Abstract
Purpose
Chordoma is a rare but highly aggressive primary bone sarcoma that arises commonly from the sacrum. While en bloc resection has been the mainstay of the treatment, the role of resection margin in millimetres with/without adjuvant radiotherapy (RT) has been unknown. We investigated the prognostic impact of surgical margin width, adjuvant RT, and their combined factor for sacral chordoma.
Methods
Forty-eight patients who underwent surgical treatment between 1996 and 2016 were studied. Of these, 11 patients (23%) received adjuvant RT; photon RT in 7 (15%) and proton RT in 4 (8%). Margins were microscopically measured in millimetres from the resection surface to the closest tumour on histologic slides.
Results
The five year and ten year disease-specific survival was 88% and 58%, respectively, and the local recurrence (LR) rate was 48%. The LR rate with 0-mm, < 1.5-mm, and ≥ 1.5-mm margin was 50% (group 1), 50% (group 2: RT−, 61%; group 3: RT+, 14%), and 0% (group 4), respectively. We observed a significantly lower LR rate in patients with adjuvant photon/proton RT (18%) than without it (57%; p = 0.026), and no LR was observed after post-operative proton RT. The combined factor of margin with RT clearly stratified the LR risk: patients of group 1 (positive margin) and 2 (< 1.5-mm margin, RT−) had approximately 7.5× LR risk (p = 0.049) compared with those of group 3 (< 1.5-mm margin, RT+) and 4 (≥ 1.5-mm margin).
Conclusion
This study identified the lowest risk of local failure in tumour resection with ≥ 1.5-mm margin or negative but < 1.5-mm margin with the use of adjuvant photon/proton radiotherapy for sacral chordoma. Early results of adjuvant proton RT demonstrated excellent local control.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chordoma is a rare primary bone tumour accounting for 1–4% of all bone malignancies [1,2,3,4,5], arising in the sacrum (50–60%), skull base (35%) or vertebral bodies (15%) [5, 6]. En bloc resection has been the mainstay of the treatment [5, 7,8,9,10,11], although recent evidence has underlined the increasing role of adjuvant radiotherapy [12,13,14,15,16]. Although chordoma is histologically characterized as low grade, it is highly recurrent and aggressive and associated with a poor prognosis [17]. The reported local recurrence (LR) rate after surgery is 30–75% [4, 11], and five and ten year overall survival (OS) rates are 70–86% [13, 14, 18, 19] and 35–59% [7, 9, 19,20,21,22,23], respectively.
To date, several prognostic factors influencing local control and survival have been reported: age (LR-free survival [LRFS] [24]; OS [21, 25]), location (LRFS [19, 24]; OS [19, 25, 26]), surgical margin (LRFS [5, 7,8,9,10, 21, 26, 27]; OS [21, 27]), size (OS [10, 27]), volume (LRFS [9, 26]; OS [26]), micro-skip metastasis (LRFS [11]), vascular invasion (OS [11]), and previous diagnostic/surgical procedure (LRFS [22, 26]). Despite the discrepancy among previous studies in terms of the prognostic factor, most of these publications described resection margin a crucial factor among the others.
There is no consensus on how surgical margins for bone and soft-tissue sarcomas are reported among different institutes worldwide, with most of the existing investigations having been performed on the system of Enneking et al.: intralesional, marginal, wide, and radical [28]. In addition, the interpretation of what is a marginal and wide resection remains inherently subjective and may vary depending on the investigators [29]. The adoption of a simple system incorporating measurable and reproducible variables would allow standardisation of treatment and monitoring and reduce bias. The closest surgical margin in millimetres could replace any subjective methods of surgical margin. Recently, the Birmingham classification for osteosarcoma, devised on the bases of the margin in millimetres and the response of chemotherapy, was superior to the conventional margin classification by Enneking for predicting LR [29]. The predictive role of margins in millimetres was also investigated in chondrosarcoma, which clearly stratified LRFS between groups in all histological grades with a surgical margin of > 4 mm [30]. However, no study has investigated the role of surgical margin in chordoma with a focus on the closest margin in millimetres.
