distance). In addition, we calculated the distance at which a call has attenuated to the ambient sound level plus a masked threshold of 11.14 dB (in the following referred to as the minimum detection distance). Because vertebrates may employ a number of perceptional mechanisms leading to a masking release (e.g., Verhey et al. 2003), the actual detection distance will be located between those two extremes.
To minimize observer bias, blinded methods were used when all data were recorded and/or analyzed.
Data availability
The sound files, selection tables (location of elephant calls and background measurements), and acoustic array geometry used during the current study are available for download in the persistent repository at the following URL: https://cornell.box.com/s/vg417uhpbudw2815f1t0jt29qhqbesuv.
Results
Attenuation
Received amplitude measured at the different recorder locations decreased significantly with distance from the caller and decreased as the dominant frequency increased (Table 2). Surprisingly, the interaction between dominant frequency and distance was not significant, indicating that, within the range of dominant frequencies in our sample, the rate of attenuation did not increase with frequency as would be expected (Table 2 and Fig. 1). The estimated attenuation rate was less than theoretical predictions of spherical spreading loss, suggesting that reflection of the sound waves within the forest results in constructive interference (Fig. 2). The harmonic structure of calls attenuated rapidly. For calls traveling only through the clearing, the likelihood that harmonics other than the second were recorded on the edge of the clearing dropped to 50% at 103 m distance (logistic regression model: χ2 = 21.53, p < 0.001; distance parameter estimate: − 0.0209, Wald χ2 = 18.12, p < 0.001).
Effect of distance to the caller and dominant frequency on received amplitude. Dots represent the measured amplitude values and lines the predicted values for received amplitudes of rumbles with mean and minimum and maximum frequency against the distance to the caller. Distance was log- and z-transformed
Attenuation of a rumble of average frequency and source amplitude (31.07 Hz, 97 dB) modeled assuming transmission loss due to spherical spreading and cylindrical spreading only, and as modeled based on our received amplitude measurements in a Central African rain forest. Note that attenuation as estimated in our study was less rapid than predicted by spherical spreading, indicating that reflection in the forest environment may cause constructive interference
Source amplitude
Based on the model of call attenuation in the forest, we estimated that calls with low (25 Hz), average (37 Hz), and high (56 Hz) dominant frequency were emitted at 98, 95, and 92 dB, respectively. In the nearby forest clearing, where we based our estimate of source amplitude on the assumption of spherical spreading loss alone, source amplitudes for the same frequencies were more than double those in the forest (106.6, 105, and 102.1 dB, respectively). As found in the forest context, the center frequency of the call was significantly negatively related to source amplitude (GLM Fs (1, 155) = 4.41, p < 0.05) but explained only 3% of variation in source amplitude.
Ambient sound levels
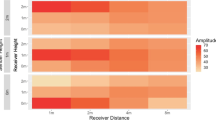
Ambient sound levels within the main study site ranged from a minimum of 20 dB under calm conditions to a maximum of 86 dB during rainstorms. Ambient sound levels were higher in the low frequency as compared to the high frequency band and differed between sites, time of day, and season (Fig. 3, GLM Fs = 384, p < 0.0001, r2 = 0.75; interaction frequency band and site: p < 0.0001; season: p < 0.0002; hour of day: p < 0.0001).
Diel pattern of ambient sound levels within 13 Hz wide frequency bands, centered on the average (31.07 Hz, solid curves) and maximum (60.56 Hz, dashed curves) center frequency of sampled forest elephant rumbles. Plotted are least square means that control the effect of season at three forested sites in Gabon: the main study site (circles), site A 55 km away within the same forestry concession (triangles), and site B in Loango National Park, 390 km away at the Gabonese coast (squares). Curves use cubic spline smoothing with lambda = 0. Note that ambient sound levels were higher in low-frequency as compared to the high-frequency band and differed between sites and time of day
Detection distance
For a rumble of average frequency and source amplitude (31.07 Hz, 96.6 dB), we estimated a maximum detection distance of 812 m (i.e., the distance at which the call amplitude has reached ambient sound level) and a minimum detection distance of 182 m (i.e., the distance at which the call amplitude has reached ambient sound level plus a masked threshold of 11.14 dB). Variation in ambient sound conditions had considerable influence on the detection distance. Under calm ambient sound conditions, we estimated a maximum and minimum detection distance of 3221 and 713 m for rumbles of average frequency and source amplitude (Table 3). Although frequency did not affect attenuation, because ambient sound levels were lower at higher frequencies, detection distance was longer for the high-frequency rumbles (maximum detection distance 2636 m, minimum detection distance 583; under average ambient sound conditions, Table 3).
