Abstract
Purpose
Sacral chordomas (SC) are rare, locally invasive, malignant neoplasms. Despite surgical resection and adjuvant therapies, local recurrence (LR) is common and overall survival (OS) is poor. The objective of this study was to identify prognostic factors that have an impact on the local recurrence-free survival (LRFS) and OS of patients with SC.
Methods
Utilizing the AOSpine Knowledge Forum Tumor multicenter ambispective database, surgically treated SC cases were identified. Cox regression modeling was used to assess the effect of several clinically relevant variables on OS and LRFS.
Results
A total of 167 patients with surgically treated SC were identified. The male/female ratio was 98/69 with a mean age of 57 ± 15 years at the time of surgery. The LR was 35 % (n = 57), death occurred in 30 % of patients (n = 50) during the study period. The median OS was 6 years post-surgery and LRFS was 4 years. In the univariate analysis, previous tumor surgery at the same site (P = 0.002), intralesional resection (P < 0.001), and larger tumor volume (P = 0.030) were significantly associated with LR. Increasing age (P < 0.001) and a preoperative motor deficit of C or D (P = 0.003) were significantly associated with poor OS, and nerve root sacrifice showed a trend towards significance (P = 0.088). In the multivariate models, previous surgery and intralesional resection were significantly related to LR, while increasing age and motor deficit of C or D were associated with poor OS.
Conclusions
This study identified two predictive variables for LRFS (previous tumor surgery and type of surgical resection) and two for OS (age and impaired motor function) in surgically treated SC patients. Our results indicate that en bloc resection reduces LR but does not influence OS. However, this was likely due to short follow-up (3.2 years).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chordoma is a rare malignant neoplasm, arising from notochordal remnants, thus it is located almost exclusively in the axial skeleton [1]. It has an overall incidence of 0.08 per 100,000 individuals and accounts for 40 % of all primary sacral tumors [2]. Sacral chordoma is a typically slow growing and locally aggressive tumor, with a reduced ability to metastasize [3]. The diagnosis is often delayed because of the long standing, nonspecific initial symptoms, allowing the tumor to reach large sizes [4]. Because chordomas have shown to have a poor sensitivity towards radiotherapy and chemotherapy, they are mainly treated by surgical resection; a daunting undertaking given the complex, resource intensive, impairment inducing nature of the procedures and the high preponderance for local recurrence (LR) and eventual death [5].
Enneking oncologic management principles would recommend wide surgical en bloc resection of chordomas; however, this is difficult, even in the hands of the most experienced spine oncology surgeons [6]. Wide resection is not uniformly achieved in 35–75 % of cases, primarily due to the relatively inaccessible anatomical location, preference for neurological preservation and large size at the time of diagnosis [7–13]. The fact that chordomas grow in a lobulated fashion and have distant microscopic tumor outgrowths also makes wide surgical resection difficult [14]. Based on low-quality evidence, insufficient tumor resection is probably the main cause of LR and subsequently death [9, 10, 13]. Other factors that possibly influence survival and LR have been previously reported and include increased age, high sacral localization, lack of radiotherapy, prior resections, higher tumor grade, and increasing extent of tumor invasion [9, 10, 15–22]. Based on the dire consequences related to mortality and morbidity with the mismanagement of sacral chordomas, higher levels of evidence are needed to improve decision making and consequently patient outcome.
This study aims to identify prognostic factors that have an impact on the local recurrence-free survival (LRFS) and overall survival (OS) of surgically treated sacral chordomas (SC) patients from a multicenter ambispective database.