The efficacy of adjuvant radiotherapy for sacral chordoma remains controversial [12, 13, 31,32,33]. Several retrospective studies at a single institution demonstrated that neo-adjuvant and/or adjuvant radiotherapy in combination with surgical resection led to better local relapse-free survival than surgical treatment alone [12, 13, 32, 33]. Conversely, a recent multicentre study reported that radiotherapy was not associated with local disease control, but it was associated with complications [31]. These inconsistencies might be attributed to the unstandardized treatment paradigms among multiple institutions, such as timing of treatment (pre- or post-operative), type of radiotherapy (photon, proton, or carbon ion), and radiation dose. Notably, the benefits of proton radiotherapy in an adjuvant setting have been unclear [34,35,36], while carbon ion [15, 18, 37] or proton radiotherapy [12, 15, 18, 38] plays a role in unresectable cases.
In the era of modern multidisciplinary treatment, prognostic risk prediction based on a single treatment-related factor may not provide precise information. We hypothesised that an approach combining surgical margin and the use of adjuvant radiotherapy might provide more precise prognostic risk stratification. The purpose of this study was to investigate the prognostic role of surgical margin width, adjuvant radiotherapy, and the combined factor of these in patients with sacral chordoma.
Patients and methods
We conducted a retrospective study of patients with sacral chordoma who underwent surgical treatment between 1996 and 2016 at a single institution. During this period, a total of 82 consecutive patients were diagnosed with a chordoma and 61 of these underwent surgical resection as initial treatment, excluding those who were treated with definitive radiotherapy, treated palliatively, and initially treated elsewhere and referred for the management of a recurrence. Patients with chordoma arising from sites other than the sacrum, those with minimum follow-up less than two years for patients alive, and those whose resection margin in millimetres was unavailable were also excluded.
Details of the clinical data which were collected from the notes included age at diagnosis, sex, tumour site, tumour size, level of resection, details of operations, surgical margin, pathological reports, and oncological outcome. Tumour volume was measured on histopathologic specimens or on coronal, transverse, and sagittal magnetic resonance (MR) imaging of the lesion; the volume was calculated using the formula of an ellipsoid mass volume: (π/6) × height × width × depth [26]. Margins were microscopically measured in millimetres from the resection surface to the closest tumour on histologic slides following gross examination of the specimen by pathologists highly experienced in bone and soft-tissue sarcomas.
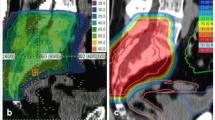
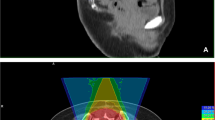
Histological diagnosis was pre-operatively confirmed and a treatment plan was discussed at a multidisciplinary meeting for all patients. The surgical indications were determined under considerations for the location and size of the lesion, its potential margins, absence of metastatic disease at presentation, the predicted surgical morbidity and mortality, and the patient's preference. For tumours at or below S3 level, resections were performed through a posterior approach, whereas tumours at S1/2 levels were resected using a combined anterior and posterior approach [5]. Depending on the cases, a vertical rectus abdominis myocutaneous flap was used to fill the dead space after tumour resection. Radiotherapy was considered in the postoperative setting if the resection margin was marginal or intralesional but also considered regardless of the margin width since 2014. Proton radiotherapy was recommended since 2012, due to the proximity of the rectum and side effects of external beam radiotherapy. Decision on the use of radiotherapy was made according to the post-operative local conditions such as wound healing after a multidisciplinary team discussion.
Post-operatively, patients remained on bed rest for the first five days. Following review of the wound, they were mobilised fully weight bearing under the guidance of a physiotherapist. Routine follow-up for patients was performed every three months for the first two years, every six months for the next three years, and then annually thereafter unless the patient developed new symptoms. Patients underwent spinal MR imaging at each visit and a chest radiograph annually for the screening of pulmonary metastases.
