Abstract
Purpose
Eight patients treated with kinematically-aligned (KA) total knee arthroplasty (TKA) presented with tibial component failure. We determined whether radiographic measurements and clinical characteristics are different between patients with and without tibial component failure to identify mechanisms of failure and strategies to reduce the risk.
Methods
Out of 3,212 primary TKAs (2,725 TKAs with a two-year minimum follow up), of which all were performed with KA, eight patients presented with tibial component failure. Radiographic measurements, clinical characteristics (e.g. age, gender, BMI, etc.), revision surgical records, and Oxford knee scores were compared to control cohort patients matched 1:3.
Results
Tibial component failure presented at an average of 28 ± 15 months after primary TKA. Patients with tibial component failure had a 6 kg/m2 greater body mass index (p = 0.034) and 5° greater posterior slope of the tibia component (p = 0.002) than controls. Final follow-up averaged 56 ± 19 months after the primary TKA and 28 ± 24 months after the revision TKA. The final Oxford knee score was 39 ± 4.6 for patients with tibial component failure and 44 ± 6.5 for the controls (p = 0.005).
Conclusions
The incidence of tibial component failure after KA TKA was 0.3% and was caused by posterior subsidence or posterior edge wear and not varus subsidence. The strategy for lowering the risk of tibial component failure when performing KA is to set the tibial component parallel to the flexion-extension plane (slope) and varus-valgus plane of the native joint line.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aseptic tibial component failure from either subsidence of the tibial baseplate or insert wear accounts for approximately 2% of revisions after mechanically-aligned (MA) total knee arthroplasty (TKA), and is associated with varus alignment of the tibial component, high body mass index (BMI), short-keeled tibial baseplates, and young age of patients [1,2,3,4]. A common mechanism of failure is varus subsidence of the tibial baseplate, which results from high medial loads that subsequently cause medial bone collapse [2, 4]. In the coronal plane, mechanical alignment strives to set the tibial component perpendicular to the mechanical axis of the tibia as varus deviation from this target increases the risk of tibial component failure, especially in the obese patient [4].
Kinematic alignment has gained interest in the last few years because of some recent randomized trials, and a national multicenter study showed that patients treated with kinematic alignment using patient-specific instrumentation reported significantly better pain relief, function, flexion and a more normal-feeling knee than patients treated with mechanical alignment [5,6,7,8,9]. In contrast to mechanical alignment, the target for kinematic alignment is to set the tibial component tangent to the varus-valgus plane and parallel to the posterior slope of the native proximal tibial joint [10, 11]. Although, kinematic alignment has a high rate of implant survival at two, three, and six-years [5, 6, 9, 12, 13], there are concerns that kinematic alignment predisposes the tibial component to high medial loads and varus failure because 75–80% are set in varus with respect to the mechanical axis of the tibia (Fig. 1) [14, 15].
Composite shows that kinematic alignment (KA) of a primary TKA (right) restored the alignments of the limb, distal femoral joint line, and proximal tibial joint line of the arthritic knee to that of the contralateral ‘native’ or normal leg (left). Aligning the components to the joint lines of the native knee aligns the tibial component in varus, creating concerns that KA predisposes the tibial component to varus failure [14, 15]
A database of a single surgeon’s consecutive series of 3,212 kinematically-aligned TKAs treated over a nine-year period with 2,725 TKAs having a follow up of a minimum of two years contained eight patients that presented with tibial component failure. Because a series of patients with tibial component failure after kinematic alignment has not been reported, the present study determined whether radiographic measurements and clinical characteristics (e.g. age, sex, BMI, etc.) are different between patients with and patients without tibial component failure to identify mechanisms of failure and strategies that might reduce their risk.