Discussion
In Central Africa, forest elephants may communicate acoustically over only relatively short distances, with potentially significant implications for the organization and maintenance of social relationships. Against average ambient sound levels and at average source amplitude, we estimated that forest elephants can detect a conspecific’s rumble only up to about 812 m and more likely at distances closer to 182 m. Moreover, we found that the harmonic structure of rumbles was completely attenuated at a median distance of only 100 m from the caller. This stands in strong contrast to playback experiments demonstrating that savanna elephants are able to detect each other’s rumbles over a distance of at least 4 km (Langbauer et al. 1991) and extract socially relevant information encoded in the harmonic structure of rumbles at distances up to 1 km (McComb et al. 2003). Why such an apparent disparity between the two species of African elephant?
Playback experiments involving savanna elephants uniformly presented stimuli at near the maximum source amplitude for the species (110 dB: Langbauer et al. 1991; 105 dB: McComb et al. 2000; 107 dB: McComb et al. 2003) and were mainly conducted in the morning to early afternoon and avoided windy conditions (Langbauer et al. 1991; McComb et al. 2000). Although no systematic measurements of ambient sound levels in savanna environments are available, these experimental conditions appear to reflect a relatively optimal communication environment. In contrast, our estimate of 0.18–0.8 km constitutes the estimated detection distance of a rumble of average frequency and source amplitude under average ambient sound conditions. Using our attenuation model and a source amplitude similar to savanna elephant studies (106 dB, also estimated for forest elephants in clearings), at lowest ambient sound levels, we estimate a minimum and maximum detection distance for forest elephants of 2.9 to 13.3 km for rumbles of average dominant frequency, which is nearly in line with the measurements for savanna elephants. Under optimal conditions, communication ranges in forest and savanna elephants may be similar, but it is not known whether “typical” ambient sound levels in savanna environments limit detection distances as strongly as they do in the forested environments of Central Africa.
Another factor that might contribute to the apparent difference in the proposed detection distances for forest elephants and those measured for savanna elephants is that savanna elephants are more than 50% larger than forest elephants and consequently have larger vocal folds and a lower average fundamental frequency of rumbles, which are predicted to attenuate more slowly (14.7 vs. 19.5 Hz in forest elephants, Poole 2011 and Thompson 2009, respectively). However, within our sampled range of frequencies, we found no evidence that high-frequency rumbles attenuated faster compared to low-frequency rumbles. Although we omitted from analysis, all rumbles with very low frequency because we were unable to calibrate our recording system at frequencies below 25 Hz, the lowest recorded dominant frequency was only 17 Hz, and so more than 80% of the frequency range was used in the model. Unless there is some discontinuity in how frequency affects attenuation, variation in the fundamental frequency of rumbles, by itself, appears to have little influence on determining detection distances.
Our estimates of detection distances show the influence of ambient sound on the effective listening area during communication in forest elephants. Comparison with savanna elephants highlights the importance of clearly defining the communication context of an experiment or set of measurements. Is the focus on “what is possible” assuming optimal sound transmission (e.g., Larom et al. 1997), or is the focus on characterizing long-distance communication given typical ambient sound levels? Ideally, either focus would be combined with investigating whether the animals behaviorally exploit optimal conditions, and whether this reflects acoustic adaptation at the species level, or flexibility in vocal production of individual animals.
How might the constrained communication distance available to forest elephants affect their social system and coordination of movements? Compared to savannah elephants, family units of forest elephants are significantly smaller (mean group size = 2 to 3, e.g., Turkalo et al. 2013), and the multi-tiered aggregations that characterize fusion episodes in savanna elephants (Wittemyer et al. 2005) have not been observed in forest elephants (but see Fishlock and Lee 2013). Although it has been suggested that low-frequency vocalizations play a critical role in maintaining coordination among extended family subunits moving through the forest (e.g., Payne 2003), the relatively small typical detection ranges estimated here suggest that such communication may be limited. Significant in this regard is the finding that the information-rich harmonic structure of elephant rumbles is severely degraded at very short distances. This suggests limitations similar to those suggested by McComb et al. (2003) for savanna elephants: that elephants may be able to perceive the presence of other individuals at distance, but only at relatively close distance are they able to decode socially relevant information contained in rumbles. In forest elephants, rumbles may function primarily in the context of the nuclear family group and at relatively close distances as they move through the forest.