Disease-specific survival and local recurrence-free survival were analysed using the Kaplan-Meier analysis. Disease-specific survival was defined as the period from the date of diagnosis to the last date when the patient was recorded to be alive or the date of tumour-related death. LRFS was defined as the period from the date of treatment and censored at the date of LR. The variables of each group were compared using the chi-square test, Student t test, or Mann-Whitney U test. All statistical analyses were performed using the SPSS software (version 23; IBM, Armonk, New York, USA). Differences were considered to be statistically significant at a p value < 0.05.
Results
Patient characteristics
After exclusion criteria, a total of 48 patients were available for analysis. The patient demographics and therapies are summarised in Table 1. The mean age at diagnosis was 61 years (range, 16 to 86 years) with a slight male predominance (n = 28; 58%). The mean tumour size was 9.3 cm (range, 3.0 to 17.5 cm), and the mean tumour volume was 347 cm3 (range, 5 to 2136 cm3). The highest level of tumour was at S1 in seven patients (14.5%), S2 in 7 (14.5%), S3 in 12 (25%), S4 in 12 (25%), S5 in six (13%), and coccyx in four (8%). Of 48 patients who underwent surgical treatment, 11 patients (23%) received post-operative radiotherapy. Histological investigation identified micro-satellite lesion in three (6%) and vascular invasion in two (4%). The mean follow-up period was 77 months (range, 8 to 206 months).
The role of surgical margin in local control
The surgical margins measured in millimetres are shown in Table 2. The closest margins were observed at the anterior resections in 27 patients (56%), posterior resections in ten (21%), lateral resections in seven (15%), and superior resections in four (8%). The overall LR rate was 48% (n = 23). The mean duration from primary surgery to LR was 22.4 months (range, 3 to 52 months). LR-free survival was 46% at both five and ten years. The relationship of the closest margin in millimetre and LR is shown in Table 2. All of the LRs were observed in patients with a margin below 1.5 mm; hence, this was chosen as our cut-off for further analysis. Univariate analysis revealed that a larger tumour volume (over 205 cm3) and the use of adjuvant radiotherapy were positive factors for LR (Table 2). The surgical margin width below 1.5 mm did not significantly stratify the LRFS (p = 0.104; Supplementary Fig. 1).
The role of adjuvant radiotherapy in local control
The LR rate was significantly lower in patients with post-operative radiotherapy (18%) than those without it (57%; p = 0.026; Table 3) as described. The five year LRFS in patients with and without post-operative radiotherapy was 82% and 37%, respectively (p = 0.045; Table 1, Supplementary Fig. 2A). We observed no LR in patients who underwent post-operative proton radiotherapy, while two of seven patients (29%) had LR after post-operative conventional photon radiotherapy (p = 0.053; Table 2, Supplementary Fig. 2B).
Combined risk stratification in local control according to surgical margin and adjuvant radiotherapy
The relationship between margin width in millimetres and LR stratified by post-operative radiotherapy is shown in Table 2. When we focus on the patients with close margin below 1.5 mm, there was a significant difference in LR rate in patients with and without radiotherapy (61% and 14%, respectively; p = 0.033). We then divided patients into four groups based on the combined factor of margin and adjuvant radiotherapy: group 1, intralesional margins; group 2, margins less than 1.5 mm without post-operative radiotherapy; group 3, margins less than 1.5 mm with post-operative radiotherapy; and group 4, margins over 1.5 mm (Table 4). The LR rate was 57%, 56%, 14%, and 0%, respectively (p = 0.048; Table 4). Although LRFS was not statistically stratified among these four groups because of the limited number of patients in each (p = 0.102; Fig. 1), the combination of these groups (group 1 + 2 vs 3 + 4) demonstrated a significant difference in the incidence of LR between high and low risk of local failure (p = 0.013; Fig. 2a), suggesting that obtaining a clear margin with the addition of radiotherapy is crucial for local control. Multivariate analysis revealed that tumour volume (≥ 205 cm3 hazard ratio [HR], 3.746; 95% confidence interval [CI], 1.383–10.141; p = 0.009 versus < 205 cm3 HR, 1) and the combined factor of surgical margin and the use of adjuvant radiotherapy (group 1 + 2 HR, 7.502; 95% CI, 1.008–55.837; p = 0.049 versus group 3 + 4 HR, 1) were independent prognostic predictors for LRFS.