Methods
Study design
AOSpine International, through their Knowledge Forum Tumor conducted one of the first multicenter studies on primary spinal tumors [23]. They developed a database containing clinical and outcome data about surgically treated primary spinal tumor cases. A retrospective review of prospectively collected data, or ambispective design with cross-sectional follow-up, was performed by 12 of 13 leading spine oncology referral centers (Fig. 1); seven centers from North America (Johns Hopkins University School of Medicine, Baltimore, USA; University of British Columbia, Vancouver, Canada; MD Anderson Cancer Center, Houston, USA; University of Toronto, Toronto, Canada; Memorial Sloan-Kettering Center, New York, USA; Mayo Clinic, Rochester, USA; University of California San Francisco, San Francisco, USA), five from Europe (National Center for Spinal Disorders, Budapest, Hungary; Rizzoli Institute, Bologna, Italy; Queens Medical Centre, Nottingham, UK; Instituto Ortopedico Galeazzi, Milan, Italy; Oxford University Hospital NHS Trust, Oxford, UK), and one from Australia (Princess Alexandra Hospital, Brisbane, Australia). Patients met the inclusion criteria if they were diagnosed with a primary spinal tumor, received a surgical resection, and participated in at least one clinical follow-up. Patients with a secondary spinal tumor, spinal cord tumor, spinal lymphoma, or myeloma were excluded. The study was approved by the Scientific and Research Ethics Committee in each of the participating centers. The reporting of this work follows the guidelines of the strengthening the reporting of observational studies in epidemiology (STROBE) initiative [24].
Thirteen leading spine oncology referral centers. 1 University of British Columbia, Vancouver, Canada; 2 University of Toronto, Toronto, Canada; 3 University of California San Francisco, San Francisco, USA; 4 Mayo Clinic, Rochester, USA; 5 MD Anderson Cancer Center, Houston, USA; 6 Johns Hopkins University School of Medicine, Baltimore, USA; 7 Memorial Sloan-Kettering Center, New York, USA (this site did not contribute cases to the sacral chordoma cohort); 8 Queens Medical Centre, Nottingham, UK; 9 Oxford University Hospital NHS Trust, Oxford, UK; 10 Instituto Ortopedico Galeazzi, Milan, Italy; 11 Rizzoli Institute, Bologna, Italy; 12 National Center for Spinal Disorders, Budapest, Hungary; 13 Princess Alexandra Hospital, Brisbane, Australia. Centers in yellow contributed to the collection of sacral chordomas, while centers in gray did not
Data collection
Data about demographics, baseline patient characteristics, surgical treatment, local disease recurrence, morbidity, and cross-sectional survival were gathered and entered into a database. The database was built and managed through a secure, web-based application (REDCap—Research Electronic Data Capture), hosted at AOSpine International [25].
Preoperative
Preoperative inpatient and outpatient clinical records were used to identify demographic and clinical data including age, gender, detailed medical history, preoperative symptoms, presence of pathologic vertebral fractures, and different neurological signs. Previous tumor surgery was defined as a surgical intervention beyond biopsy before the surgical resection. Motor deficit was assessed according to the Frankel or ASIA scales. Preoperative motor deficit was combined into two categories distinguishing the intact motor function (Frankel E) from paresis (Frankel D-A). Signs of cauda equina syndrome were also recorded. Results from imaging (CT, MRI, X-Ray, bone scan, PET-CT) and histological diagnosis were used to determine the localization, the local extension, and the oncologic stage of the tumor. The staging was performed according to the main categories of the Enneking surgical staging system (Ia—low grade malignant, confined to compartment; Ib—low grade malignant, invasive; IIa—high grade malignant, confined to compartment; IIb—high grade malignant, invasive; III—metastasis) [26].
Intraoperative
Intraoperative surgical data including surgical approach, nerve root and cauda equina sacrifice, type of resection, type of reconstruction, and the amount of blood loss were recorded. The parameters for type of resection (wide, marginal, intralesional or palliative) were determined by the surgeon. The surgeon’s impression about the surgical margins was validated by the pathologist during the histological analysis. The resections were also categorized according to the Enneking principles. Based on this classification, the resection of a malignant primary tumor such as chordoma (Enneking Ia, Ib, IIa, IIb, III) is defined as Enneking appropriate (EA) when a wide or marginal pathology is reported and Enneking inappropriate (EI) in the case of intralesional or palliative resection [27]. Tumor volume was measured on the histopathologic specimens. The height, width, and depth of the tumor were recorded, and the volume was calculated using the formula of an ellipsoid mass (volume = π/6 × height × width × depth) [28]. Tumor volume was transformed into a categorical variable where tumors were grouped as <100 and ≥100 cm3.
Postoperative
Follow-up data were obtained by direct examination of the patient and by performing the required imaging modalities. Follow-up data included any early and late postoperative complications, adjuvant radiation and chemotherapy, LR, any further surgeries for complications or recurrence, and current vital status. Postoperative complications were considered “early” if they occurred within 6 weeks after surgery and “late” if they occurred more than 6 weeks postoperative.