Interestingly, despite the overall short proposed detection distances for forest elephant rumbles, our results indicate that rumbles attenuated at a slower rate than expected by spherical spreading. Studies on sound transmission in closed environments, like forests, emphasize the negative effect of the high vegetation density within these, which is generally assumed to result in higher attenuation rates than expected by spherical spreading. However, forest environments can provide acoustic conditions similar to concert halls in which sound waves reflected at the forest canopy or off the ground and direct sound waves reinforce each other (Sakai et al. 1998). The often long duration of elephant rumbles should enhance such constructive interference. For instance, longer vocalizations of rainforest thrush species experience less attenuation due to superimposing reflections of sound waves (Nemeth et al. 2006). Similar to the temperature inversion in the atmosphere above savanna habitats (Garstang et al. 1995), such a concert hall effect may contribute to an improvement of an otherwise very short detection distance.
The detection distances estimated in this study varied broadly, mostly due to variation in ambient sound levels. This raises the intriguing question of whether forest elephants behaviorally exploit this variation to improve communication, for example timing calling activity to specific time windows (e.g., Larom et al. 1997; Garcia-Rutledge and Narins 2001). The diel pattern of ambient sound recorded at three different sites do not show any obvious “windows” where sound levels are particularly low. In Loango, for which long-term data are available on the diel pattern of forest elephant calling behavior (Fig. 1 in Wrege et al. 2017), calling activity increased in the early evening, when ambient sound levels were lower. But without similar data from other forests, it is unknown whether this pattern represents exploitation of quieter conditions or is a consequence of diel activity patterns and increased communication needs irrespective of the acoustic environment. Another behavioral mechanism could be to increase the dominant frequency of rumbles to avoid the higher ambient noise we measured at low frequencies in all three forests. For calls centered on 60 Hz, the range of detection distance increased to between 0.58 and 2.6 km at average ambient levels and 2.3–10.4 km under quiet conditions. In forest elephants, dominant frequencies (i.e., the 2nd harmonic, or F1) above about 41 Hz are nearly all produced by juveniles (Thompson 2009) and it is unclear how often this age-class participates in long-distance communication, but it would be intriguing if adult elephants shifted the fundamental frequency of their rumbles to take advantage of the lower ambient sound levels at higher frequencies. Moreover, elephants could increase call duration and the rate at which they produce rumbles (Ey et al. 2009), as well as source amplitude (the “Lombard Effect,” see Brumm and Zollinger 2011), to increase the probability of being heard. Previous studies have identified several structurally distinct, yet intergraded, classes of rumbles, with potentially different functions (McComb et al. 2003; Soltis et al. 2005, 2014; Wood et al. 2005; Poole 2011; Stoeger et al. 2012). It will be important to investigate variation in rumble structure in relation to their suitability for long-distance communication and encoded informational content to fully understand their functional use within the forest elephants’ social system. Elephants also have an unusually long and complex vocal tract that includes the trunk, and rumble vocalizations can be emitted either from the mouth or from the tip of the trunk, rumbles from the latter significantly lowering formant positions (Stoeger et al. 2012). This anatomical and behavioral complexity provides the potential for considerable adaptive response to changing communication challenges.
Although we empirically measured attenuation of elephant rumbles in one representative forest environment, with attendant variation in habitat structure and the physical orientation of the caller with respect to recording devices, the estimated detection distances of such calls are based on a number of important assumptions regarding the sound perception of elephants and will require further refinement. Estimates for the hearing sensitivity and audiograms of elephants are based on ex-vivo studies on elephant cochleae (Von Békésy and Wever 1960) and on a playback study with one captive Asian elephant (Heffner and Heffner 1982). Data on the structure of auditory filters and noise perception, such as critical bandwidths and ratios, are not available for elephants, nor for frequencies below 100 Hz for terrestrial mammals in general. Our estimation of detection distance represents a range in which the detection distance must fall, assuming a noise perception approximately within 1/3 octave bands as found in other vertebrates (e.g., Erbe et al. 2016). Perception mechanisms such as auditory scene analysis (see Bee and Micheyl 2008) and co-modulation masking release (e.g., Verhey et al. 2003), might enable elephants to segregate acoustic signals from ambient noise at larger distances than expected given the masked threshold used in this study. Studies on multiple subjects to provide a detailed quantification of sound perception in African elephants are needed to enhance our understanding of their long-distance vocal communication. We here provide a theoretical estimate of the detection distance of rumbles and no direct evidence about the ability of forest elephants to perceive rumbles. Ultimately, playback studies are required to evaluate the extent of long-distance communication in forest elephants. Behavioral playback studies, while logistically difficult in the forest elephant’s densely vegetated habitat, could be possible with elephants in clearings and rumbles broadcast from the surrounding forest, or by combining recorder-accelerometer devices on individual elephants (that could monitor both vocal and postural response to a stimulus) with an acoustic array that would allow localization of naturally produced vocalizations. For savanna elephants, studies evaluating how natural variation in ambient conditions affects sound transmission would provide a more comprehensive understanding of long-distance communication and coordination in this species. Understanding the constraints and opportunities that different environments impose on these species’ communication capability may be critical to understanding the known and suspected differences in social complexity that these species express.