Material and methods
With Institutional Review Board Approval (IRB 813565–1), a retrospective review of a single surgeon’s database was performed containing all patients treated with a primary TKA between January 2006 and January 2015. Eight patients out of 2,725 primary kinematically-aligned TKAs with a minimum follow-up time from surgery of two years were identified with tibial component failure. Kinematic alignment was performed on each knee with one of four brands of cemented cruciate-retaining implants unless the posterior cruciate ligament was torn pre-operatively or inadvertently damaged intra-operatively in which case a cruciate-substituting implant was used. The indications were (1) disabling knee pain and functional loss unresolved with non-operative treatment modalities; (2) radiographic evidence of Kellgren-Lawrence Grade 2, 3 or 4 arthritic change or osteonecrosis; (3) any severity of varus or valgus deformity; (4) and any severity of flexion contracture. Patients with prior femoral fracture, tibial fracture, and high tibial osteotomy were included. The implant brand, dates of use, and surgical technique (patient-specific or manual instruments) for all kinematically aligned TKAs were recorded. There were 821 knees treated with Vanguard CR (Zimmer Biomet, Warsaw, IN), 1368 with Triathlon CR (Stryker, Inc., Mahwah, NJ), 489 with Sigma CR (Depuy, Inc. Warsaw, IN), 487 with Persona CR (Zimmer Biomet, Warsaw, IN), and 47 with cruciate-substituting implants (Table 1). Standard-length tibial components were used without stem extensions. Characteristics of the eight patients that presented with tibial component failure are listed in Table 2. A control cohort of patients without tibial component failure was randomly selected and matched 3:1 to patients with tibial component failure based on date of surgery (± 3 months), age (± 10 years), sex, knee deformity (varus or valgus), and implant brand (Table 3).
Kinematic alignment was performed with patient-specific instrumentation (PSI) in 995 knees (OtisMed Corporation, Alameda, CA, USA) until October 2009 and manual instruments were used in 2,217 knees with use of previously-described techniques [7, 11]. Both surgical techniques had the goal of setting the femoral component tangent to the distal and posterior native joint lines of the knee. The patient-specific instrumentation technique used a custom femoral cutting guide [12, 16]. The manual instrument technique used a distal offset cutting block to set the flexion-extension, varus-valgus, and proximal-distal positions of the femoral component [11]. Restoration of the distal and posterior native joint lines of the femur was achieved by adjusting the calipered measured thickness of the distal and posterior femoral resections until they equaled the thickness of the distal and posterior medial and lateral femoral condyles of the femoral component within ±0.5 mm after compensating for cartilage wear and kerf [11, 17, 18]. Both surgical techniques set the internal-external rotation of the A-P axis of the tibial component parallel to the flexion-extension plane of the extended knee and set the tibial component tangent to the varus-valgus plane and slope (i.e. flexion-extension) of the native proximal tibial joint line (Fig. 2) [11, 18]. Kinematic alignment with manual instruments set the slope of the tibial component by adjusting the inclination of an angel wing placed in the saw slot until parallel to the slope of the medial joint line [10, 11]. The varus-valgus plane of the tibial cut was fine-tuned without release of soft tissues until the varus-valgus laxity with trial components was negligible in full extension as in the native knee [11, 19]. All components were cemented. A post-operative X-ray of the operated knee was done in the recovery room, and on the day of discharge an anteroposterior, rotationally controlled, long-leg CT scanogram of the limb was obtained. Beginning in January 2010, axial CT scans of the knee were obtained with use of a previously described technique and were available for six of eight patients with tibial component failure and 18 of 24 patients in the matched control cohort [17, 18, 20].
Intra-operative photographs of a right knee with a varus deformity in 90° of flexion show the measurement of the anterior offset of the tibia from the worn distal medial articular surface of the femur at the time of exposure (left) and with best-fitting trial components (right). Adjusting the anterior-posterior slope and the thickness of the tibial component until the offset of the anterior tibia from the distal medial femoral condyle matches that of the knee at the time of exposure is used to restore the slope of the native proximal tibial joint line [10, 11]
Tibial component loosening was diagnosed by patient complaints of an insidious onset of new pain and radiographs showing posterior subsidence of the tibial component. Posterior wear of the tibial insert was diagnosed by patient complaints of an insidious onset of rotatory instability confirmed by physical examination, and radiographs showing rotatory subluxation or dislocation of the tibial component on the femoral component. For each patient with tibial component failure, the history and clinical presentation, physical examination findings, and non-surgical treatment and operative management from the patient record were recorded (Table 2).
The post-operative radiographic measurements and clinical characteristics were analyzed to determine differences between the TKAs with and without tibial component failure. Seven post-operative radiographic measurements were compared including flexion-extension of the femoral component, varus-valgus angle of the femoral component to the mechanical and anatomic axis of the femur, hip-knee-ankle angle of the limb, varus-valgus angle of the tibial component to the mechanical axis of the tibia, slope of the tibial component to the anatomic axis of the tibia, and internal-external rotation of the tibial component on the femoral component using previously described techniques (Fig. 3) [13, 16,17,18, 21]. One author (AJN) blinded to the patient group measured each radiographic measurement using previously described and validated techniques with use of image-analysis software (OsiriX Imaging Software, http://www.osirix-viewer.com) [20] (Fig. 3). Eight pre-operative clinical characteristics were compared including age, sex, body mass index (kg/m2), range of motion, varus-valgus deformity, and function scores (Table 3).