At the end of the study period, a cross-sectional follow-up of the vital status was performed in the form of an outpatient visit, telephone interview or accessing governmental vital statistic databases, if necessary.
Statistical analysis
The Kaplan–Meier method (K–M) was used to estimate OS and the LR. LR-free survival was defined as the length of time from the spine tumor surgery to the diagnosis of the first LR. The analysis was restricted to events that occurred within the first 10 years to adjust for patients who recently were diagnosed and had shorter follow-up times. Similarly, OS was defined as the length of time from the spine tumor surgery to death. Observations were censored when the patient was tumor free (LRFS analysis) or was alive (OS analysis) at the time of last clinical follow-up. The effect of individual variables on LR and OS was evaluated by assessing K–M curves with log-rank tests. To test for significance, selected continuous and categorical variables were re-categorized. Age, previous surgery, motor deficit, presence of cauda syndrome, tumor volume, adjuvant therapy, pathology, reconstruction, nerve root sacrifice, and tumor recurrence were evaluated. Variables with at least a marginally significant effect on survival (P < 0.1) were selected for the multivariate proportional hazards regression modeling. In the multivariate analysis, prognostic variables were identified when P ≤ 0.05. Statistical analysis was performed using STATA 12.0 software.
Results
Patient demographic and clinical characteristics
Between December 1985 and May 2012, a total of 1,495 primary spinal tumors were treated and the data were entered in the AOSpine Tumor Knowledge Forum Primary Spinal Tumor database (Fig. 2). Three hundred and forty-four patients had a chordoma and 173 patients received surgical treatment for a primary chordoma localized in the sacrum. Six patients who had Enneking Grade III (metastases) tumors were excluded from the study. Table 1 shows the demographic characteristics of the final cohort (167 patients). The male/female ratio was 98/69 with a mean age of 57 ± 15 years at the time of surgery (range 18–89). The majority of patients (n = 152, 96 %) presented with tumor-related spinal pain at the time of the diagnosis. Presence of motor deficit (Frankel/ASIA C and D) was also relatively common (n = 37, 24 %), and serious neurological deterioration was also a frequent symptom, where 41 (27 %) patients had cauda equina syndrome. Fifteen (9 %) patients had at least one previous spinal tumor surgery.
Tumor characteristics
Sixty-three (38 %) patients had chordomas with only sacral involvement, 89 (54 %) patients had sacrococcygeal chordomas, nine (6 %) patients had a sacral tumor involving the lumbar spine, and three (2 %) patients had only coccygeal chordomas (Table 2). The majority of the tumors (n = 128, 79 %) were Enneking Ib tumors (conventional and chondroid chordoma), and only 30 (19 %) tumors were Enneking IIb tumors (dedifferentiated chordoma). Only four (2 %) patients had a relatively small size tumor, that was confined only to the sacrum (two patients Enneking Ia and two IIa tumors). The mean tumor volume was 588.1 ± 1,423.1 cm3 (range 0.8–14,137 cm3).
Treatment
The majority of tumors (n = 125, 76 %) were removed by a posterior only approach, 38 (23 %) by a combined anterior/posterior approach, and only two (1 %) were managed by an anterior only approach (Table 3). In 125 (82 %) patients, the sacrifice of one or more nerve roots was necessary during the tumor resection; in 10 (7 %) patients, the whole cauda equina was resected. The mean blood loss was 2,646 ± 3,613.5 ml (range 100–22,000 ml).
The surgeon rated the intervention as marginal or wide in 131 (86 %) patients, and as intralesional in 21 (14 %) patients. The final pathologist rated specimen was widely or marginally resected in 129 (81 %) patients and intralesionally resected in 30 (19 %) patients. The difference between the two ratings was not significant (P = 0.34, Chi2 = 0.907, df = 1). Based on Enneking principles, 129 (81 %) patients had EA resection and 30 (19 %) patients had EI resection.
Thirty-nine (23 %) patients received adjuvant chemotherapy, conventional radiotherapy, carbon beam irradiation, or a combination.
