Abstract
To evaluate the effects of calling site on call degradation, we broadcast synthetic advertisement calls of male gray treefrogs through forest, over open terrain, and across pond water. Calls were recorded at distances of 1, 2, 4, 8, 16, and 32 m. We varied speaker and microphone heights for a total of five elevation combinations ranging from surface level to a height of 1.5 m. We quantified structural degradation in recorded calls using “∆V,” a measure of relative sound energy in call pulses and interpulse intervals. A subset of recorded calls was used in two-speaker discrimination tests with females. Finally, we examined male selection of perch height by recording locations of calling males on ladder-like trellises positioned around the periphery of a breeding pond. We found the greatest degradation for calls broadcast through forest followed by calls transmitted across open terrain and then pond water. At relatively small source-receiver separations, elevation had only small effects on degradation. However, for separations greater than 4 m (especially through forest), elevation had a significant impact on ∆V—with calls broadcast and recorded near the substrate particularly vulnerable to degradation. Choice tests demonstrated that such levels of degradation could significantly reduce a male’s attractiveness. This may, in part, explain why males only seldom called from low rungs of trellises.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Across numerous taxa, including frogs, considerable effort has been expended to learn how specific attributes of signals (such as duration and complexity) and signaling behaviors (such as rate and timing patterns) influence female discrimination and, by inference or measurement, mate choice and mating success (Ryan and Keddy-Hector 1992; Andersson 1994). In contrast, although research on insects, anurans, primates, and especially birds has sought to determine how the habitat in which a male signals impacts signal structure at potential receiver locations (e.g., Waser and Waser 1977; Wiley and Richards 1982; Dabelsteen et al. 1993; Kime et al. 2000; Lang 2000), fewer data are available on how these habitat-induced effects, in turn, influence the behavior of potential mates and thus possibly mating success (Gerhardt 1976; Lang 2000; Murphy 2008; Kuczynski et al. 2010). We also know relatively little about whether and how animals adjust their signaling behavior to features of the environment (Ey and Fischer 2009; Ziegler et al. 2011; Erdtmann and Lima 2013). Of particular and growing interest, although a topic neglected in most studies of communication, is choice of signaling location (for examples of exceptions, see Fellers 1979a; Wells and Schwartz 1982; Wilczynski et al. 1989; Arak and Eiriksson 1992; Lang 2000; Barker et al. 2009). In chorusing animals, the precise location of individuals, relative to structural elements of the environment as well as other signalers, should be especially important in determining the extent to which receivers can assess important features of communication sounds (Schwartz and Gerhardt 1995; Bee and Micheyl 2008).
Many species of anuran amphibians form aggregations (i.e., choruses) at wetlands in which males vocally advertise to attract mates. These choruses generate high levels of background noise that pose serious challenges to acoustic communication (Gerhardt and Klump 1988; Narins and Zelick 1988; Wollerman 1999; Schwartz and Bee 2013; Vélez et al. 2013). Moreover, call characteristics are modified as signals travel through the environment, posing further challenges to communication (Ryan and Sullivan 1989; Kime et al. 2000; Castellano et al. 2003; Penna et al. 2006, 2013; Kuczynski et al. 2010; Röhr and Junca 2013). In addition to attenuation due to spherical spread, higher frequencies typically undergo greater excess attenuation relative to lower frequencies. Sound scattering and reverberation also affect call structure and can have significant effects on the fine temporal structure of calls. The resulting degradation has been found to increase with source-receiver distance and may vary dramatically among habitat types and, as with other effects, can change with elevation of signalers and receivers (Wiley and Richards 1982; Ryan and Kime 2003; Slabbekoorn 2004).
Previous studies (Zimmerman 1983; Ryan and Sullivan 1989; Ryan et al. 1990; Kime et al. 2000; Castellano et al. 2003; Bosch and de la Riva 2004; Penna et al. 2006) have addressed whether the calls of different anuran taxa (species, subspecies, or diploids and tetraploids) are best suited to their habitats, as predicted by the acoustic adaptation hypothesis (Morton 1975; Wiley and Richards 1982). Although the evidence for such structural acoustic adaptation is at best mixed (Gerhardt and Huber 2002; Wells 2007; Ey and Fischer 2009; Erdtmann and Lima 2013), there can nevertheless be significant impacts of habitat type, elevation, and transmission distance on the post-emission features of anuran vocalizations. For example, the calls of subspecies of Acris crepitans exhibited greater degradation when broadcast through forested habitats than grassland areas (Ryan et al. 1990). Ryan and Sullivan (1989) assessed call degradation in the toads Bufo valliceps and Bufo woodhousii. The advertisement calls of the former species, which have a slower rate of amplitude modulation than calls of the latter species, exhibited greater declines in the structural integrity of pulses with broadcast distance. Based on the neurophysiological data of Rose and Capranica (1984) on two other toad species, the authors speculate that species discrimination abilities of females could be compromised at distances of 80 m or greater from calling males. Indeed, Ratnam et al. (2004), also see Feng and Schul 2006), showed that structural alterations to the fine temporal components of the calls of Rana pipiens pipiens as a result of echoes and reverberation can affect the response of neurons in the auditory midbrain in a way likely to impair call discrimination. In a study of neotropical species, Kime et al. (2000) observed that calls suffered less degradation when broadcast from greater elevation and when broadcast through more open habitats.
Attenuation of signals can contribute to major limitations on communication distance in frogs (e.g., Penna et al. 2006, 2013), and as has been repeatedly demonstrated for insects (Römer 2012), birds (Slabbekoorn 2004; Brumm and Naguib 2009), and mammals (Waser and Waser 1977; Sabatini and Ruiz-Miranda 2008), its magnitude is influenced by the calling venue. For example, Wells and Schwartz (1982), working with glassfrogs (Hyalinobatrachium fleischmanni), found that call transmission through dense vegetation caused greater excess attenuation than did call transmission at greater height through less obstructed habitat. A mathematical modeling study of call propagation in the spring peeper (Pseudacris crucifer) found that calling away from the chorus from elevated perches and over water increased signal active space relative to calling near the ground or near other males (Parris 2002). Interestingly, Sun et al. (2000) found that excess attenuation of higher frequencies in the call of túngara frogs resulted in increased concordance between the tuning of the basilar papillae in the auditory periphery of females and the distribution of call spectral energy. However, the opposite result was obtained using similar data on cricket frogs.
The research we describe below builds on knowledge accumulated over more than three decades on communication and sexual selection in the gray treefrog, Hyla versicolor (Gerhardt and Huber 2002; Wells 2007). Gray treefrogs, like other acoustically signaling animals, are often found in environments that are complex as a result of patterns of individual distribution, natural vegetation, and additional habitat attributes. Because these factors contribute to sonic complexity and signal integrity, they will necessarily influence the effectiveness of communication; yet, we know relatively little about these influences (Feng and Ratnam 2000; Gerhardt and Huber 2002; Slabbekoorn 2004). Indeed, in spite of recent efforts to increase the realism of experimental conditions (e.g., Schwartz et al. 2001, 2013; Christie et al. 2010), critical gaps remain in our understanding of how H. versicolor and other species of frogs communicate in the real world (Feng and Ratnam 2000).
Choruses of male gray treefrogs form in and near wetlands in much of the eastern half of the USA during late spring and early summer, and females approach these aggregations from the surrounding habitat and then listen to males calling from different locations before selecting a mate (Fellers 1979a; Schwartz et al. 2004; Johnson et al. 2007). In our study sites in southeastern New York (and in other treefrog breeding areas), males call in a variety of locations (e.g., on the ground, water surface, floating logs/trees, branches, tree trunks, within bushes and dense stands of cattails, and on sparse emergent or floating vegetation) and broadcast their calls across a variety of habitat types (pond, forest, grassy).
Males of H. versicolor produce spectrally bimodal advertisement calls consisting of a chain of pulses (NY ~18 pulses at ~20 pulses/s at 20 °C). Each pulse has spectral peaks (and relative amplitudes) at, on average, about 1.1 kHz (−6 dB) and 2.2 kHz (0 dB), and frequency varies among males of different size (Gerhardt 1991, 2005a). However, call preferences of females based on frequency differences are fairly weak (Gerhardt 2005a), and our New York animals exhibit no discrimination among calls lacking energy in different spectral bands (Gerhardt et al. 2007). Call recognition (and species discrimination) by females of H. versicolor is primarily mediated by the durations of pulses and interpulse intervals and to a lesser degree by pulse shape (Schul and Bush 2002). Thus, the recognition mechanisms themselves may exhibit sensitivity to corruption of signal elements (Schwartz et al. 2010). The number of pulses in calls can vary both among and within males (Wells and Taigen 1986; Gerhardt 1991). In phonotaxis tests, all else being equal, females typically discriminate in favor of longer relative to shorter calls (Gerhardt et al. 1996, 2000; Schwartz et al. 2001). Males append pulses to their calls in response to acoustic stimulation while simultaneously reducing call rate. Females often, but not always, prefer longer calls produced at lower rates over shorter calls at higher rates (Klump and Gerhardt 1987; Gerhardt et al. 1996; Schwartz et al. 2001, 2008).
The goals of our study were threefold. First, given the range of possible calling sites and signal transmission routes from males to listening females as well as the importance of the pulse structure within advertisement calls to females, we wanted to ascertain the effects of perch elevation, habitat type, and distance from the signaler on the fine temporal structure of the advertisement calls. Studies of signal transmission have identified significant impacts on signal structure and attenuation of transmission close to the ground and through dense vegetation (Wiener and Keast 1959; Aylor 1972; Marten and Marler 1977; Dabelsteen et al. 1993; Forrest 1994; Wiley 2009). Therefore we were particularly, but not exclusively, interested in how calling at low elevation and through forest would affect the structural integrity and amplitude of the pulsed calls of gray treefrogs relative to other elevations or habitat types. Second, we wanted to learn how changes in call structure during transmission influence the behavior of female receivers. To accomplish this objective, we offered females a choice between paired subsets of the calls recorded under different transmission conditions as well as an opportunity to approach individual, rather than paired, stimulus calls. Kuczynski et al. (2010) found that females of Cope’s gray treefrog, H. chrysoscelis, were relatively tolerant of declines in modulation depth of synthetic calls consisting of sinusoidally amplitude-modulated tones. We predicted, therefore, that females would not discriminate among pulsed calls transmitted over short distances or through habitats lacking woody vegetation, especially at higher elevations above the habitat’s surface. However, as articulated in Kuczynski et al. (2010), we predicted that females would discriminate when there were large differences in the salience of pulse structure within call alternatives.