Predictors of disease-specific survival
The disease-specific survival rate was 88% at 5 years and 58% at 10 years. Univariate analysis revealed that the highest level of tumour involvement above S3 (p = 0.045) was a negative prognostic factor. The use of adjuvant radiotherapy was not a prognostic factor for disease-specific survival, but the patients treated with adjuvant proton radiotherapy are all alive with a mean follow-up period of 45 months (range, 27 to 81 months). Although the combined factor of surgical margin and post-operative radiotherapy was not a significant prognostic factor for disease-specific survival (p = 0.157), we observed a trend toward better prognosis in patients with group 3 + 4 (Fig. 2b). None of these factors achieved statistical significance in multivariate analysis because of the limited number of patients.
Discussion
It is well known that surgical margin remains a crucial prognostic factor for sacral chordoma; however, previous literature has described margins by the Enneking system (intralesional, marginal, or wide) [28] or R classification (intralesional or clear) [39]. Importantly, a surgical margin defined as wide and marginal varies depending on the reporter and not fully reproductive [29]. This study is the first to describe the role of margin width in millimetres for sacral chordoma. Obtaining a wide margin greater than 1.5 mm is technically demanding for this disease because of the anatomical proximity to the rectum, which was confirmed in this study. Therefore, it is important to stratify and predict the oncologic risk especially for patients with a close margin below 1.5 mm. We propose the adoption of a simple system incorporating measurable and reproducible variables, replacing the subjectivity of ‘wide’, ‘marginal’, or ‘microscopic tumour at margin’, which will allow standardisation of treatment and improve communication and future research.
The role of adjuvant radiotherapy for sacral chordoma remains a subject of debate, and its value in local control is inconclusive [4, 8, 21, 27, 31, 33]. In this study, we identified a significantly lower LR rate in patients who received adjuvant radiotherapy (18%) than in those who did not (57%). Our results were consistent with those by van Wulfften et al. [33]. In a multicentre study, Houdek et al. reported no significant benefit by neo- and/or adjuvant radiotherapy but suggested that this might be attributed to no standardised treatment strategy existing among multiple institutions including regarding radiation dose, field, or indication [31]. Our study was based on the results of post-operative radiotherapy, which we believe would provide useful information based on the safer use of radiotherapy. Further research involving larger cohorts with less bias of therapeutic details would be necessary to clarify the benefits of the use of radiotherapy for sacral chordoma.
The effect of proton radiotherapy in an adjuvant setting has been unclear, whereas recent studies have reported favourable early results with proton radiotherapy as definitive treatment, with local control rates of over 90% at 3 years [15, 19]. As an adjuvant treatment, proton radiotherapy was reported to result in a local control rate of 33–95% with a relatively short follow-up (Table 5) [15, 19, 34, 35, 38]. The wide range of local control rates among these studies might be attributed to the heterogeneous settings among the study cohorts, such as margin status and pre-/postoperative use of proton radiotherapy, which hinder effective comparisons of the outcomes associated with the adjuvant use of conventional photon radiotherapy [40]. In a multicentre study by Houdek et al., there was no association between local control and the use of proton radiotherapy [31]. However, the authors postulated that this outcome might be related to the insufficient number of patients in specific subsets. In the current study, we found excellent local control with the use of adjuvant proton radiotherapy despite the relatively short follow-up period in patients with negative resection margins. Further investigation with longer follow-up is necessary to elucidate the effects of adjuvant proton radiotherapy.