Follow-up
The average follow-up of the patients was 3.2 years (range 5 days–16.2 years). The majority of the patients (n = 106, 63 %) were alive with no evidence of local or systemic disease at last clinical follow-up (Table 4). Twenty-six (15 %) patients were alive with evidence of local disease only, 11 (6 %) patients with systemic disease only, and 9 (5 %) patients with both local and systemic disease. Nine patients died due to propagation of the disease or due to disease-related complications. The cause of death for six patients was possibly unrelated to the sacral chordoma. The cross-sectional follow-up revealed that after the last clinical follow-up, 35 additional patients died from different causes.
Local recurrence analysis
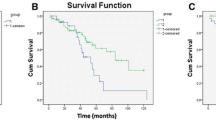
Fifty-seven (35 %) patients had LR after surgery. The median LRFS was 4 years (Fig. 3a). In the univariate analyses, previous tumor surgery at the same site (P = 0.002), type of resection (P < 0.001), and tumor volume (P = 0.030), were significantly associated with LR (Table 5). When these three variables were combined in a multivariate model, previous surgery and type of resection were significantly related to LR (P = 0.048, HR = 2.05, CI 95 % = 1.00–4.18 and P = 0.009, HR = 2.43, CI 95 % = 1.25–4.73, respectively; Table 6). Undergoing a previous spine tumor operation and having an intralesional resection are associated with an increased risk of LR.
Survival analysis
By the end of the study period, 50 (30 %) patients died and 117 (70 %) patients were alive. The median OS was 6 years (Fig. 3b). In the univariate analyses, age at surgery (P < 0.001) and motor deficit (P = 0.003) were significantly associated with OS (Table 5). The nerve root sacrifice was only trending towards significance (P = 0.088). When these three variables were combined in a multivariate model, age and motor deficit remained significantly associated with OS (P = 0.039, HR = 1.02, CI 95 % = 1.00–1.04 and P = 0.002, HR = 0.83, CI 95 % = 1.46–5.48, respectively; Table 6). Increasing age and a motor deficit of C or D are associated with a poor OS.
Discussion
Sacral chordomas (SC) are rare and thus difficult to manage and study. We report, to our knowledge, the largest multicentric ambispective cohort study of surgically treated SC. Our survival analysis of 167 patients with sacral chordoma assessed the effect of several variables both on LRFS and OS. The results from Kaplan–Meier and log-rank analyses were first evaluated to identify variables for multivariate Cox modeling. The multivariate model showed that EA surgery (en bloc resection with wide or marginal margins based on the pathology data) does improve the LRFS. Another interesting finding was the negative effect of previous surgery on LR. Furthermore, age and motor deficit (Frankel or ASIA score of C or D) were independently associated with poor survival.
The postoperative LR and the mortality can be influenced by several factors (Table 7). Several publications tried to identify prognostic factors, but the majority of these studies are statistically underpowered. In contrast, this study uses a large population-based multicentric database and statistical modeling to identify prognostic factors.
The first study which used survival analysis to assess the effect of different factors on LRFS in 21 surgically treated SC was published by Samson et al. in 1993 [17]. The authors used univariate Cox regression analysis and found old age to have an impact on LR, but only showing a trend towards significance. Cheng et al. reviewing their 31-year experience with sacral chordoma resection had similar findings, old age and higher sacral localization with or without lumbar involvement were independently associated with high LR [20]. In our analysis, old age had a negative impact only on OS.
In 1999, York et al. [9] reported a survival analysis of 27 surgically treated sacral chordoma cases. They assessed only the LRFS, which was negatively influenced in the univariate survival analysis by subtotal tumor resection and by the lack of radiotherapy after surgery. One year later in 2000, Bergh et al. [18] analyzed 39 consecutive patients, and found that inadequate surgical margins have a negative impact on LRFS and on disease-specific survival. In the case series of Fuchs et al. [10] the authors reported that surgical margins were the most important predictor of OS and LRFS. In 2010, Ruggieri et al. analyzing their institutional experience with sacral chordoma resection (56 patients during 30 years practice) found that surgical margins and previous intralesional surgery had a negative impact on LRFS. The inadequate surgical margin and the previous surgery was a prognostic factor for LRFS in our multivariate model. This indicates that EA resection reduces LR. In the group of patients who underwent EI resection, the occurrence of LR was higher 64 % versus the 29 % of cases where the EA resection was feasible (P < 0.001, Chi2 = 12.383, df = 1). This difference was not significant in the term of survival (Table 8).