Signal efficacy (Guilford and Dawkins 1991; Hebets and Papaj 2005; Ryan and Cummings 2005) can be improved via signal design as well as by behaviors influencing when and where the signal is used. For example in anurans, mating success may be correlated with characteristics of the calling site likely to influence signal efficacy such as obstructing vegetation (in gray treefrogs, Fellers 1979a) and perch elevation (in a dart-poison frog, Pröhl and Hödl 1999). Accordingly, a third goal was to learn whether males do or can adopt behaviors that compensate for negative impacts of call degradation occurring during transmission. We investigated whether adding pulses to their calls could help males compensate for deleterious impacts of call degradation on their relative attractiveness. We tested also whether the elevation of calling perches selected by males is concordant with expectations based on relative vulnerabilities of calls to degradation at those locations and the responses of receivers. Only a few previous studies have described calling sites of gray treefrogs (Johnson 1966; Ralin 1968; Fellers 1979a; Ptacek 1992; Höbel and Barta 2014).
Materials and methods
Call broadcasts in the field
We assessed the impact of signaler and receiver elevation on the structural degradation of advertisement calls at different transmission distances in three habitat types. These were through deciduous forest, over grassy open terrain, and across pond water at areas where gray treefrogs breed. In each of the three habitat types, we ran three transects. The three transects in deciduous forest were located adjacent to a pond at the Blue Mountain Reservation in Peekskill, NY (latitude 41.268848, longitude −73.898678). Two of the grassy open terrain transects were also at the Blue Mountain Reservation site. The third open terrain transect was a lawn at the edge of a large marsh on private property (latitude 41.437708, longitude −73.703778) within 1 km of the Putnam County Veterans’ Park. The aspect of vegetation on the private property site was similar, but not identical, to grassy open areas at our other sites; however, its size allowed us to easily run a sufficiently long transect. The three different transects across pond water were located along the edge of a large pond (~0.60 ha) at the Putnam County Veterans’ Park (latitude 41.442726, longitude −73.709335). Although a large pond (~0.25 ha) is present, the extremely muddy substrate and sloping bottom along sections near the shore made it too difficult to perform our broadcasts across pond water at the breeding venue in the Blue Mountain Reservation site. We conducted broadcasts on three different nights, for each habitat type, during the late summer of 2009 and 2010 (N = 9 nights total). Choruses of H. versicolor often exhibit noise levels of between 70 and 80 dB (Schwartz et al. 2001), and overlap of calls is common (Schwartz et al. 2002). Therefore, we waited until after the breeding season of H. versicolor had ended (typically by the third week in July) so that vocalizations of males would not obscure recordings of our broadcast calls. We conducted our tests from approximately 9:00 PM until midnight which coincides with the hours of peak chorus activity. Accordingly, the environmental conditions were similar to those present when males naturally advertise for mates except that background noise levels were relatively low (<58 dB, sound pressure level (SPL) re to 20 μPa; C-weighting, slow root mean square (RMS) response, Radio Shack model #33-2050 meter).
For each test condition, we broadcast from a battery-powered PA system (Behringer model Europort EPA40), either resting on a pad on the substrate or mounted on a tripod (Zhumell model ZHUL024), 10 repetitions of a synthetic 18-pulse advertisement call that was stored on a memory card in a digital recorder (Zoom model H2 Handy Recorder). We synthesized calls (16 bits per sample, 20 kHz sampling rate), modeled after the natural advertisement calls of H. versicolor calling at 20 °C, using a custom program written by the first author. Calls were digitally synthesized by first creating and combining 2200 and 1100 Hz sinusoids (0° phase offset) with the 25-ms duration of a single pulse within a call. The relative amplitude of the two main frequency components of the call was adjusted during synthesis to compensate for the frequency response of the playback system so that during broadcast the relative amplitude of the lower frequency peak was approximately −6 dB SPL. The pulse was shaped to give it a nearly linear 20 ms rise and a 5 ms concave down fall and then, together with a 25-ms interpulse interval, copied to produce a full call of 18 pulses. The call was subsequently shaped to impart a 50 ms linear rise. The software has also been used to synthesize calls of other species of anurans including bullfrogs, Lithobates catesbeianus, (Schwartz and Simmons 1990), túngara frogs, Physalameus pustulosus, (Wilczynski et al. 1995), and Cope’s gray treefrog, H. chrysoscelis, (Gerhardt 2005a, b).
Using the volume control of the Zoom H2, prior to experimental broadcasts, the amplitude of an unmodulated sound with the frequency structure of the call was adjusted to 100 dB (SPL re to 20 μPa; C-weighting, slow RMS response) with a sound level meter (Radio Shack model #33-2050) held 1 m from the speaker (speaker and meter height = 0.75 m). This level was not changed during broadcasts. We used an unmodulated sound because this provided for more precise level adjustment than the pulsatile advertisement call. Broadcast calls were recorded with an omnidirectional condenser microphone (Audio Technica model AT8010; frequency response flat ±1 dB between 200 and 3000 Hz) in the manufacturer supplied windscreen and a digital recorder (Marantz model PMD 670; WAV files; 16 bits per sample; 22,050 Hz sampling rate). Once adjusted at a distance of 1 m from the speaker, recording level was not changed. We used an omnidirectional rather than a directional microphone to more effectively capture broadcast sounds that traversed an indirect path from speaker to microphone. The directionality of the former microphone type also more closely resembles that of the treefrog’s ear than does a more directional unit (Gerhardt and Huber 2002).
We chose areas for transects that were judged representative of the desired habitat type and then selected a speaker location and orientation pseudo-randomly (using the second hand of a watch) but making minor adjustments if large trees or other obstructions would prevent placement of equipment at desired positions. For broadcasts over pond water, our transects traversed shallow water near the shore. To facilitate making recordings at the appropriate distances from the speaker, we placed red flags (on thin metal stakes) at positions 1, 2, 4, 8, 16, and 32 m from the speaker face (these were placed in mud/sand on or near the shoreline for broadcasts over pond water).
At each of the aforementioned distances from the speaker, we recorded the broadcast audio file using five elevation combinations for the microphone and speaker (0.00/0.00, 0.75/0.00, 0.75/0.75, 0.75/1.50, and 1.50/1.50 m). Among nights, we varied the distance (either 1 or 32 m) and elevation (either 0.00 or 1.50 m) at which we started our broadcasts and recording. Except for very quiet evenings, the speaker-microphone separations probably more than span distances at which females evaluate the calling performance of male conspecifics (Gerhardt and Klump 1988; Murphy and Gerhardt 2002; Christie et al. 2010). The range of elevations encompasses those at which males are most frequently found calling (JJS personal observations and data herein).
Analysis of recordings
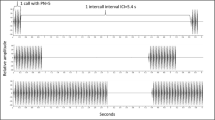
Using Cool Edit Pro (Syntrillium Software Corp., version 1.2), we deleted from WAV files for each experimental condition material (e.g., voice, relative silence) recorded prior to the start of the first recorded synthetic advertisement call. The presence of an initial unmodulated sound burst in the recordings that was broadcast loudly just prior to the first advertisement call helped us to locate the initiation of this call in the recordings at the largest transmission distance. We used a custom program, written in C, that utilized the known time positions of the calls’ pulses relative to the initial sound, to calculate the root mean square amplitude of individual pulses (RMS P) and the following interpulse intervals (RMS IPI) within each advertisement call (N = 16 of each calculated per call). We quantified degradation using “∆V” (Ryan and Sullivan 1989; Kuczynski et al. 2010). ∆V, calculated as 1 − (RMS IPI / RMS P), provides a measure of the spread of sound energy from the pulses to the interpulse intervals (Fig. 1). This spread typically becomes more pronounced with increased source—receiver separations. A ∆V of 0.0 would mean that the sound energy within a pulse is equal to that in the subsequent interpulse interval, and a ∆V of 1.0 would mean that sound energy within the interpulse interval is absent. ∆V values for the pulses and intervals were subsequently averaged to provide a mean value for each of these elements per call.
We also quantified attenuation of calls, using RMS amplitudes, relative to that obtained for each habitat and elevation at a distance of 1 m from the broadcast speaker. These calculations, those of ∆V, and statistical analyses were performed using SAS (SAS Institute, Cary, NC).
Tests with females
Subjects
We acquired females of H. versicolor along the periphery of a woodland pond in the Blue Mountain Reservation in Peekskill, NY on evenings between approximately 10:00 PM and midnight of May, June, and July of 2009 to 2013. Subjects were captured in amplexus to increase the probability that they would exhibit positive phonotaxis in our tests. The females were placed in plastic containers and transported to our laboratory at the Pleasantville, NY campus of Pace University. There they were maintained overnight in an ice-filled cooler or refrigerator to prevent them from releasing their eggs and thus becoming non-responsive to advertisement calls. All individuals were returned to the reservation pond following testing (usually within 24 h; handling and use of frogs was approved by the Pace University IACUC).
We used 200 females in 828 two-stimulus phonotaxis trials (43 different tests). Each subject was used in only one completed trial per choice test although females could be used in one to 10 different tests (mean = 4.14). There is no indication of carryover effects in females employed in multiple tests (Gerhardt et al. 2000). As described below, we used 17 additional females in single speaker tests (one trial per female in each of five tests).
General testing procedures
The morning following capture, females were placed individually in a small plastic container with a small amount of water and allowed to warm to room temperature (19–21 °C) in a darkened stall away from the choice chamber where testing began following approximately 30 min. We conducted the phonotaxis tests within a constant temperature (~20 °C) chamber (inner dimensions 255 cm long × 225 cm wide × 195 cm high, Ultimate Walk-Ins, Inc.) walled with echo-attenuating acoustic foam (Silent Source HFX-4) and floored with waterproof low-pile carpet marked with 10 × 10 cm squares. The testing “arena” within the chamber was surrounded by an acoustically transparent circular barrier (2 m diameter, 25.4 cm high) of thin black cloth draped over wire mesh (1.27 × 1.27 cm).