Regardless of the use of adjuvant radiotherapy, intralesional resection was associated with a high rate of LR, as described in a number of previous publications. In this study, we identified that the LR rate in patients with a clear but close margin below 1.5 mm was worse (61%) than that of intralesional margins (50%) without adjuvant radiotherapy. However, the LR decreased to 14% in patients with a clear but close (< 1.5 mm) margin when adjuvant radiotherapy was administered. These data indicated that the risk of local failure with narrow margins (< 1.5 mm) may be salvaged by adjuvant radiotherapy. Although we identified no LR in patients with a margin over 1.5 mm, we cannot determine the necessity of adjuvant radiotherapy for them because of the limited numbers and follow-up periods. Considering the possible presence of a micro-satellite lesion and the reported LR rate even in the patients with a wide margin over 1.5 mm [41, 42], adjuvant radiotherapy could be effective regardless of the margin extent.
In the era of multidisciplinary treatment for sarcomas, we face the limitation in risk assessment only by the single clinicopathological factor. A recent study has demonstrated that more precise risk stratification is achieved by combining two prognostic factors in patients with osteosarcoma. The best predictor of LR was the combination of a resection margin of ≤ 2 mm and a chemotherapy response of < 90% necrosis compared with a single factor of either resection margin or response to chemotherapy [29]. In this study, clear stratification in LR was possible using the combined factor of margin in millimetres and adjuvant radiotherapy, which was impossible only by the surgical margin. This was attributed to not a small number of patients with clear but close margin below 1.5 mm, indicating the importance of risk stratification with the use of adjuvant radiotherapy among these patients. Collectively, the combination of the key therapeutic factors could provide more precise information for the prognostic prediction and help in the decision-making of further treatment.
We acknowledge several limitations of this study. First, data on the quality of surgical margin were not available but further analysis considering margin quantity would provide a more accurate prognostic indicator. If the margin quality could be predicted prior to resection, particularly when the resection may require close dissection or even the excision of nearby vital structures, it may be possible to give more accurate information for preoperative planning. Second, the follow-up periods in patients with adjuvant radiotherapy, especially with proton radiotherapy, were relatively short, despite no statistical difference among them. Longer follow-up for them would further determine the efficacy of postoperative photon/proton radiotherapy. Third, this study was retrospective in nature and was based on the records of the patients at a single institution. Further well-designed, retrospective studies with a larger cohort would be useful for decision-making of treatment. Despite these limitations, we believe that our analyses, which first demonstrate the role of margin in millimetres in combination with the use of adjuvant radiotherapy, provide more precise oncological prediction and help physicians and patients in making informed decisions of treatments and follow-ups.
In summary, the present study identified the lowest risk of local failure in tumour resection with ≥ 1.5 mm margin and adjuvant photon/proton radiotherapy for sacral chordoma. In patients with clear margin but narrower than 1.5 mm, risk of LR is as high as those with the intralesional margin unless adjuvant radiotherapy is performed. Although longer follow-up is required, early results demonstrated the excellent local control by adjuvant proton radiotherapy.