McGirt et al. published the only population-based study until now, which assessed surgically treated chordoma patients (67 sacral chordoma and 47 mobile spine chordoma patients). They revealed from the SEER registry that increasing age, increasing extent invasion, more recent year of surgery and sacral localization is associated with poor survival in chordoma [16]. In the publications of Bergh and McGirt, large tumor size was a prognostic factor of poor survival. In our analysis, it was significant only in the univariate model (P = 0.03).
In the primary spinal tumor literature, there is no reference on the preoperative neurological deficit as a prognostic factor of mortality. However, Tokuhashi et al. [29] reported the severity of spinal cord injury as an important factor of poor prognosis in patients with secondary spinal tumors. In SC, neurological deficit is rare and is limited mainly to the L5, S1 nerve roots. In the most severe cases, the whole cauda equina can be affected. In our analysis, the presence of cauda equina syndrome was not a prognostic factor. In contrast, we identified Frankel or ASIA score below E as a negative prognostic factor for survival in the multivariate analysis. Another interesting observation is that patients with postoperative neurologic deficit due to planed nerve root sacrifice have poorer survival. However, this prognostic factor was significant only in the univariate analysis. Patients with neurologic deficit usually have an impaired quality of life (QOL) which was suggested to shorten the survival [30].
In the majority of these publications, old age and inadequate surgical margins were common prognostic factors for OS and LRFS. However, a common drawback in interpreting their results is that they used the simplest form of survival analysis (Kaplan–Meier and log-rank analysis) based on statistically underpowered studies and retrospective limitations. Only McGirt et al. used multivariate Cox regression modeling—which is the gold standard in survival analysis—to identify the possible prognostic factors associated with OS. The problem with this population-based study is that it lacked granularity due to its registry design, specifically around surgical details and pathology. In our multivariate Cox models, the number of events per variable was 19.5 and 16.7 in LFRS and OS analyses, respectively, which is superior than the literature recommendation [31].
Despite an ambispective design and dedicated, detailed data collection, our study has numerous limitations. The major limitation is with respect to follow-up. Based on best available literature, the current 5- and 10-year survival for chordomas is 72 and 48 % [32]. The follow-up in our study, therefore, is too short to specifically deal with the issue of OS. It is not unreasonable from a theoretical perspective however that if LR occurs the OS is likely reduced; only longer term follow-up data would answer this question. Similarly, the follow-up is probably a little early for LR, but the results of statistical significance of EA and decreased LR are probably very robust. Furthermore, the fact that the analysis was based on a retrospective review of prospective data constitutes a limitation. To overcome this, we performed a cross-sectional follow-up of the vital status at the end of the study period. The final limitations are around the error and variability in surgical and pathology impressions, which are difficult to control at rare conditions and a multicenter design. Multicenter collection has been initiated.
Due to the intensive research in oncology, the therapeutic strategies in the management of primary spinal tumors are changing. On one hand, the proton and carbon beam therapies are showing promising effect in the cases of otherwise radioresistant solid tumors (chordoma and chondrosarcoma). On the other hand, in the past years, some molecular pathways and possible target molecules were identified (e.g., brachyury in the case of chordoma), which can lead in the near future to the development of novel therapeutic agents. Until then, regardless of its morbidity, the surgical intervention is the treatment of choice in PSTs. To improve the surgical decision making, and to better understand the positive and negative effects of surgery, prospective multicenter studies are needed—incorporating health-related QOL assessment; but the results of this study would suggest that surgeons treating SC strongly adhere to EA surgical margins to minimize the risk of LR and its miserable, relentless sequelae.