For each test, we transferred a female treefrog to the interior of an inverted acoustically transparent centrally located screen cup on the floor of the arena. We used an externally controlled pulley to raise the cup and grant the female access to the remainder of the floor of the arena. We subsequently observed her actions under infrared illumination (Marlin P. Jones & Assoc. Inc. part # 11665OP) from outside the chamber on the monitor of a closed circuit video security system. Digitized stimuli were amplified (M-audio model Firewire 410 interface and a Sonance model Sonamp 1230), and unless otherwise indicated (see below), alternative stimulus calls were broadcast in alternating fashion every 2 s (15 calls per min per speaker) from two speakers (RCA model PRO-X33AV) on the floor of the arena separated by 180°. The face of each speaker was flush with the outer surface of the arena barrier and so located ~1 m from the release point. We exposed each female to the broadcast calls for approximately 30 s before raising the cup and freeing the female. We scored a positive response if a female moved to within 10 cm of a speaker within 10 min. If a female failed to respond, she was provided a second opportunity following at least 15 min that day. For each tested female (see Figs. 4, 5, 6, and 7 for numbers used per test), we changed the starting point within our digital playback file using our audio software (Adobe Audition, version 1.3). We also varied the broadcast location (right or left speaker) for alternative stimuli and shuffled the relative positions of the calls (within playback files) among groups of females tested at different times of the day and on different days.
Test stimuli
For each choice stimulus, we created a 24 s digital loop of six calls from our recordings from multiple transects (two calls from each of three of our field recordings for the desired treatment; call period = 4 s). Using CoolEdit Pro, these were filtered (Bessel, Low Pass, cutoff = 4000 Hz, order = 6) and adjusted to the maximum amplitude possible without digital clipping. This ensured that the different calls of a looped file were, to the extent possible, of equivalent peak amplitude.
Because of the potentially huge number of pairwise choice tests (90 field treatment conditions, 4005 possible two-stimulus tests), we used calls obtained from just a subset of the field digital recordings in tests of female discrimination (N = 18 different pairs of call types). Given the aforementioned findings of Kuczynski et al. (2010), we deemed it unlikely that females would exhibit significant discrimination when offered call alternatives that showed either relatively low levels of structural degradation or small differences in degradation between stimuli. In addition, we believed that masking by background chorus noise would often make it unlikely that females could either detect calls or assess differences among them (Gerhardt and Klump 1988; Wollerman 1999; Murphy and Gerhardt 2002; Christie et al. 2010) when females were separated from males by more than about 8 m (call SPL = 84.5–95 dB at 1 m, fast RMS, Gerhardt 1975). Accordingly, all but two of our 43 tests of female discrimination were conducted using recordings made when the speaker and microphone were separated by 8 m. This was also a separation sufficient to result in at least moderate degradation in calls broadcast through some habitat types at some elevations (e.g., speaker and microphone near the ground at a distance of 8 m).
Effect of habitat
We addressed the consequences of transmission across different habitat types by comparing the three possible combinations of habitat type and utilizing calls recorded at 8 m from the speaker (14 different pairs of call types, including those with different numbers of pulses, Table 1). For all three pairings of habitat type, we used call alternatives from our recordings made along (1) the highest elevation transmission path, (2) the lowest elevation transmission path, and (3) a sloped transmission path mimicking a male at the highest (1.5 m) elevation and a female at an intermediate elevation (0.75 m). These choice tests, therefore, approximated a situation in the field in which a female, located near the boundary of two habitat types, was exposed to the calls of two males each calling in a different habitat but at the same height. We ran one test using call alternatives transmitted through forest and open terrain that also had an angled transmission path. However, in this test, the speaker broadcasting the forest calls in the field had been located on the ground and the speaker broadcasting the open terrain calls had been located 1.5 m above the ground. For both stimuli, the microphone had been located 0.75 m above the ground. Therefore, this test mimicked a situation in which a female at intermediate height was exposed to calls from two males located in different habitats and at different elevation extremes. Based on the lack of significant discrimination in this test and others using sloped and partially intermediate transmission paths, we did not conduct additional tests of this type for other pairs of habitat types.
Effect of elevation within habitat
For each habitat type, we assessed the impact of elevation by testing for discrimination among calls both broadcast and recorded at a height of 1.5 m versus those both recorded and broadcast at ground or water level. In some cases, we used signals recorded at distances of 4 or 16 m (forest habitat: two tests) as well as those at other elevations (three tests on effect of elevation, Table 2). We conducted the former tests with the 4 and 16 m signals to assess the impact on female discrimination ability of lesser or greater amounts of call degradation than present in 8 m signals. The ability of females to discriminate relatively subtle differences in degradation might also be revealed by employing, in the latter tests, call alternatives recorded following transmission across paths differing only moderately in elevation. We chose recordings made in forest habitat for such tests and compared female responses to calls from (1) a low elevation and an intermediate level transmission path and (2) a low-elevation sloped path and a high-elevation sloped path. We also ran a choice test using the stimulus alternatives with sloped paths obtained during broadcasts through open terrain. This test situation mimics a natural situation in which a female perched at intermediate elevation compares the calls of a male perched at high elevation and a male near the ground. Because of the absence of female discrimination in this experimental case using open terrain calls and the relatively small differences in degradation among low and intermediate elevation calls through open terrain at 8 m, we did not conduct a choice test offering low and intermediate elevation calls recorded in this habitat. Our data also obviated the need to conduct additional choice tests using pond calls.
When investigating the effects of habitat and elevation, we performed the majority of discrimination tests (N = 26 including tests with different numbers of pulses described below) with the amplitudes of call alternatives equalized at 84 dB (SPL re 20 μPa; C-weighting, fast RMS response; Gen Rad model 1982 Precision Sound Level meter calibrated with a Gen Rad model 1562-A sound level calibrator) at the female release point in our testing chamber. This is a realistic call amplitude for the initial source (i.e., speaker)—female separation of 1 m and is identical or close to that typically used by us and others in tests of phonotaxis of female gray treefrogs (e.g., Schwartz et al. 2004; Gerhardt 2005a, b; Bee and Schwartz 2009). However, signal amplitude is another variable that obviously is influenced by habitat, elevation, and separation of the signal source and receiver. Moreover, in this species, signal amplitude can influence female selectivity for fine temporal call features (Gerhardt and Schul 1999; Beckers and Schul 2004). For this reason, we believed that it was important to conduct some tests with both call alternatives at equivalent lower amplitudes (i.e., four tests at 72 dB SPL) as well as amplitudes similar to those expected at signaler-receiver separations corresponding to the speaker-microphone separations and elevations we employed when making our recordings in the field. For example, in one choice test using forest calls, the amplitude of the call broadcast and recorded at a higher elevation (1.5 at 8 m) was presented at 72 dB and the lower elevation call (ground level at 8 m) was presented at 64 dB (see attenuation data for forest broadcasts in Fig. 3). In another test, we broadcast these forest calls at 84 dB and 76 dB. We expected that such tests with call alternatives of different amplitude would exacerbate any female preferences because the calls more attenuated during transmission were typically those that experienced greater structural degradation (and female frogs usually orient toward louder conspecific calls; Ryan and Keddy-Hector 1992; Gerhardt et al. 2000; Bee et al. 2012). Accordingly, tests at equal SPLs of 84 dB were conservative in that they were less likely to reveal discrimination against more degraded calls.
Following an initial set of tests in which we found significant discrimination among call alternatives with 18 pulses, we estimated the strength of some preferences by lowering the number of pulses in the preferred call (tested at both equal and unequal amplitudes: forest highest elevation versus forest lowest elevation and forest lowest elevation versus pond lowest elevation; tested at equal amplitudes: open lowest elevation versus open highest elevation, N = 292 additional trials). This enabled us to evaluate possible tradeoffs between calling site of a signaler and call duration as well as determine how males might compensate for degradation of their calls by appending pulses to their calls. Longer calls, with more pulses, are not only more expensive to produce (Taigen and Wells 1985) but, at equivalent call rates, are typically more attractive to females than are shorter calls (Klump and Gerhardt 1987; Gerhardt et al. 1996; Schwartz et al. 2001). We also evaluated the strength of preferences by reducing the amplitude of the previously preferred high-elevation calls relative to ground-level calls broadcast through forest (72 vs 84 dB) or open areas (72 vs 84 dB; 78 vs 84 dB).
Single speaker tests and phonotaxis scores
In addition to the tests of discrimination described above, we performed one single speaker experiment. Our main purpose was to assess whether highly degraded calls were inherently attractive to females at a realistic and highly attenuated sound level. The stimulus was created from the calls broadcast and recorded through the forest at ground level with a speaker-microphone separation of 8 m. This “experimental” stimulus was presented to females at 64 dB SPL, and subjects’ approach times to the speaker were compared to those for calls obtained from those broadcast and recorded in the forest at 1.5-m elevation. The latter experimental stimulus was presented at 72 dB SPL. Our metric of attractiveness was the “phonotaxis score” (e.g., Bush et al. 2002; Schwartz and Marshall 2006; Bee and Schwartz 2009), the calculation of which utilized response times to a standard stimulus (18-pulse synthetic call broadcast at 84 dB SPL). We gave each of 17 females two tests with this standard stimulus. This was followed by one test with each of the experimental stimuli before a final (fifth) test with this standard stimulus. Within this sequence, successive trials of the same female were separated by a minimum of 5 min and the order of testing with the experimental stimuli switched among days. The approach time to initial presentation of the standard calls was used as an acclimation trial, and the data were not incorporated in our calculations (see Bush et al. 2002). For each experimental stimulus, we computed a phonotaxis score for each female by forming the ratio of the average approach time to the standard stimulus (obtained from the second and final trial) and the approach time to an experimental stimulus (Tavstand/Texp). Therefore, a phonotaxis score of one indicates an equivalent response to the experimental stimulus and the standard call: A score less than one indicates that the experimental stimulus was less attractive than the standard, and a score of more than one indicates that the experimental stimulus was more attractive than the standard. Subjects that did not reach the broadcast speaker within 10 min received a score of “0.” All but one female made contact with the speaker within the allotted time period.