The socio-spatial dynamics characteristic of fission-fusion systems create distinctive selective pressures acting on underlying communicative and cognitive abilities (Aureli et al. 2008). The challenges associated with the extent of, and variation in, detection distances of long-distance vocalizations used by animals living in fission-fusion societies to mediate social interactions have rarely been investigated. However, the distance over which individuals are able to communicate is critical to their ability to coordinate reunions and maintain social relationships when separated. Our results emphasize that environmental variation, such as changing ambient sound levels, can severely impede this ability. In order to maintain their social relationships given such variation in communication conditions, animals may employ advanced cognitive skills and high levels of behavioral flexibility, including vocal plasticity. Investigating the reciprocal effects of detection distance and intergroup distance in relation to environmental constraints is a fascinating field to further study the coordination of social interactions in fission-fusion societies in both a cognitive and a socio-ecological framework.
References
Archie EA, Moss CJ, Alberts SC (2006) The ties that bind: genetic relatedness predicts the fission and fusion of social groups in wild African elephants. Proc R Soc Lond B 273(1586):513–522. https://doi.org/10.1098/rspb.2005.3361
Aureli F, Schaffner CM, Boesch C et al (2008) Fission-fusion dynamics: new research frameworks. Curr Anthropol 49:627–654
Baotic A, Stoeger AS (2017) Sexual dimorphism in African elephant social rumbles. PLoS One 12(5):e0177411. https://doi.org/10.1371/journal.pone.0177411
Bee MA, Micheyl C (2008) The cocktail party problem: what is it? How can it be solved? And why should animal behaviorists study it? J Comp Psychol 122(3):235–251. https://doi.org/10.1037/0735-7036.122.3.235
Bradbury JW, Vehrencamp SL (2011) Principles of animal communication. Sinauer Associates, Sunderland
Brenowitz EA (1982) The active space of red-winged blackbird song. J Comp Physiol 147(4):511–522. https://doi.org/10.1007/BF00612017
Brumm H, Zollinger SA (2011) The evolution of the Lombard effect: 100 years of psychoacoustic research. Behaviour 148(11):1173–1198. https://doi.org/10.1163/000579511X605759
Calupca TA, Fristrup KM, Clark CW (2000) A compact digital recording system for autonomous bioacoustic monitoring. J Acoust Soc Am 108(5):2582–2582. https://doi.org/10.1121/1.4743595
Charif RA, Ramey RR, Langbauer WR, Payne KP, Martin RB, Brown LM (2005) Spatial relationships and matrilineal kinship in African savanna elephant (Loxodonta africana) clans. Behav Ecol Sociobiol 57(4):327–338. https://doi.org/10.1007/s00265-004-0867-5
Charlton BD, Reby D, Ellis WA, Brumm J, Fitch WT (2012) Estimating the active space of male koala bellows: propagation of cues to size and identity in a eucalyptus forest. PLoS One 7(9):e45420. https://doi.org/10.1371/journal.pone.0045420
Eckhardt N, Polansky L, Boesch C (2015) Spatial cohesion of adult male chimpanzees (Pan troglodytes verus) in Taï National Park, Côte d’Ivoire. Am J Primatol 77(2):125–134. https://doi.org/10.1002/ajp.22316
Erbe C, Reichmuth C, Cunningham K, Lucke K, Dooling R (2016) Communication masking in marine mammals: a review and research strategy. Mar Pollut Bull 103:15–38
Ey E, Rahn C, Hammerschmidt K, Fischer J (2009) Wild female olive baboons adapt their grunt vocalizations to environmental conditions. Ethology 115(5):493–503. https://doi.org/10.1111/j.1439-0310.2009.01638.x
Fay R (1988) Hearing in vertebrates—a psychophysics Databook. Hill-Fay Associates, Winnetka
Figueroa H, Robbins M (2008) XBAT: an open-source extensible platform for bioacoustic research and monitoring. In: Frommolt K-H, Bardeli R, Clausen M (eds) Computational bioacoustics for assessing biodiversity. Bundesamt für Naturschutz, Bonn, pp 143–155
Fishlock V, Lee PC (2013) Forest elephants: fission–fusion and social arenas. Anim Behav 85(2):357–363. https://doi.org/10.1016/j.anbehav.2012.11.004