References
Walcott BP, Nahed BV, Mohyeldin A, Coumans JV, Kahle KT, Ferreira MJ (2012) Chordoma: current concepts, management, and future directions. Lancet Oncol 13(2):e69–e76. doi:10.1016/S1470-2045(11)70337-0
Ropper AE, Cahill KS, Hanna JW, McCarthy EF, Gokaslan ZL, Chi JH (2012) Primary vertebral tumors: a review of epidemiologic, histological and imaging findings, part II: locally aggressive and malignant tumors. Neurosurgery 70(1):211–219. doi:10.1227/NEU.0b013e31822d5f17 (discussion 219)
Fourney DR, Gokaslan ZL (2003) Current management of sacral chordoma. Neurosurg Focus 15(2):E9
Fourney DR, Rhines LD, Hentschel SJ, Skibber JM, Wolinsky JP, Weber KL, Suki D, Gallia GL, Garonzik I, Gokaslan ZL (2005) En bloc resection of primary sacral tumors: classification of surgical approaches and outcome. J Neurosurg Spine 3(2):111–122. doi:10.3171/spi.2005.3.2.0111
Hsu W, Kosztowski TA, Zaidi HA, Dorsi M, Gokaslan ZL, Wolinsky JP (2009) Multidisciplinary management of primary tumors of the vertebral column. Curr Treat Options Oncol 10(1–2):107–125. doi:10.1007/s11864-009-0102-8
Varga PP, Szövérfi Z, Lazary A (2014) Surgical treatment of primary malignant tumors of the sacrum. Neurol Res 36(6):577–587. doi:10.1179/1743132814Y.0000000366
Ahmed AR (2009) Safety margins in resection of sacral chordoma: analysis of 18 patients. Arch Orthop Trauma Surg 129(4):483–487. doi:10.1007/s00402-008-0674-y
Hanna SA, Aston WJ, Briggs TW, Cannon SR, Saifuddin A (2008) Sacral chordoma: can local recurrence after sacrectomy be predicted? Clin Orthop Relat Res 466(9):2217–2223. doi:10.1007/s11999-008-0356-7
York JE, Kaczaraj A, Abi-Said D, Fuller GN, Skibber JM, Janjan NA, Gokaslan ZL (1999) Sacral chordoma: 40-year experience at a major cancer center. Neurosurgery 44(1):74–79 (discussion 79–80)
Fuchs B, Dickey ID, Yaszemski MJ, Inwards CY, Sim FH (2005) Operative management of sacral chordoma. J Bone Joint Surg Am 87(10):2211–2216. doi:10.2106/JBJS.D.02693
Hulen CA, Temple HT, Fox WP, Sama AA, Green BA, Eismont FJ (2006) Oncologic and functional outcome following sacrectomy for sacral chordoma. J Bone Joint Surg Am 88(7):1532–1539. doi:10.2106/JBJS.D.02533
Atalar H, Selek H, Yildiz Y, Saglik Y (2006) Management of sacrococcygeal chordomas. Int Orthop 30(6):514–518. doi:10.1007/s00264-006-0095-x
Baratti D, Gronchi A, Pennacchioli E, Lozza L, Colecchia M, Fiore M, Santinami M (2003) Chordoma: natural history and results in 28 patients treated at a single institution. Ann Surg Oncol 10(3):291–296
Kayani B, Hanna SA, Sewell MD, Saifuddin A, Molloy S, Briggs TW (2014) A review of the surgical management of sacral chordoma. Eur J Surg Oncol. doi:10.1016/j.ejso.2014.04.008
Ruggieri P, Angelini A, Ussia G, Montalti M, Mercuri M (2010) Surgical margins and local control in resection of sacral chordomas. Clin Orthop Relat Res 468(11):2939–2947. doi:10.1007/s11999-010-1472-8
McGirt MJ, Gokaslan ZL, Chaichana KL (2011) Preoperative grading scale to predict survival in patients undergoing resection of malignant primary osseous spinal neoplasms. Spine J 11(3):190–196. doi:10.1016/j.spinee.2011.01.013
Samson IR, Springfield DS, Suit HD, Mankin HJ (1993) Operative treatment of sacrococcygeal chordoma. A review of twenty-one cases. J Bone Joint Surg Am 75(10):1476–1484
Bergh P, Kindblom LG, Gunterberg B, Remotti F, Ryd W, Meis-Kindblom JM (2000) Prognostic factors in chordoma of the sacrum and mobile spine: a study of 39 patients. Cancer 88(9):2122–2134
Schwab JH, Healey JH, Rose P, Casas-Ganem J, Boland PJ (2009) The surgical management of sacral chordomas. Spine 34(24):2700–2704. doi:10.1097/BRS.0b013e3181bad11d
Cheng EY, Ozerdemoglu RA, Transfeldt EE, Thompson RC Jr (1999) Lumbosacral chordoma. Prognostic factors and treatment. Spine 24(16):1639–1645