Trellis experiment
To evaluate calling perch elevation selected by males, at Blue Mountain Reservation, we positioned pairs of custom-made wooden ladder-like trellises (see Fig. 8) at 15 stations around the periphery of the pond at locations where males most frequently call. Perch choice is likely influenced by the characteristics (plant species, form, orientation, availability, density, etc.) of natural perches, and using trellises provided a convenient way to generate data on perch selection while reducing the influence of the natural factors just listed on perch choice. Each trellis had eight rungs and measured 178 × 61 cm (70″×24″; inter-rung separation = 25.4 cm) exclusive of 10 cm (4″) legs below the bottom rung. Trellises were located so that we sampled from areas that provided a good representation of the variation in the forest and pond edge at our field site. On nights (N = 129) when we searched for females (late May–early July, 2009–2012; approximately 10:00–11:30 PM), we recorded the height (i.e., rung number) of males that we found calling on each trellis. Typically, we checked trellises once per night. Because of the presence of an enormous number of natural potential calling perches for males, it was necessary to run this experiment for 4 years to obtain a reasonable sample size. So that we could test for the influence of weather conditions on perch height, temperature and humidity were recorded at the field site using a data logger (HOBO Model H21-002) equipped with a relative humidity and temperature sensor (HOBO Model S-THB-M002).
We intentionally did not toe clip males observed on trellises for later identification if recaptured. Given that the estimated size of our breeding season population is large (150+) and the high number of natural perches, we believed that the chance of observing the same male more than once on our trellises was relatively low. Nevertheless, we repeated our analysis of perch heights in a way that we believe reduces the possibility of non-independence. In this second analysis, only the first male captured per year was counted per trellis station (i.e., trellis pair) because males who return on subsequent nights call in the same general area (Runkle 1992; for data on male movement during breeding season, see Johnson et al. 2007).
Statistical analyses
For our analyses of data on structural degradation and decline in call amplitude during call transmission, we used a mixed linear model with the three habitats, five elevations, six transmission distances as fixed effects, and transect (N = 9) as a random effect nested within habitat (SAS v. 9.2, Proc Mixed; arcsine square root transformed ∆Vs). We present the results of our two-stimulus tests of discrimination as the percent of females choosing each alternative. The null hypothesis of no preference was evaluated with two-tailed binomial tests. For each test result, we also provide the Bayesian central confidence interval (i.e., credible interval; see Gerhardt et al. 2007). For the single speaker tests, we used the two significance criteria of Schul and Bush (2002). First, we judged the responses of females as significant (t test; significance level P < 0.05) if our sample of phonotaxis scores was greater than a sample of zeros (i.e., as would be obtained in a sample of identical size from a hypothetical population in which females fail to approach the speaker in the allotted time). Second, significance required that the mean response to the experimental stimulus was at least 50 % of that to the control stimulus (i.e., mean phonotaxis score ≥0.50). The null hypothesis of no difference in time of approach to the standard and experimental stimulus in single speaker tests of phonotaxis at 64 and 72 dB SPL was tested with a Wilcoxon signed-rank sum test (SAS v. 9.2, Proc Univariate). The null hypothesis of no difference in phonotaxis scores of the two experimental stimuli was also tested with a Wilcoxon signed-rank sum test. In the trellis experiment, the possibility of a preference for perch height was evaluated using two-tailed binomial tests, and we tested for a relationship between weather variables and perch height using multiple linear regression (SAS v. 9.2, Proc Reg).
Results
Call broadcasts in the field
Habitat type and elevation have significant effects on call degradation and attenuation (Figs. 2 and 3; F > 10.95, P < 0.01 for habitat, elevation, distance, and all two and three-way interactions; mixed linear model; degrees of freedom (DF) habitat 2, 6; elevation 4, 2604; distance 5, 2604; habitat × elevation 8, 2604; habitat × distance 10, 2604; elevation × distance 20, 2604; habitat × elevation × distance 40, 2604). Experimental conditions had a statistically significant effect on ∆V even for transmission over pond water at less than 8 m (F > 18.6, P < 0.001 for elevation DF = 4, 433, distance 2, 433, and their interaction 8, 433; for biological significance, see Tests with females). The impact of habitat type was most pronounced when calls were broadcast and recorded near the substrate but usually had a more limited impact at higher elevations. For example, even at the greatest transmission distance (32 m), the drop in ∆V relative to that at a distance of 1 m averaged only 0.34 for the forest and open habitat types at speaker and microphone elevations of 1.5 m. However, at the lowest elevation, broadcast through forest resulted in a dramatic drop in ∆V and increase in excess attenuation at separations greater than 4 m. Call transmission over pond water had the least impact on these signal attributes and broadcast over open terrestrial terrain intermediate effects. At lowest elevation, the decline in ∆V was sharpest for broadcast through forest and open terrain for the first 16 m. Although differences in structural degradation were generally small between pond and open terrain conditions in which the speaker, microphone, or both were elevated, there was a relatively sharp decline in ∆V in the forest with the microphone at 0.75 m (speaker at 0.0 m).
Effect of habitat type on signal degradation (top) and attenuation (bottom) as a function of distance. For clarity, only results for both the microphone and speaker at the highest (left, 1.5 m) and lowest elevations (right, adjacent to ground or pond surface) are shown. Broadcasts across pond (blue), across open terrain (green), and through woodland (orange) (forest). ∆Vs are presented as means ± 1 SE of the values calculated for each call (N = 30 calls per condition; 10 calls per condition × 3 dates). Attenuation levels (dB) are relative to those obtained at 1 m from the broadcast speaker. Red dots show attenuation expected for spherical sound spread only (i.e., 6 dB drop for each doubling of separation)
Effect of elevation on signal degradation (top) and attenuation (bottom) as a function of distance for all habitat types. Elevations of speaker and microphone are color coded as indicated. ∆Vs are presented as means ± 1 SE of the values calculated for each call (N = 30 calls per condition; 10 calls per condition × 3 dates). Attenuation levels (dB) are relative to those obtained at 1 m from the broadcast speaker. Red dots show attenuation expected for spherical sound spread only (i.e., 6 dB drop for each doubling of separation)
Except for the forest habitat, attenuation effects were generally relatively close to those expected due to spherical spread (means within ±6 dB of expectation). In the forest at ground level, we observed a sharp decline in call amplitude within the first 8 m followed by an approximate leveling off between 16 and 32 m. Curiously, at a microphone elevation of 0.75 m and a speaker elevation of 1.5 m, plots of mean attenuation versus distance revealed reduced attenuation as compared to that based on spherical spread in all three habitats. For terrestrial habitats, relative to those with the two lowest transmission paths, attenuation was also usually significantly lower when both the microphone and speaker were at a height of 1.5 m (t 2604 > 4.84, P < 0.001; Tukey-Kramer adjustment for multiple comparisons with all distances pooled; exception: forest highest path versus forest with microphone at 0.75 m and speaker at 0.0 m). In fact, elevation of microphone and speaker from near the substrate most often increased signal strength.
Tests with females
Considering our choice results collectively, we generally observed significant discrimination (two-tailed binomial tests, P < 0.05; see Figs. 4, 5, 6, and 7 for sample sizes other than N = 20) by females when call alternatives exhibited large differences in ∆V. In fact, such differences in degradation among alternative calls were significantly greater when females showed significant discrimination (N = 7) than when females failed to do so (N = 11; two-sided t test, t 16 = −2.88, P = 0.0278, Cochran approximation), and the % difference in number of females choosing call alternatives was a function of the difference in ∆V between alternatives (r 2 = 0.499; F 1,16 = 15.94, P < 0.001, arcsine-transformed percentages; choice tests using calls with identical numbers of pulses and equal amplitudes).
Results of female choice tests with call alternatives obtained from broadcast through different habitats: a forest versus pond, b forest versus open, and c pond versus open. Transmission distance is indicated at the upper right in each section. For each section, an oscillogram of a representative for each of the alternative stimulus types is shown on the left. The percent of tested females choosing each alternative, located at the approximate vertical position of its oscillogram, is indicated by a dot with the horizontal line providing the Bayesian central confidence interval (i.e., credible interval). Asterisks indicate P < 0.05 using a two-tailed binomial test and the number of females tested, if other than 20, is given in parentheses. The average ∆V value for each call stimulus type is indicated above dots. Within each figure section, results for different choice tests are indicated using different kinds of horizontal lines and/or presence or absence of dot fill. Solid lines with filled dots are for 18-pulse alternatives presented at 84 dB SPL. In other cases, stimulus levels are indicated. For example, in the uppermost section of Fig. 4a, the dashed line shows that 0 % of 10 tested females chose the ground-level forest call (F8s0.0m0.0) at 64 dB relative to the 100 % choosing the low-elevation pond call (P8s0.0m0.0) at 72 dB (see dashed line at the bottom of this section of the figure). In this figure section, the dotted lines show the result when the forest and pond calls were offered to females at 72 dB. The solid lines show the result at 84 dB. See text (Results: Tests with females) for explanation of the descriptive names of the stimulus calls shown with each oscillogram
Results of female choice tests with call alternatives obtained from broadcasts through forest or across pond at an elevation of ~0 m. Within each figure section, the number of pulses in pond calls is indicated to the right of each horizontal line. Calls obtained from broadcasts through forest were offered with 18 pulses. An oscillogram of an 18-pulse representative for each of the alternative stimulus types is shown on the left. The percent of tested females choosing the pond call alternative is indicated by a dot with the horizontal line providing the Bayesian central confidence interval (i.e., credible interval). Dots to the right of the vertical dashed line indicate that more than 50 % of females chose the pond call. Asterisks indicate P < 0.05 using a two-tailed binomial test and the number of females tested, if other than 20, is given in parentheses. Call intensities of alternatives are given at the top of each figure section
Results of female choice tests with 18-pulse call alternatives obtained from broadcasts at different elevations: a forest, b open, and c pond. For additional details, see legend of Fig. 4.