References
Chugh R, Tawbi H, Lucas DR, Biermann JS, Schuetze SM, Baker LH (2007) Chordoma: the nonsarcoma primary bone tumor. Oncologist 12:1344–1350
Healey J, Lane J (1989) Chordoma: a critical review of diagnosis and treatment. Orthop Clin N Am 20:417–426
McMaster ML, Goldstein AM, Bromley CM, Ishibe N, Parry DM (2001) Chordoma: incidence and survival patterns in the United States, 1973–1995. Cancer Causes Control 12:1–11
Walcott BP, Nahed BV, Mohyeldin A, Coumans J-V, Kahle KT, Ferreira MJ (2012) Chordoma: current concepts, management, and future directions. Lancet Oncol 13:e69–e76. https://doi.org/10.1016/s1470-2045(11)70337-0
Xie C, Whalley N, Adasonla K, Grimer R, Jeys L (2015) Can local recurrence of a sacral chordoma be treated by further surgery? Bone Joint J 97:711–715
Eriksson B, Gunterberg B, Kindblom L-G (1981) Chordoma: a clinicopathologic and prognostic study of a Swedish national series. Acta Orthop Scand 52:49–58
Schwab JH, Healey JH, Rose P, Casas-Ganem J, Boland PJ (2009) The surgical management of sacral chordomas. Spine 34:2700–2704
Dubory A, Missenard G, Lambert B, Court C (2014) “En bloc” resection of sacral chordomas by combined anterior and posterior surgical approach: a monocentric retrospective review about 29 cases. Eur Spine J 23:1940–1948. https://doi.org/10.1007/s00586-014-3196-z
Varga PP, Szoverfi Z, Fisher CG, Boriani S, Gokaslan ZL, Dekutoski MB, Chou D, Quraishi NA, Reynolds JJ, Luzzati A, Williams R, Fehlings MG, Germscheid NM, Lazary A, Rhines LD (2015) Surgical treatment of sacral chordoma: prognostic variables for local recurrence and overall survival. Eur Spine J 24:1092–1101. https://doi.org/10.1007/s00586-014-3728-6
Radaelli S, Stacchiotti S, Ruggieri P, Donati D, Casali PG, Palmerini E, Collini P, Gambarotti M, Porcu L, Boriani S, Gronchi A, Picci P (2016) Sacral chordoma: long-term outcome of a large series of patients surgically treated at two reference centers. Spine 41:1049–1057. https://doi.org/10.1097/BRS.0000000000001604
Akiyama T, Ogura K, Gokita T, Tsukushi S, Iwata S, Nakamura T, Matsumine A, Yonemoto T, Nishida Y, Saita K, Kawai A, Matsumoto S, Yamaguchi T (2018) Analysis of the infiltrative features of chordoma: the relationship between micro-skip metastasis and postoperative outcomes. Ann Surg Oncol 25:912–919. https://doi.org/10.1245/s10434-017-6268-6
Hug EB, Fitzek MM, Liebsch NJ, Munzenrider JE (1995) Locally challenging osteo-and chondrogenic tumors of the axial skeleton: results of combined proton and photon radiation therapy using three-dimensional treatment planning. Int J Radiat Oncol Biol Phys 31:467–476
Zabel-du Bois A, Nikoghosyan A, Schwahofer A, Huber P, Schlegel W, Debus J, Milker-Zabel S (2010) Intensity modulated radiotherapy in the management of sacral chordoma in primary versus recurrent disease. Radiother Oncol 97:408–412. https://doi.org/10.1016/j.radonc.2010.10.008
Imai R, Kamada T, Sugahara S, Tsuji H, Tsujii H (2014) Carbon ion radiotherapy for sacral chordoma. Br J Radiol
Mima M, Demizu Y, Jin D, Hashimoto N, Takagi M, Terashima K, Fujii O, Niwa Y, Akagi T, Daimon T, Hishikawa Y, Abe M, Murakami M, Sasaki R, Fuwa N (2014) Particle therapy using carbon ions or protons as a definitive therapy for patients with primary sacral chordoma. Br J Radiol 87:20130512. https://doi.org/10.1259/bjr.20130512