Fishlock V, Schuttler S, Breuer T (2015) Forest elephant biology and behaviour: research questions for conservation. In: Fischlock V, Breuer T (eds) Studying Forest elephants. Neuer Sportverlag, Stuttgart, pp 32–41
Fletcher H (1940) Auditory patterns. Rev Mod Phys 12(1):47–65. https://doi.org/10.1103/RevModPhys.12.47
Garcia-Rutledge EJ, Narins PM (2001) Shared acoustic resources in an old world frog community. Herpetologica 57(1): 104–116
Garstang M, Larom D, Raspet R, Lindeque M (1995) Atmospheric controls on elephant communication. J Exp Biol 198(Pt 4):939–951
Heffner RS, Heffner HE (1982) Hearing in the elephant (Elephas maximus): absolute sensitivity, frequency discrimination, and sound localization. J Comp Physiol Psychol 96(6):926–944. https://doi.org/10.1037/0735-7036.96.6.926
Janik VM (2000) Source levels and the estimated active space of bottlenose dolphin (Tursiops truncatus) whistles in the Moray Firth, Scotland. J Comp Physiol A 186(7-8):673–680. https://doi.org/10.1007/s003590000120
Langbauer WR, Payne KB, Charif RA, Rapaport L, Osborn F (1991) African elephants respond to distant playbacks of low-frequency conspecific calls. J Exp Biol 157:35–46
Larom D, Garstang M, Lindeque M, Raspet R, Zunckel M, Hong Y, Brassel K, O'Beirne S (1997) Meteorology and elephant infrasound at Etosha National Park, Namibia. J Acoust Soc Am 101(3):1710–1717. https://doi.org/10.1121/1.418180
Marten K, Marler P (1977) Sound transmission and its significance for animal vocalization. Behav Ecol Sociobiol 2(3):271–290. https://doi.org/10.1007/BF00299740
McComb K, Moss C, Sayialel S, Baker L (2000) Unusually extensive networks of vocal recognition in African elephants. Anim Behav 59(6):1103–1109. https://doi.org/10.1006/anbe.2000.1406
McComb K, Reby D, Baker L, Moss C, Sayialel S (2003) Long-distance communication of acoustic cues to social identity in African elephants. Anim Behav 65(2):317–329. https://doi.org/10.1006/anbe.2003.2047
Mennill DJ, Burt JM, Fristrup KM, Vehrencamp SL (2006) Accuracy of an acoustic location system for monitoring the position of duetting songbirds in tropical forest. J Acoust Soc Am 119(5):2832–2839. https://doi.org/10.1121/1.2184988
Miller PJO (2006) Diversity in sound pressure levels and estimated active space of resident killer whale vocalizations. J Comp Physiol A 192(5):449–459. https://doi.org/10.1007/s00359-005-0085-2
Moore BC (ed) (1995) Hearing. Academic Press, San Diego
Morton ES (1975) Ecological sources of selection on avian sounds. Am Nat 109(965):17–34. https://doi.org/10.1086/282971
Nemeth E, Dabelsteen T, Pedersen SB, Winkler H (2006) Rainforests as concert halls for birds: are reverberations improving sound transmission of long song elements? J Acoust Soc Am 119(1):620–626. https://doi.org/10.1121/1.2139072
Payne K (2003) Sources of social complexity in the three elephant species. In: de Waal FBM, Tyak PL (eds) Animal social complexity: intelligence, culture, and individualized societies. Harvard University Press, Cambridge, pp 57–85. https://doi.org/10.4159/harvard.9780674419131.c5
Poole JH (2011) Behavioral contexts of elephant acoustic communication. In: Moss CJ, Croze H, Lee PC (eds) The Amboseli elephants: a long-term perspective on a long-lived mammal. University of Chicago Press, Chicago, pp 125–161. https://doi.org/10.7208/chicago/9780226542263.003.0009
Poole JH, Payne K, Langbauer WR, Moss CJ (1988) The social contexts of some very low frequency calls of African elephants. Behav Ecol Sociobiol 22(6):385–392. https://doi.org/10.1007/BF00294975
R Core Team (2016) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Sakai H, Sato S, Ando Y (1998) Orthogonal acoustical factors of sound fields in a forest compared with those in a concert hall. J Acoust Soc Am 104(3):1491–1497. https://doi.org/10.1121/1.424360
Soltis J, Leong K, Savage A (2005) African elephant vocal communication II: rumble variation reflects the individual identity and emotional state of callers. Anim Behav 70:589–599