Results of female choice tests with call alternatives obtained from broadcasts at different elevations in particular habitats (upper three sections: forest, lower section: open). For each habitat type, the number of pulses in the higher elevation calls (1.5 m) is indicated to the right of each horizontal line. Calls obtained from broadcasts at ground level (0.0 m) were offered with 18 pulses. For each section, an oscillogram of an 18-pulse representative for each of the alternative stimulus types is shown on the left. The percent of tested females choosing the elevated call alternative is indicated by a dot with the horizontal line providing the Bayesian central confidence interval (i.e., credible interval). Dots to the right of the vertical dashed line indicate that more than 50 % of females chose the elevated call. Asterisks indicate P < 0.05 using a two-tailed binomial test and the number of females tested, if other than 20, is given in parentheses. Call intensities of alternatives are given at the top of each section unless these were both 84 dB SPL
We first consider the responses of females to call alternatives broadcast through different habitats and then responses to call alternatives broadcast through the same habitat at different elevations. In the text below, the names of the stimulus calls indicate the habitat type (F, P, O for forest, pond, or open, respectively), followed by the separation in meters between speaker and microphone, followed by the speaker (s) height in meters, followed by the microphone (m) height in meters.
Effect of habitat
Habitat and elevation-related effects on call degradation could influence female discrimination, although females were tolerant of small to moderate differences in levels of degradation. For example, first considering the effect of habitat at a distance of 8 m, females failed to discriminate between calls broadcast at the lowest elevation across pond (P8s0.0m0.0, ∆V = 0.88514) and across open terrain (O8s0.0m0.00, ∆V = 0.78620) (Fig. 4c, bottom panel; calls at 84 dB SPL). This was also the case at the highest and intermediate elevations (Fig. 4c, top and middle panels). However, when the alternative to the pond calls were those broadcast and recorded through the forest at ground level (F8s0.0m0.0, ∆V = 0.41162), females showed significant discrimination in favor of the pond calls (Fig. 4a, top panel; 84 dB). At higher broadcast and recording elevations, degradation of calls through forest was reduced (also see below) and females failed to discriminate between calls transmitted through the forest and over a pond (Fig. 4a, bottom 2 panels; 84 dB). Females also discriminated against forest calls in favor of those transmitted across open terrain at ground level (Fig. 4b, bottom panel; 84 dB). At higher elevations, results were mixed (Fig. 4b, top and middle panels). In fact, at a broadcast and recording height of 1.5 m, females preferred the calls transmitted across open terrain in spite of their nearly equivalent level of degradation with the elevated forest calls as reflected by the values of ∆V (Fig. 4b, top panel; 84 dB).
Reduction in the amplitude of alternative stimuli to 72 dB SPL eliminated discrimination in favor of lowest elevation calls from the pond habitat relative to the forest habitat (Fig. 4a, top panel). In contrast, presenting the more degraded forest calls at 64 dB SPL and the pond calls alternative stimulus at 72 dB SPL yielded an even more pronounced preference of females for the latter (Fig. 4a, top panel). At a distance of 8 m near the substrate, a male calling in the forest must give 18-pulse calls to match the attractiveness of 12 pulse calls broadcast over pond water when calls are received at an equivalent amplitude of 84 dB SPL (Fig. 5, top). This pulse number differential was doubled when the effect of excess attenuation through forest was incorporated into the choice tests (Fig. 5, bottom).
Effect of elevation within habitat
Within habitats, differences in elevation of the signal transmission path resulted in female discrimination of recorded calls—again generally provided that there were more than minor to moderate differences in levels of degradation among choice alternatives. For example, transmission across pond water at different elevations typically yielded high values of ∆V that in most cases differed only slightly (Fig. 3), and females failed to discriminate between the calls transmitted across the lowest (0.0 m) and the highest elevation (1.5 m) paths when offered stimuli recorded at a distance of 8 m (Fig. 6c; difference in mean ∆V = 0.0346). Therefore, we did not test further for elevation-based discrimination preferences among our pond recordings. In spite of similar ∆V values, over open terrain, females preferred calls transmitted across the more elevated 8 m path (1.5 m) as compared to those transmitted across a ground-level path (0.0 m; Fig. 6b, top panel, 84 dB calls) that could exhibit greater corruption of pulse shape. When using call alternatives of 18 pulses, the only other test using open terrain calls in which females discriminated significantly occurred when we reduced the relative amplitude of the formerly preferred 1.5 m elevation stimulus by 12 dB (Fig. 6b, top, 84 vs 72 dB). The full duration open terrain ground-level call stimulus was also preferred when we removed six pulses from the 1.5 m elevation stimulus (Fig. 7, bottom). Females did not discriminate between call alternatives when open terrain calls had been transmitted at different but intermediate elevation paths (Fig. 6b, bottom panel).
For broadcasts through forest habitat at a transmission distance not at (Fig. 6a, top panel) but greater than 4 m, an elevation differential of 1.5 m yielded signals that elicited statistically significant discrimination when 18-pulse call alternatives were presented to females at 84 dB (Fig. 6a, second and third panels down). At a transmission distance of 8 m, mean ∆V was nearly halved in our 0.0 m stimulus relative to our 1.5 m stimulus and a preference for the latter was maintained for all stimulus amplitudes we employed (Fig. 6a, second panel down). This includes the result showing a preference for the high-elevation stimulus in the test providing a 12 dB relative amplitude advantage for the more degraded ground-level stimulus calls transmitted across 8 m (84 dB ground vs 72 dB elevated). At equal stimulus amplitudes of 84 dB SPL, we found that a twofold pulse number advantage was sufficient to reverse the aforementioned preference for the elevated call (Fig. 7, top). Thus, all else being equal, a male calling through the forest at ground level (to a ground-level female) needs to give 18-pulse calls to exceed the attractiveness of a male with 9-pulse calls transmitted well above the ground. When the effect of exacerbated excess attenuation of a ground-level transmission path was incorporated into a test, even a threefold pulse difference (18 pulses at 76 dB vs 6 pulses at 84 dB) could be insufficient to reverse the female preference for calls transmitted across the most elevated relative to the ground-level path (Fig. 7, second panel down; also see results for 18 pulses at 64 dB vs 9 pulses at 72 dB; 9-pulse elevated call preferred). Not surprisingly considering the difference in average ∆V, females also showed significant discrimination for calls transmitted across a forest path 0.75 m high as compared to at substrate level (Fig. 6a, fourth panel down). However, when the stimulus alternatives came from recordings of calls with angled sound transmission paths and less extreme elevation differences (Fig. 6a, bottom), females failed to discriminate at SPLs of either 84 or 72 dB.
Single speaker tests and phonotaxis scores
Discrimination appeared strong, and thus, positive phonotaxis in two-choice tests was low to the highly degraded calls obtained from broadcasts and recordings at ground level through the forest (8 m transmission path). Nevertheless, based on our two aforementioned criteria (P < 0.001, 64 dB t = 8.45, N = 17, 72 dB t = 8.38, N = 17, t test; mean phonotaxis scores ≥ 0.5), responses were significant, and so this stimulus at 64 dB as well as the high-elevation forest call at 72 dB were still inherently attractive to females. When the calls were presented without an alternative, at both 72 and 64 dB SPL, females displayed robust phonotaxis although their approach to the speaker was, on average, only significantly slower to the ground-level calls presented at 64 dB SPL than it was to the standard call at 84 dB SPL (S = 73.5, N = 17, P < 0.0001, Wilcoxon signed-rank sum test, phonotaxis scores (mean ± std) 0.576 ± 0.281 for ground-level call at 64 dB; S = 30.5, N = 17, P = 0.1558, 0.906 ± 0.446 for elevated call at 72 dB). Phonotaxis scores were also significantly lower for the ground-level call than for the high-elevation call (S = 57, N = 17, P = 0.0017, Wilcoxon signed-rank sum test).
Trellis experiment
We recorded 75 instances of males perched and vocalizing on trellises over four breeding seasons. These observations revealed a non-random distribution with respect to elevation. Individuals seldom called at the lowest two levels (Fig. 8, two-tailed binomial test, P = 0.00031, N = 75). We obtained a qualitatively similar result when we confined our data set to the subset of males less likely to include repeated observations of the same individuals (two-tailed binomial test, P = 0.0039, N = 30). Multiple linear regression indicated that there was no relationship between the weather variables of temperature and humidity and perch height (r 2 = 0.0015, F 2,72 = 0.06, P = 0.9462).
Distribution of the males (N = 75) found calling on the eight rungs of 30 trellises at the edge of our study pond. Frogs are not drawn to scale, and the relative width of the trellis has been adjusted to accommodate the number of frogs illustrated (http://www.free-clipart-pictures.net/frog_clipart.html)
Discussion
We emphasize that our results and interpretation of our findings apply to calls we recorded at the nine transects used in our study. Although we believe that our field sites provided good representations of the pond, forest, and open habitat types at areas where choruses of gray treefrogs form, there obviously exist other venues at which the environmental impacts during call transmission would deviate quantitatively and perhaps qualitatively from those described here. Thus, readers should not overgeneralize from our data.
With that caveat, our results indicate that, with respect to communication, the location at which a male gray treefrog advertises and receivers listen is biologically significant. Impacts are most likely when signals are transmitted close to the ground through thickly vegetated habitat, such as forest. At sufficient distances between source and receiver, the synthetic advertisement calls we broadcast exhibited significant declines in the salience of their pulse structure. Under circumstances where females can compare such highly degraded signals with those broadcast at higher elevation or through more acoustically benign habitat, all else being equal, our data indicate that calling venue may have a significant influence on a male’s probability of mating. Our data further indicate that male preference for elevated calling perches can reduce levels of call degradation and, by appending pulses to calls, males could sometimes compensate for transmission-related reductions in the relative attractiveness of their signals.