Pennicooke B, Laufer I, Sahgal A, Varga PP, Gokaslan ZL, Bilsky MH, Yamada YJ (2016) Safety and local control of radiation therapy for chordoma of the spine and sacrum: a systematic review. Spine 41(Suppl 20):S186–S192. https://doi.org/10.1097/BRS.0000000000001831
Atalar H, Selek H, Yıldız Y, Sağlık Y (2006) Management of sacrococcygeal chordomas. Int Orthop 30:514–518
Nishida Y, Kamada T, Imai R, Tsukushi S, Yamada Y, Sugiura H, Shido Y, Wasa J, Ishiguro N (2011) Clinical outcome of sacral chordoma with carbon ion radiotherapy compared with surgery. Int J Radiat Oncol Biol Phys 79:110–116. https://doi.org/10.1016/j.ijrobp.2009.10.051
Chen YL, Liebsch N, Kobayashi W, Goldberg S, Kirsch D, Calkins G, Childs S, Schwab J, Hornicek F, DeLaney T (2013) Definitive high-dose photon/proton radiotherapy for unresected mobile spine and sacral chordomas. Spine 38:E930–E936. https://doi.org/10.1097/BRS.0b013e318296e7d7
Baratti D, Gronchi A, Pennacchioli E, Lozza L, Colecchia M, Fiore M, Santinami M (2003) Chordoma: natural history and results in 28 patients treated at a single institution. Ann Surg Oncol 10:291–296. https://doi.org/10.1245/aso.2003.06.002
Fuchs B, Dickey ID, Yaszemski MJ, Inwards CY, Sim FH (2005) Operative management of sacral chordoma. JBJS 87:2211–2216
Ruggieri P, Angelini A, Ussia G, Montalti M, Mercuri M (2010) Surgical margins and local control in resection of sacral chordomas. Clin Orthop Relat Res 468:2939–2947. https://doi.org/10.1007/s11999-010-1472-8
Stacchiotti S, Casali PG, Vullo SL, Mariani L, Palassini E, Mercuri M, Alberghini M, Pilotti S, Zanella L, Gronchi A (2010) Chordoma of the mobile spine and sacrum: a retrospective analysis of a series of patients surgically treated at two referral centers. Ann Surg Oncol 17:211–219
Samson IR, Springfield DS, Suit HD, Mankin HJ (1993) Operative treatment of sacrococcygeal chordoma A review of twenty-one cases. JBJS 75:1476–1484
McGirt MJ, Gokaslan ZL, Chaichana KL (2011) Preoperative grading scale to predict survival in patients undergoing resection of malignant primary osseous spinal neoplasms. Spine J 11:190–196
Angelini A, Pala E, Calabrò T, Maraldi M, Ruggieri P (2015) Prognostic factors in surgical resection of sacral chordoma. J Surg Oncol 112:344–351
Bergh P, Kindblom LG, Gunterberg B, Remotti F, Ryd W, Meis-Kindblom JM (2000) Prognostic factors in chordoma of the sacrum and mobile spine: a study of 39 patients. Cancer: Interdisciplinary International Journal of the American Cancer Society 88:2122–2134
Enneking WF, Spanier SS, Goodman MA (1980) A system for the surgical staging of musculoskeletal sarcoma. Clin Orthop Relat Res 153:106–120
Jeys LM, Thorne CJ, Parry M, Gaston CL, Sumathi VP, Grimer JR (2017) A novel system for the surgical staging of primary high-grade osteosarcoma: the Birmingham Classification. Clin Orthop Relat Res 475:842–850. https://doi.org/10.1007/s11999-016-4851-y
Stevenson JD, Laitinen MK, Parry MC, Sumathi V, Grimer RJ, Jeys LM (2018) The role of surgical margins in chondrosarcoma. Eur J Surg Oncol
Houdek MT, Rose PS, Hevesi M, Schwab JH, Griffin AM, Healey JH, Petersen IA, TF DL, Chung PW, Yaszemski MJ, Wunder JS, Hornicek FJ, Boland PJ, Sim FH, Ferguson PC, Other Members of the Sacral Tumor S (2019) Low dose radiotherapy is associated with local complications but not disease control in sacral chordoma. J Surg Oncol 119:856–863. https://doi.org/10.1002/jso.25399