Soltis J, Leighty KA, Wesolek CM, Savage A (2009) The expression of affect in African elephant (Loxodonta africana) rumble vocalizations. J Comp Psychol 123(2):222–225. https://doi.org/10.1037/a0015223
Soltis J, King LE, Douglas-Hamilton I, Vollrath F, Savage A (2014) African elephant alarm calls distinguish between threats from humans and bees. PLoS One 9(2):e89403. https://doi.org/10.1371/journal.pone.0089403
Spehar SN, Di Fiore A (2013) Loud calls as a mechanism of social coordination in a fission–fusion taxon, the white-bellied spider monkey (Ateles belzebuth). Behav Ecol Sociobiol 67(6):947–961. https://doi.org/10.1007/s00265-013-1520-y
Stoeger AS, Heilmann G, Zeppelzauer M, Ganswindt A, Hensman S, Charlton BD (2012) Visualizing sound emission of elephant vocalizations: evidence for two rumble production types. PLoS One 7(11):e48907. https://doi.org/10.1371/journal.pone.0048907
Thompson M (2009) African forest elephant (Loxodonta africana cyclotis) vocal behavior and its use in conservation. PhD thesis, Cornell University
Todd NPM (2007) Estimated source intensity and active space of the American alligator (Alligator Mississippiensis) vocal display. J Acoust Soc Am 122(5):2906–2915. https://doi.org/10.1121/1.2785811
Turkalo AK, Fay JM (2001) Forest elephant behavior and ecology. Yale University Press, New Haven
Turkalo AK, Wrege PH, Wittemyer G (2013) Long-term monitoring of Dzanga Bai forest elephants: forest clearing use patterns. PLoS One 8(12):e85154. https://doi.org/10.1371/journal.pone.0085154
Verhey JL, Pressnitzer D, Winter IM (2003) The psychophysics and physiology of comodulation masking release. Exp Brain Res 153:405–417
Von Békésy G, Wever EG (1960) Experiments in hearing. McGraw-Hill, New York
Wittemyer G, Douglas-Hamilton I, Getz WM (2005) The socioecology of elephants: analysis of the processes creating multitiered social structures. Anim Behav 69:1357–1371
Wood JD, McCowan B, Langbauer WR Jr, Viljoen JJ, Hart LA (2005) Classification of African elephant Loxodonta africana rumbles using acoustic parameters and cluster analysis. Bioacoustics 15(2):143–161. https://doi.org/10.1080/09524622.2005.9753544
Wrege PH, Rowland ED, Keen S, Shiu Y (2017) Acoustic monitoring for conservation in tropical forests: examples from forest elephants. Methods Ecol Evol (published online, doi:10.1111/2041-210X.12730). https://doi.org/10.1111/2041-210X.12730)
Acknowledgments
This study was supported by a grant to PHW from the US Fish and Wildlife Service, the Robert G. and Jane V. Engle Foundation, and through a generous gift from Lisa Yang to the Cornell Lab of Ornithology. Research clearance was approved by the Gabon government’s Centre National de la Recherché Scientifique et Technologique. We thank the reviewers for their careful review and very useful suggestions. Special thanks go to Abbey Doyno for analysis help; Yu Shiu, Holger Klinck, and Dean Hawthorne for discussions; Lynn Marie Johnson for statistical advice; Elizabeth D. Rowland and Herve Londo for superb assistance with data collection; and Precious Woods Gabon for critical logistics support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
This work was carried out using a non-invasive method, which required no direct observations or contact with the animals. This research was conducted in accordance with the national laws of the Republic of Gabon. Research clearance was approved by the Gabon government’s Centre National de la Recherché Scientifique et Technologique.
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Communicated by K. E. Ruckstuhl
Rights and permissions
About this article
Cite this article
Hedwig, D., DeBellis, M. & Wrege, P.H. Not so far: attenuation of low-frequency vocalizations in a rainforest environment suggests limited acoustic mediation of social interaction in African forest elephants. Behav Ecol Sociobiol 72, 33 (2018). https://doi.org/10.1007/s00265-018-2451-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-018-2451-4