Call broadcasts in the field
Over many years of work with H. versicolor, we have observed unpaired females in locations at which received calls of likely potential mates have been transmitted through forest, across water, or over open terrain. However, given that a male cannot control the position of distant females, he is better off calling from an elevated perch than one near the substrate. If the sound transmission path to a female is through forest, the impact of elevation can be significant at sufficient source-receiver separations. For example, although the magnitude of pulse energy spread was relatively small for a high-elevation forest path, at ground level for separations of greater than 4 m, we observed a rapid decline in ∆V up to separations of 16 m—at which point the contrast in sound energy within pulses and interpulse intervals was essentially nil. However, based on our data, if the sound transmission path to the female is over water, the effect on the energy spread as quantified in ∆V is expected to be comparatively minor. Accordingly, elevation of the calling male and listening female is largely irrelevant, even over relatively long distances. The density of emergent vegetation should exacerbate degradation, and this may be one explanation for the more rapid decline of ∆V with distance for signals broadcast and recorded near the surface of some ponds in the study of Kuczynski et al. (2010) using synthetic calls of Cope’s gray treefrog H. chrysoscelis. At low elevation, transmission across open terrain had intermediate effects on the structure of our broadcast calls.
Transmission close to the ground and through vegetation has long been known to significantly impact sound signals transmitted over long distances (Wiener and Keast 1959; Aylor 1972; Marten and Marler 1977; Attenborough 2002). Accordingly, sound absorption, reflection, diffraction, and refraction, whose effects typically increase with separation of source and receiver, density of objects in direct and indirect sound paths and proximity to the ground are likely important factors contributing to the declines of ∆V that we observed (Wiley and Richards 1982; Dabelsteen et al. 1993; Forrest 1994; Wiley 2009). Pulses within calls will also become more obscured by background noise as signal amplitude declines with distance (Christie et al. 2010). However, while a factor in natural choruses, the role of noise in reducing ∆V was minimal in our recordings because of the conditions at the times and dates we intentionally chose to perform our broadcasts.
Unamplexed females are difficult to locate although most females likely approach the chorus from the surrounding forest (Johnson et al. 2007). Based on decades of work with gray treefrogs at Blue Mountain Reservation and other field sites (Schwartz 1987; Schwartz et al. 2004), when discovered, the first author has usually found such attracted females within 2–3 m of calling males and the ground. Males, as mentioned earlier, call from different heights, and thus, there will frequently be differences in elevation between calling males and approaching females. Results of our transmission tests indicate that when receiver and source heights differ, the impact on call attributes reflected in ∆V is often, but not always, intermediate to that observed when these entities are both at the two elevation extremes. For example, in the case of transmission over a distance of 16 or 32 m through forest, elevating our microphone by 0.75 m but keeping the speaker at ground level, moderately reduced pulse energy spread. A similar effect was observed at 16 m during broadcasts across open terrain. However, for all three habitat types, for transmission distances of less than about 8 m, as long as at least the sound source or sound receiver is elevated, differences in energy spread based on caller location are likely to be small. Given the results of our choice tests, such differences, per se, would rarely if ever influence female decisions.
As with pulse energy spread, sound transmission through forest at ground level produced the greatest excess attenuation (over 10 dB at a distance of 8 m). Our data are therefore qualitatively consistent with expectations based on the physics of sound transmission near porous surfaces (Forrest 1994; Embleton 1996). However, for the forest at smaller microphone-speaker separations and the other habitats at all separations, reductions in signal amplitude beyond those predicted based on spherical spread were at most trivial. Numerous studies on insects and a variety of vertebrates have demonstrated that elevation can reduce excess attenuation of signals (e.g., Waser and Waser 1977; Brenowitz et al. 1984; Wilczynski et al. 1989; Römer and Lewald 1992; Lang 2000; Parris 2002) in part because of sound scattering by low level vegetation (and non-living material) and the positive impact of soft ground on excess attenuation (Forrest 1994; Embleton 1996). The lower attenuation often observed when the speaker was elevated 1.50 m in forest or open terrain was therefore not surprising. In some situations (e.g., forest and open terrain with the microphone at 0.75 m and the speaker at 1.5 m), we consistently observed an increase of signal level relative to that predicted. Transmission over water, because of water’s large impedance difference with air and accompanying reflection of sound, can more than counteract signal attenuation due to spherical spread (Forrest 1994). This or a temperature gradient (Embleton 1996) may, in part, account for the reduced attenuation often observed when we broadcast calls across the pond. However, we have no definitive explanation(s) for the higher than expected amplitudes observed in different habitats.
Tests with females
Our female choice tests demonstrate an ability to discriminate among calls that have arrived via different transmission paths. The structural degradation experienced by calls is present in many of our choice stimuli and is not only evident in pulse energy spread but also distortion of pulse shape. We know from previous studies that females of the gray treefrog are sensitive to both kinds of changes. For example, Schul and Bush (2002) have documented how call recognition (and species discrimination) by females of H. versicolor is primarily mediated by the static temporal features of pulse and interpulse interval duration and pulse shape (also see Gerhardt 2005b). In fact, alteration of just a single interpulse interval can negatively impact the relative attractiveness of an advertisement call (Schwartz et al. 2010). Kuczynski et al. (2010), working with H. chrysoscelis, found that response rates of subjects during single speaker tests of phonotaxis declined with large reductions of modulation depth of synthetic stimuli. Such reductions in modulation depth were similar to structural changes that would yield declines in ∆V. These alterations could potentially attenuate the responses of interval-counting neurons in the auditory mid-brain of gray treefrogs (Rose et al. 2015). Nevertheless, Kuczynski et al. (2010) judged females fairly tolerant of drops in modulation depth, especially at lower signal amplitudes. Results from our single speaker tests using calls transmitted at ground level through forest, in which all but one female approached the speaker, are consistent with this assessment as are the bulk of our results from females that involved tests of discrimination. The finding that such degraded calls elicit phonotaxis is also consistent with findings that female treefrogs can exhibit phonotaxis toward a source of natural or synthetic chorus sounds (Swanson et al. 2007; JJS unpublished data; but see Christie et al. 2010 and Murphy 2003).
Based on our data, significant discrimination is only likely when the difference in ∆V of equal and sufficiently high-amplitude stimulus alternatives is greater than about 0.25. Differences in the corruption of pulse shape, perhaps due to reflection of sound from one or more trees, may, however, contribute to discrimination between calls with smaller differences in the energy spread of pulses. For example, we suspect that the greater and more consistent corruption of pulse shape in the forest calls—relative to open calls at the highest transmission elevation (see Fig. 4b, top panel)—accounts for the observed female preference for the latter (also see low- and high-elevation open calls in Fig. 6b, top panel). This means that unless there is significant alteration of pulse shape during signal transmission, calling site may not exert a meaningful effect on female choice unless females are comparing at sufficient distance, calls transmitted through (1) forest at low versus high or perhaps intermediate elevation, (2) forest (at or partially at low elevation) versus over pond water, or (3) forest at low elevation versus across open terrain. However, even for calls transmitted at low elevation through forest, at male-female separations of less than ~6–8 m, structural degradation may be insufficient, unless augmented by amplitude differences, to impact female discrimination against such calls versus those transmitted through other habitats or at other elevations. Discrimination of alternating calls based on sound level has been estimated in the related gray treefrog, H. chrysoscelis, to occur at a difference of 2–4 dB, with or without a simulated background chorus at a realistic level (Bee et al. 2012). Moreover, Fellers (1979b) observed discrimination for overlapped calls of 92 dB over those of 90 dB for females of H. versicolor. Therefore, the influence of elevation-related impacts on amplitude, such as we observed, should not be dismissed.
Although not as problematic for some calls that occur during dips in the chorus din (see Vélez and Bee 2011), at greater separations than 6–8 m, during evenings of very active chorusing, masking by background noise may render habitat-induced structural degradation largely irrelevant (Kuczynski et al. 2010). It should be remembered, however, that as a female moves through the habitat, acoustic attributes of the calls, as she hears them, will change. Thus, at times when or locations where calls are not masked, perceived differences among males in the amplitude and fine pulse structure of their calls will shift in a way that could alter a female’s travel route and perhaps strengthen her decision to approach a particular male. Additionally, under circumstances when only a lone male is audible, even highly degraded calls are likely to elicit phonotaxis (also see Kuczynski et al. 2010).
Call duration and perch height
Our tests using calls with different numbers of pulses demonstrate that males whose calls suffer influential levels of degradation can, to some extent, counteract potential negative impacts on female attraction by giving longer calls than males whose calls are less degraded. The necessary differential in number of call pulses will increase with the difference in degradation level and, when the effects of attenuation are included, can be considerable. Of course, as mentioned above, a male is ignorant of the location of distant females and thus could not actively compensate in this knowledge-based way for the level of degradation of his calls at females’ locations. However, a male calling at low elevation in forest as opposed to one calling where, on average, his calls would suffer less degradation and excess attenuation, such as near the surface of a pond or at a higher elevation in forest, could possibly benefit from appending more pulses to his calls. All else being equal, this would necessitate additional energy expenditure (Taigen and Wells 1985; Wells and Taigen 1986; Reichert and Gerhardt 2012) and a similar benefit might be more easily achieved through prudent choice of calling venue.
At our main study pond (Blue Mountain Reservation), as expected, only a relatively small percentage of males were found calling on our trellises (just 75 captures over four field seasons). Nevertheless, because their simple and identical structure helped control for potentially influential natural perch attributes, we believe that our observations provide valuable information on males’ preferred elevation of calling perches. A range of factors potentially contribute to calling site choice in anurans (e.g., availability of perch types, premating isolation, competitors for mates, noise, predation risk, UV exposure, microclimate: Etges 1987; Arak and Eiriksson 1992; Ptacek 1992; Amézquita and Hödl 2004; Kats et al. 2012; Höbel and Barta 2014). For example, early in the breeding season when air temperatures are relatively cool, Höbel and Barta (2014) found that male gray treefrogs are more likely to call on the surface of the water than later in the season. Our trellises were located along the shore throughout the breeding season and provided a range of options for perch elevation to males. It seems reasonable that, as has been posited for male songbirds (e.g., Barker and Mennill 2009), the potential for degradation and excess attenuation explains in part why the male treefrogs we observed on trellises avoided calling close to the ground. Although Ptacek (1992) found that males of H. versicolor in Missouri often vocalize on or close to the substrate (albeit near the shoreline where elevated perch sites were scarce), Fellers (1979a) data from Maryland suggest that males of H. versicolor may be selective with respect to perch features likely to influence transmission of their calls. In fact, in locations bordering our main study pond where a large range of perch site elevations are naturally available to males, in agreement with our data from trellises, we only rarely find treefrogs calling within 30 cm of the ground. Because males were often found on trellis rungs 2–4, our perch selection data also are consistent with our general result that, even through forest, calls transmitted at intermediate elevations do not suffer levels of degradation likely to seriously compromise a male’s relative attractiveness.