Composite shows the radiographic measurements from computer tomographic scanograms and axial views which are: flexion-extension of the femoral component to the anatomic axis of the distal femur (A), varus-valgus angle of the femoral component to the anatomic and mechanical axes of the femur (B & C), hip-knee-ankle angle of the limb (D), varus-valgus angle of the tibial component to the mechanical axis of the tibia (E), slope of the tibial component to the anatomic axis of the proximal tibia (F), and internal-external rotation of the tibial component on the femoral component (G)
Statistical analysis
The intraclass correlation coefficient (ICC) was computed to quantify the reproducibility of the measurements of the seven radiographic parameters made on ten randomly-selected imaging studies by two observers (AJN and RAH) independent from the treating surgeon. The range of the ICCs from 0.84 to 0.99 indicated excellent (> 0.9) to good (0.75–0.90) agreement [22].
Continuous variables were reported as mean ± standard deviation (SD) or median (range), and discrete variables were reported as number (percentage). To determine whether a clinical or radiographic characteristic was associated with tibial component failure, the significance of the difference of each characteristic between the tibial component failure and matched control cohort groups was assessed with either a Wilcoxon–Mann-Whitney test for continuous and discrete variables, or a Chi-square test for categorical variables (JMP, 12.0.1, http://www.jmp.com). Significance was p < 0.05.
Results
Of the total study population of 2,725 consecutive primary kinematically aligned TKAs with a minimum time from surgery of two years, 2678 were performed with cruciate retaining implants and 47 with a posterior cruciate substituting implants. The posterior cruciate substituting implant was used when there was a pre-existing tear (N = 6) or inadvertent damage to the posterior cruciate ligament at the time of surgery (N = 41).
Eight patients presented with tibial component failure from either posterior subsidence of the baseplate (N = 5) or posterior edge wear of the polyethylene insert (N = 3) (Table 2). The onset of tibial component failure was insidious and atraumatic and presented at an average of 28 ± 15 months from the primary TKA. Five patients with posterior subsidence of the tibial baseplate and one with posterior edge wear of the insert had revision using a longer stemmed tibial component with retention of the cruciate retaining femoral component and preservation of the posterior cruciate ligament. Two patients with posterior edge wear had an exchange using a thicker insert. The proportion of failures per implant brand was five of 1,368 Triathlon CR (3 posterior subsidence, 2 posterior-lateral edge wear of insert), two of 821 Vanguard CR (1 posterior subsidence, 1 posterior-medial edge wear of insert), and one of 489 Sigma CR (1 posterior subsidence). Patient-specific instrumentation was used in one patient in whom the tibial baseplate was errantly placed in anterior or reverse tibial slope and who subsequently developed posterior subsidence and in one patient with posterior medial wear of the insert. Manual instrumentation was used in the other six patients.
Comparisons of the seven post-operative radiographic measurements between the patients with tibial component failure and the matched control cohort (Table 4) showed slope of the tibial component was the one of seven radiographic measurements with a significant difference. Patients with tibial component failure had 5° greater posterior slope (mean 11.2 ± 3.1°, p = 0.002) after excluding the one patient that had a 10° anterior or reverse slope from use of a patient-specific guide.
The comparison of the eight preoperative clinical characteristic between the patients with tibial component failure and the matched control cohort are listed in Table 3. Preoperatively, patients with tibial component failure had a 6 kg/m2 greater BMI (mean 36 ± 5.4 kg/m2, p = 0.034), 15° less knee flexion (mean 101 ± 15.5°, p = 0.006), and 14 point lower Knee Society function score (mean 44 ± 12.4, p = 0.046) than the matched control cohort (Table 3). The time from primary TKA to final follow-up averaged 56 ± 19 months. The time from revision surgery to final follow-up averaged 28 ± 24 months. At final follow-up, the Oxford knee score averaged 39 ± 4.6 for patients with tibial component failure, which was five points lower than the average of 44 ± 6.5 for the controls (p = 0.005).
Discussion
This case control study was performed because there are concerns that kinematic alignment predisposes the tibial component to high medial loads and varus failure because 75–80% are set in varus with respect to the mechanical axis of the tibia (Fig. 1) [14, 15]. Accordingly, differences in radiographic measurements and clinical characteristics between patients with and without tibial component failure were determined to identify mechanisms of failure and propose strategies that might reduce the risk. The most important findings were that patients with a kinematically-aligned TKA: (1) have a low 0.3% incidence of early failure, (2) present with pain or instability from the mechanisms of posterior subsidence of the tibial baseplate or posterior edge wear of the insert, (3) are associated with 5° greater posterior slope of the tibial component, 6 kg/m2 greater pre-operative BMI and 15° less pre-operative flexion than a matched control cohort, and (4) that revision is successful in the short term by implanting a long-stemmed tibial component with less posterior slope or exchange to a thicker insert with retention of the cruciate-retaining femoral component.