Park L, Delaney TF, Liebsch NJ, Hornicek FJ, Goldberg S, Mankin H, Rosenberg AE, Rosenthal DI, Suit HD (2006) Sacral chordomas: impact of high-dose proton/photon-beam radiation therapy combined with or without surgery for primary versus recurrent tumor. Int J Radiat Oncol Biol Phys 65:1514–1521. https://doi.org/10.1016/j.ijrobp.2006.02.059
van Wulfften Palthe OD, Tromp I, Ferreira A, Fiore A, Bramer JA, van Dijk NC, DeLaney TF, Schwab JH, Hornicek FJ (2019) Sacral chordoma: a clinical review of 101 cases with 30-year experience in a single institution. Spine J 19:869–879
Baumann BC, Lustig RA, Mazzoni S, Grady SM, O'Malley BW, Lee JYK, Newman JG, Schuster JM, Both S, Lin A, Dorsey JF, Alonso-Basanta M (2019) A prospective clinical trial of proton therapy for chordoma and chondrosarcoma: feasibility assessment. J Surg Oncol 120:200–205. https://doi.org/10.1002/jso.25502
Rotondo RL, Folkert W, Liebsch NJ, Chen YL, Pedlow FX, Schwab JH, Rosenberg AE, Nielsen GP, Szymonifka J, Ferreira AE, Hornicek FJ, DeLaney TF (2015) High-dose proton-based radiation therapy in the management of spine chordomas: outcomes and clinicopathological prognostic factors. J Neurosurg Spine 23:788–797. https://doi.org/10.3171/2015.3.SPINE14716
Zhou J, Yang B, Wang X, Jing Z (2018) Comparison of the effectiveness of radiotherapy with photons and particles for chordoma after surgery: a meta-analysis. World Neurosurg 117:46–53. https://doi.org/10.1016/j.wneu.2018.05.209
Imai R, Kamada T, Sugahara S, Tsuji H, Tsujii H (2011) Carbon ion radiotherapy for sacral chordoma. Br J Radiol 84(spec no1):S48–S54. https://doi.org/10.1259/bjr/13783281
DeLaney TF, Liebsch NJ, Pedlow FX, Adams J, Weyman EA, Yeap BY, Depauw N, Nielsen GP, Harmon DC, Yoon SS, Chen YL, Schwab JH, Hornicek FJ (2014) Long-term results of phase II study of high dose photon/proton radiotherapy in the management of spine chordomas, chondrosarcomas, and other sarcomas. J Surg Oncol 110:115–122. https://doi.org/10.1002/jso.23617
Tunn P-U, Kettelhack C, Dürr HR (2009) Standardized approach to the treatment of adult soft tissue sarcoma of the extremities. In: Treatment of Bone and Soft Tissue Sarcomas. Springer. pp. 211–228
Boriani S, Bandiera S, Biagini R, Bacchini P, Boriani L, Cappuccio M, Chevalley F, Gasbarrini A, Picci P, Weinstein JN (2006) Chordoma of the mobile spine: fifty years of experience. Spine 31:493–503
Hanna S, Aston W, Briggs T, Cannon S, Saifuddin A (2008) Sacral chordoma: can local recurrence after sacrectomy be predicted? Clin Orthop Relat Res 466:2217–2223
Kaiser TE, Pritchard DJ, Unni KK (1984) Clinicopathologic study of sacrococcygeal chordoma. Cancer 53:2574–2578
Acknowledgements
This work was supported by overseas research fellowships of The Uehara Memorial Foundation (TF).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 346 kb)
Rights and permissions
About this article
Cite this article
Fujiwara, T., Tsuda, Y., Stevenson, J. et al. Sacral chordoma: do the width of surgical margin and the use of photon/proton radiotherapy affect local disease control?. International Orthopaedics (SICOT) 44, 381–389 (2020). https://doi.org/10.1007/s00264-019-04460-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-019-04460-5