The flexibility of male anuran vocal behavior in response to acoustic conditions and cues is well established (Gerhardt and Huber 2002; Wells and Schwartz 2006; Schwartz and Bee 2013). However, there is only a very small body of information (e.g., Lardner and Bin Lakim 2002; Ziegler et al. 2011) indicating that male anurans can dynamically adjust their call structure/features to physical aspects of their environment in ways that enhance effective transmission and thus communication. Accordingly, it could be especially interesting to test whether males of the gray treefrog use longer calls in certain locations by performing an experiment in which males are moved between perches. The height of females will also influence the characteristics of the calls that females hear and there is an indication from some studies (e.g., Padgham 2004; Mathevon et al. 2008) that elevation of receivers and signalers can have different effects. An examination of female approach paths might therefore be valuable in the context of gaining a further understanding of the behavioral consequences of (and adaptations to) signal degradation.
References
Amézquita A, Hödl W (2004) How, when, and where to perform visual displays? The case of the Amazonian frog Hyla parviceps. Herpetologica 60:20–29
Andersson M (1994) Sexual selection. Princeton University Press, Princeton
Arak A, Eiriksson T (1992) Choice of singing sites by male bushcrickets (Tettigonia viridissima) in relation to signal propagation. Behav Ecol Sociobiol 30:365–372
Attenborough K (2002) Sound propagation close to the ground. Annu Rev Fluid Mech 34:51–82
Aylor D (1972) Noise reduction by vegetation and ground. J Acoust Soc Am 51:197–205
Barker NKS, Mennill DJ (2009) Song perch height in rufous-and-white wrens: does behaviour enhance effective communication in a tropical forest? Ethology 115:897–904
Barker NKS, Dabelsteen T, Mennill DJ (2009) Degradation of male and female rufous-and-white wren songs in a tropical forest: effects of sex, perch height, and habitat. Behaviour 146:1093–1022
Beckers OM, Schul J (2004) Phonotaxis in Hyla versicolor (Anura: Hylidae): the effect of absolute call amplitude. J Comp Physiol A 190:869–876
Bee MA, Micheyl C (2008) The cocktail party problem: What is it? How can it be solved? And why should animal behaviorists study it? J Comp Psychol 122:235–251
Bee MA, Schwartz JJ (2009) Perception by frogs in the presence of chorus-shaped noise: I. Behavioral measures of signal recognition thresholds. J Acoust Soc Am 126:2788–2801
Bee MA, Vélez A, Forester JD (2012) Sound level discrimination by gray treefrogs in the presence and absence of chorus-shaped noise. J Acoust Soc Am 131:4188–4195
Bosch J, De la Riva I (2004) Are frog calls modulated by the environment? An analysis with anuran species from Bolivia. Can J Zool 82:880–888
Brenowitz EA, Wilczynski W, Zakon HH (1984) Acoustic communication in spring peepers: environmental and behavioral aspects. J Comp Physiol A 155:585–592
Brumm H, Naguib M (2009) Environmental acoustics and the evolution of bird song. Adv Study Behav 40:1–33
Bush SL, Gerhardt HC, Schul J (2002) Pattern recognition and call preferences in treefrogs (Anura: Hylidae): a quantitative analysis using a no-choice paradigm. Anim Behav 63:7–14
Castellano S, Giacoma C, Ryan MJ (2003) Call degradation in diploid and tetraploid green toads. Biol J Linn Soc 78:11–26
Christie KJ, Schul J, Feng AS (2010) Phonotaxis to male’s calls embedded within a chorus by female gray treefrogs, Hyla versicolor. J Comp Physiol A 196:569–579
Dabelsteen T, Pedersen SB, Larsen ON (1993) Habitat-induced degradation of sound signals: quantifying the effects of communication sounds and bird location on blur ratio, excess attenuation and signal-to-noise ratio. J Acoust Soc Am 93:2206–2220
Embleton TFW (1996) Tutorial on sound propagation outdoors. J Acoust Soc Am 100:31–48
Erdtmann LK, Lima AP (2013) Environmental effects on anuran call design: what we know and what we need to know. Ethol Ecol Evol 25:1–11
Etges WJ (1987) Call site choice in male anurans. Copeia 1987:910–923
Ey E, Fischer J (2009) The “acoustic adaptation hypothesis”—a review of the evidence from birds, anurans and mammals. Bioacoustics 19:21–48
Fellers GM (1979a) Mate selection in the gray treefrog, Hyla versicolor. Copeia 1979:286–290
Fellers GM (1979b) Aggression, territoriality, and mating behaviour in North American treefrogs. Anim Behav 27:107–119
Feng AS, Ratnam R (2000) Neural basis of hearing in real-world situations. Annu Rev Psychol 51:699–725
Feng SS, Schul J (2006) Sound processing in real-world environments. In: Narins PM, Feng AS, Fay RR, Popper AN (eds) Springer handbook of auditory research: hearing and sound communication in amphibians. Springer-Verlag, New York, pp 323–350
Forrest TG (1994) From sender to receiver: propagation and environmental effects on acoustic signals. Am Zool 34:644–654
Gerhardt HC (1975) Sound pressure levels and radiation patterns of vocalizations of some North American frogs and toads. J Comp Physiol 102:1–12
Gerhardt HC (1976) Significance of two frequency bands in long distance vocal communication in the green treefrog. Nature 261:692–694
Gerhardt HC (1991) Female mate choice in treefrogs. Static and dynamic acoustic criteria. Anim Behav 42:615–635
Gerhardt HC (2005a) Acoustic spectral preferences in two cryptic species of grey treefrogs: implications for mate choice and sensory mechanisms. Anim Behav 70:39–49
Gerhardt HC (2005b) Advertisement-call preferences in diploid-tetraploid treefrogs (Hyla chrysoscelis and Hyla versicolor): implications for mate choice and the evolution of communication systems. Evolution 59:395–408
Gerhardt HC, Huber F (2002) Acoustic communication in insects and frogs: common problems and diverse solutions. University of Chicago Press, Chicago
Gerhardt HC, Klump GM (1988) Masking of acoustic signals by the chorus background noise in the green tree frog: a limitation on mate choice. Anim Behav 36:1247–1249
Gerhardt HC, Schul J (1999) A quantitative analysis of behavioral selectivity for pulse-rise time in the gray treefrog, Hyla versicolor. J Comp Physiol A 185:33–40
Gerhardt HC, Dyson ML, Tanner SD (1996) Dynamic acoustic properties of the advertisement calls of gray tree frogs: patterns of variability and female choice. Behav Ecol 7:7–18
Gerhardt HC, Tanner SD, Corrigan CM, Walton HC (2000) Female preference functions based on call duration in the gray tree frog (Hyla versicolor). Behav Ecol 11:663–669
Gerhardt HC, Martínez CC, Schwartz JJ, Marshall VT, Murphy CG (2007) Preferences based on spectral differences in acoustic signals in four species of treefrog (Anura: Hylidae). J Exp Biol 210:2990–2998
Guilford T, Dawkins MS (1991) Receiver psychology and the evolution of animal signals. Anim Behav 42:1–14
Hebets EA, Papaj DR (2005) Complex signal function: developing a framework of testable hypotheses. Behav Ecol Sociobiol 57:197–214
Höbel G, Barta T (2014) Adaptive plasticity in calling site selection in grey treefrogs (Hyla versicolor). Behaviour 151:741–754
Johnson C (1966) Species recognition in the H. versicolor complex. Tex J Sci 18:361–364
Johnson JR, Knouft JH, Semlitsch RD (2007) Sex and seasonal differences in the spatial terrestrial distribution of gray treefrog (Hyla versicolor) populations. Biol Conserv 140:250–258
Kats LB, Bucciarelli GM, Schlais DE, Blaustein AR, Han BA (2012) Ultraviolet radiation influences perch selection by a neotropical poison-dart frog. PLoS ONE 7:e51364
Kime NM, Turner WR, Ryan MJ (2000) The transmission of advertisement calls in Central American frogs. Behav Ecol 11:71–83
Klump GM, Gerhardt HC (1987) Use of non-arbitray acoustic criteria in mate choice by female gray treefrogs. Nature 326:286–288
Kuczynski MV, Vélez A, Schwartz JJ, Bee MA (2010) Sound transmission and the recognition of temporally degraded sexual advertisement signals in Cope's gray treefrog (Hyla chrysoscelis). J Exp Biol 213:2840–2850
Lang F (2000) Acoustic communication distances of a gomphocerine grasshopper. Bioacoustics 10:233–258
Lardner B, Bin Lakim M (2002) Animal communication: tree-hole frogs exploit resonance effects. Nature 420:475
Marten K, Marler P (1977) Sound transmission and its significance for animal vocalization. I. Temperate habitats. Behav Ecol Sociobiol 2:271–290
Mathevon N, Dabelsteen T, Blumenrath SH (2008) Are high perches in the blackcap Sylvia atricapilla song or listening posts? A sound transmission study. J Acoust Soc Am 114:442–449
Morton E (1975) Ecological sources of selection on avian sounds. Am Nat 109:17–34
Murphy CG (2003) The cause of correlations between nightly numbers of male and female barking treefrogs (Hyla gratiosa) attending choruses. Behav Ecol 14:274–281
Murphy CG (2008) Assessment of distance to potential mates by female barking treefrogs (Hyla gratiosa). J Comp Psychol 122:264–273
Murphy CG, Gerhardt HC (2002) Mate sampling by female barking treefrogs (Hyla gratiosa). Behav Ecol 13:472–480
Narins PM, Zelick R (1988) The effects of noise on auditory processing and behavior in amphibians. In: Fritszch B, Wilczynski W, Ryan MJ, Hetherington T, Walkowiak W (eds) The evolution of the amphibian auditory system. Wiley, USA, pp 511–536
Padgham M (2004) Reverberation and frequency attenuation in forests—implications for acoustic communication in animals. J Acoust Soc Am 115:402–410
Parris KM (2002) More bang for your buck: the effect of caller position, habitat and chorus noise on the efficiency of calling in the spring peeper. Ecol Model 156:213–224