Szövérfi Z, Lazary A, Bozsódi Á, Klemencsics I, Éltes PE, Varga PP (2014) Primary spinal tumor mortality score (PSTMS): a novel scoring system for predicting poor survival. Spine J 14(11):2691–2700. doi:10.1016/j.spinee.2014.03.009
Boriani S, Saravanja D, Yamada Y, Varga PP, Biagini R, Fisher CG (2009) Challenges of local recurrence and cure in low grade malignant tumors of the spine. Spine 34(22 Suppl):S48–S57. doi:10.1097/BRS.0b013e3181b969ac
Fisher CG, Goldschlager T, Boriani S, Varga PP, Fehlings MG, Bilsky MH, Dekutoski MB, Luzzati A, Williams RP, Berven S, Chou D, Reynolds JJ, Quraishi NA, Rhines LD, Bettegowda C, Gokaslan ZL (2013) A novel scientific model for rare and often neglected neoplastic conditions. Evid-Based Spine-Care J 4(2):160–162. doi:10.1055/s-0033-1357365
von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP (2007) The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 370(9596):1453–1457. doi:10.1016/S0140-6736(07)61602-X
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG (2009) Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42(2):377–381. doi:10.1016/j.jbi.2008.08.010
Enneking WF, Spanier SS, Goodman MA (1980) A system for the surgical staging of musculoskeletal sarcoma. Clin Orthop Relat Res 153:106–120
Fisher CG, Saravanja DD, Dvorak MF, Rampersaud YR, Clarkson PW, Hurlbert J, Fox R, Zhang H, Lewis S, Riaz S, Ferguson PC, Boyd MC (2011) Surgical management of primary bone tumors of the spine: validation of an approach to enhance cure and reduce local recurrence. Spine 36(10):830–836. doi:10.1097/BRS.0b013e3181e502e5
Ruggieri P, Angelini A, Pala E, Mercuri M (2012) Infections in surgery of primary tumors of the sacrum. Spine 37(5):420–428. doi:10.1097/BRS.0b013e3182213a44
Tokuhashi Y, Matsuzaki H, Toriyama S, Kawano H, Ohsaka S (1990) Scoring system for the preoperative evaluation of metastatic spine tumor prognosis. Spine 15(11):1110–1113
Treatment of spinal metastases and MSCC (2008) In: NICE Clinical Guidelines, No. 75. Metastatic spinal cord compression: diagnosis and management of patients at risk of or with metastatic spinal cord compression National Institute for Health and Clinical Excellence: Guidance, Cardiff, p 29
Peduzzi P, Concato J, Feinstein AR, Holford TR (1995) Importance of events per independent variable in proportional hazards regression analysis. II. Accuracy and precision of regression estimates. J Clin Epidemiol 48(12):1503–1510
Smoll NR, Gautschi OP, Radovanovic I, Schaller K, Weber DC (2013) Incidence and relative survival of chordomas: the standardized mortality ratio and the impact of chordomas on a population. Cancer 119(11):2029–2037. doi:10.1002/cncr.28032
Acknowledgments
We are grateful to the collaborating centers’ local clinical research personnel and support staff for their active participation. This study was organized and funded by AOSpine International, through the AOSpine Knowledge Forum Tumor, a pathology-focused working group of up to ten international spine experts acting on behalf of AOSpine in the domain of scientific expertise. Study support was provided directly through AOSpine’s Research department and AO’s Clinical Investigation and Documentation unit.
Conflict of interest
The authors have no financial conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
P. P. Varga and Z. Szövérfi are co-first authors.
Rights and permissions
About this article
Cite this article
Varga, P.P., Szövérfi, Z., Fisher, C.G. et al. Surgical treatment of sacral chordoma: prognostic variables for local recurrence and overall survival. Eur Spine J 24, 1092–1101 (2015). https://doi.org/10.1007/s00586-014-3728-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-014-3728-6