Penna M, Marquez R, Bosch J, Crespo EG (2006) Nonoptimal propagation of advertisement calls of midwife toads in Iberian habitats. J Acoust Soc Am 119:1227–1237
Penna M, Plaza A, Moreno-Gómez FN (2013) Severe constraints for sound communication in a frog from the South American temperate forest. J Comp Physiol A 199:723–733
Pröhl H, Hödl W (1999) Parental investment, potential reproductive rates, and mating system in the strawberry dart-poison frog, Dendrobates pumilio. Behav Ecol Sociobiol 46:215–220
Ptacek MB (1992) Calling sites used by male gray treefrogs, Hyla versicolor and Hyla chrysoscelis, in sympatry and allopatry in Missouri. Herpetologica 48:373–382
Ralin DB (1968) Ecological and reproductive differentiation in the cryptic species of the H. versicolor complex (Hylidae). Southwest Nat 13:283–300
Ratnam R, Iyer N, Goense J, Feng FS (2004) Effect of reverberation on neural response to amplitude modulated signals. ARO Abstr 27:113 (#336)
Reichert MS, Gerhardt HC (2012) Trade-offs and upper limits to signal performance during close-range vocal competition in gray tree frogs Hyla versicolor. Am Nat 180:425–437
Röhr DL, Junca FA (2013) Micro-habitat influence on the advertisement call structure and sound propagation efficiency of Hypsiboas crepitans (Anura: Hylidae). J Herpetol 47:549–554
Römer H (2012) The sensory ecology of acoustic communication in insects. In: Hoy RR, Fay RR (eds) Comparative hearing: insects. Springer, New York, pp 63–96
Römer H, Lewald J (1992) High-frequency sound transmission in natural habitats: implications for the evolution of insect acoustic communication. Behav Ecol Sociobiol 29:437–444
Rose GJ, Capranica RR (1984) Processing amplitude-modulated sounds by the auditory midbrain of two species of toads: Matched temporal filters. J Comp Physiol A 154:211–219
Rose GJ, Hanson JL, Leary CJ, Graham JA, Alluri RK, Vasquez-Opazo GA (2015) Species-specificity of temporal processing in the auditory midbrain of gray treefrogs: interval-counting neurons. J Comp Physiol A 201:485–503
Runkle L (1992) Seasonal and individual variation in the vocal behavior and energetics of the gray treefrog, Hyla versicolor. Unpublished MS Thesis, University of Connecticut, Storrs CT
Ryan MJ, Cummings ME (2005) Animal signals and the overlooked costs of efficacy. Evolution 59:1160–1161
Ryan MJ, Keddy-Hector A (1992) Directional patterns of female mate choice and the role of sensory biases. Am Nat 139:S4–S35
Ryan MJ, Kime NM (2003) Selection on long distance acoustic signals. In: Simmons A, Fay R, Popper A (eds) Springer handbook of auditory research: acoustic communication. Springer Verlag, New York, pp 225–274
Ryan MJ, Sullivan BK (1989) Transmission effects on temporal structure in the advertisement calls of two toads, Bufo woodhousii and Bufo valliceps. Ethology 80:182–189
Ryan MJ, Cocroft RB, Wilczynski W (1990) The role of environmental selection in intraspecific divergence of mate recognition signals in the cricket frog, Acris crepitans. Evolution 44:1869–1872
Sabatini V, Ruiz-Miranda CR (2008) Acoustical aspects of the propagation of long calls of wild Leontopithecus rosalia. Int J Primatol 29:207–223
Schul J, Bush SL (2002) Non-parallel coevolution of sender and receiver in the acoustic communication system of treefrogs. Proc R Soc Lond B 269:1847–1852
Schwartz JJ (1987) The function of call alternation in anuran amphibians: a test of three hypotheses. Evolution 41:461–471
Schwartz JJ, Bee MA (2013) Anuran acoustic signal production in noisy environments. In: Brumm H (ed) Animal communication and noise. Springer, New York, pp 91–132
Schwartz JJ, Gerhardt HC (1995) Directionality of the auditory system and call pattern recognition during acoustic interference in the gray treefrog, Hyla versicolor. Audit Neurosci 1:195–206
Schwartz JJ, Marshall VT (2006) Forms of call overlap and their impact on advertisement call attractiveness to females of the gray treefrog, Hyla versicolor. Bioacoustics 16:39–56
Schwartz JJ, Simmons AM (1990) Encoding of a spectrally complex natural call in the bullfrog's auditory nerve. J Comp Physiol A 166:489–499
Schwartz JJ, Buchanan BW, Gerhardt HC (2001) Female mate choice in the gray treefrog (Hyla versicolor) in three experimental environments. Behav Ecol Sociobiol 49:443–455
Schwartz J, Buchanan BW, Gerhardt HC (2002) Acoustic interactions among male gray treefrogs, Hyla versicolor, in a chorus setting. Behav Ecol Sociobiol 53:9–19
Schwartz JJ, Huth K, Hutchin T (2004) How long do females really listen? Assessment time for female mate choice in the gray treefrog, Hyla versicolor. Anim Behav 68:533–540
Schwartz JJ, Brown R, Turner S, Dushaj K, Castano M (2008) Interference risk and the function of dynamic shifts in calling in the gray treefrog (Hyla versicolor). J Comp Psychol 122:283–288
Schwartz JJ, Huth K, Hunce R, Lentine B (2010) Effect of anomalous pulse timing on call discrimination by females of the gray treefrog: behavioral correlates of neurobiology. J Exp Biol 213:2066–2072
Schwartz JJ, Crimarco N, Bregman Y, Umeoji K (2013) An investigation of the functional significance of responses of the gray treefrog (Hyla versicolor) to chorus noise. J Herpetol 47:354–360
Slabbekoorn H (2004) Singing in the wild: the ecology of birdsong. In: Marler P, Slabbekoorn H (eds) Nature’s music: the science of birdsong. Academic Press/Elsevier, San Diego, pp 178–205
Sun LX, Wilczynski W, Rand AS, Ryan MJ (2000) Trade-off in short- and long-distance communication in túngara (Physalaemus pustulosus) and cricket (Acris crepitans) frogs. Behav Ecol 11:102–109
Swanson EM, Tekmen SM, Bee MA (2007) Do female frogs use inadvertent social information to locate breeding aggregations? Can J Zool 85:921–932
Taigen TL, Wells KD (1985) Energetics of vocalization by an anuran amphibian (Hyla versicolor). J Comp Physiol B 155:163–170
Vélez A, Bee MA (2011) Dip-listening and the cocktail party problem in grey treefrogs: signal recognition in temporally fluctuating noise. Anim Behav 82:1319–1327
Vélez A, Schwartz JJ, Bee MA (2013) Anuran signal perception in noisy environments. In: Brumm H (ed) Animal Communication and Noise. Springer, New York, pp 133–185
Waser PM, Waser MS (1977) Experimental studies of primate vocalization: specializations for long-distance propagation. Z Tierpsychol 43:239–263
Wells KD (2007) The ecology and behavior of amphibians. University of Chicago Press, Chicago
Wells KD, Schwartz JJ (1982) The effect of vegetation on the propagation of calls in the neotropical frog Centrolenella fleischmanni. Herpetologica 38:449–455
Wells KD, Schwartz JJ (2006) The behavioral ecology of anuran communication. In: Narins PM, Feng AS, Fay RR, Popper AN (eds) Springer handbook of auditory research: hearing and sound communication in amphibians. Springer-Verlag, New York, pp 44–86
Wells KD, Taigen TL (1986) The effect of social interactions on calling energetics in the gray treefrog (Hyla versicolor). Behav Ecol Sociobiol 19:9–18
Wiener FM, Keast DN (1959) Experimental study of the propagation of sound over ground. J Acoust Soc Am 31:724–733
Wilczynski W, Ryan MJ, Brenowitz EA (1989) The display of the blue-black grassquit: the acoustic advantage of getting high. Ethology 80:218–222
Wilczynski W, Rand AS, Ryan MJ (1995) The processing of spectral cues by the call analysis system of the túngara frog, Physalaemus pustulosus. Anim Behav 49:911–929
Wiley RH (2009) Signal transmission in natural environments. In: Squire LR (ed) Encyclopedia of neuroscience volume 8. Academic Press, Oxford, pp 827–832
Wiley RH, Richards DG (1982) Adaptations for acoustic communication in birds: sound propagation and signal detection. In: Kroodsma DE, Miller EH (eds) Acoustic communication in birds, vol 1. Academic Press, New York, pp 131–181
Wollerman L (1999) Acoustic interference limits call detection in a Neotropical frog Hyla ebraccata. Anim Behav 57:529–536
Ziegler L, Arim M, Narins PM (2011) Linking amphibian call structure to the environment: the interplay between phenotypic flexibility and individual attributes. Behav Ecol 22:520–526
Zimmerman BL (1983) A comparison of structural features of calls of open and forest habitat frog species in the central Amazon. Herpetologica 39:235–246
Acknowledgments
We thank Kirsanov Charles, Stephen Sizensky, Reginald Jemison, Stacy Thomas, Jennifer Noviski, and Michael Disimone for help in the lab and field. Jeffrey Storms helped set up the weather station, and Chris Ruthven provided access to the Veteran’s Park during evening hours. We are especially grateful to Kentwood Wells and three anonymous reviewers for valuable comments on the manuscript. This work was supported with Pace University Scholarly Research Awards to JJS.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The experiments performed comply with the current laws of the USA and were approved by the Animal Care and Use Committee of Pace University (protocol no. 2009–1).
Conflicts of interest
The authors declare that they have no conflicts of interest.
Additional information
Communicated by K. Summers
Rights and permissions
About this article
Cite this article
Schwartz, J.J., Hunce, R., Lentine, B. et al. Calling site choice and its impact on call degradation and call attractiveness in the gray treefrog, Hyla versicolor . Behav Ecol Sociobiol 70, 1–19 (2016). https://doi.org/10.1007/s00265-015-2016-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-015-2016-8