Three limitations should be discussed that might affect the generalization of the study findings. First, the inadvertent exclusion of patients treated elsewhere for tibial component failure or lost to follow-up after two years would have underestimated the 0.3% incidence of tibial component failure in the present study. The early failure rate of 0.3% was computed using all patients that returned to our centre for evaluation for tibial component failure out of 2,725 primary kinematically-aligned TKAs that had a minimum follow up time from surgery of two years. A second limitation was whether the power, based on sample sizes of eight with tibial component failure and 24 controls, was adequate to conclude that the 2° greater varus alignment of the tibial component to the tibial mechanical axis in the failure group was not a clinically important difference relative to the control group. The analysis included one patient with 10° varus and 12° posterior slope of the tibial component, which skewed the average to 2°. Paradoxically, the failure mechanism in this patient was posterior-lateral wear of the insert, which suggests the precipitant was the 12° posterior slope and not the 10° varus alignment. A post-hoc power analysis computed an adequate power of 0.86 using a clinically important difference of 3° based on an inter- observer analysis that reported 66% of repeated measurements are within 3°, sample sizes of eight and 24, standard deviation of 2.7°, and an alpha of 0.05 [23]. Hence, these observations and analyses suggest that the 2° greater varus alignment in the group with tibial component failure was not a precipitant of posterior subsidence of the tibial baseplate or posterior edge wear of the insert. A third limitation was whether the use of posterior cruciate substituting implants influences the applicability of the results to posterior cruciate retaining implants. However, as only 1.5% of implants used in this study were posterior cruciate substituting, the applicability of the results for posterior retaining implants should not be affected.
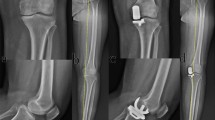
The average onset at 28 months, the initiation of the instability by an atraumatic mechanism, radiographic documentation of posterior subsidence of the tibial baseplate, and explant observations of posterior edge wear of the insert suggest that posterior tibial overload and not medial tibial overload precipitated tibial component failure. The association with posterior tibial overload is supported by the 5° greater posterior slope and a higher BMI than the matched control cohort. Greater posterior slope loosens the flexion gap and obesity compresses posterior soft-tissues especially in flexion, which in combination enables anterior translation of the tibial component on the femoral component and higher loading of the posterior portion of the tibial component (Fig. 4). For the seven patients with tibial component failure (excluding the one with reverse slope), a post-hoc comparison showed that the average post-operative slope of the tibial component of 11 ± 3.1° was 7° greater than the average pre-operative tibial slope of the native or osteoarthritic knee of 4 ± 2.5° (p < 0.0009). In these patients, surgeon error inadvertently malpositioned the tibial component in excessive flexion with respect to the native joint line. The target for minimizing the risk of tibial component failure when performing kinematic alignment is to match the flexion of the tibial component to the slope of the native knee.
Composite of radiographic images of a male patient, BMI of 40 kg/m2, treated with KA TKA. Figures parts A and B show the left limb. Tibial component in 14° posterior slope and neutral hip-knee-ankle angle. Figure part C shows, at 34 months, 4° increased posterior slope from 14° to 18°, reactive sclerosis of the posterior tibia indicative of overload (*), and anterior translation of the tibial component with posterior edge loading by the femoral component. The left leg in figure part D was imaged 36 months after revision with a long-stem tibial component and retention of the cruciate retaining femoral component. The right leg in figure part D was imaged 18 months after the revision of the left knee and shows a primary KA TKA, using a different implant brand and a long-stem tibial component based on prior history of posterior subsidence of the tibial baseplate
Both the lack of a varus mechanism and a low incidence of 0.3% of early tibial component failure after kinematic alignment are notable since varus overload causing medial bone collapse and varus subsidence of the tibial component are responsible for a comparable if not higher incidence of 0.7% revisions after mechanical alignment [2]. Differences in alignment targets and levels of soft tissue release between these mechanical and kinematic alignment techniques provide insight. The varus-valgus slope of the tibia is cut to achieve negligible varus-valgus laxity in the extended knee during clinical assessment with trial components, which replicates the laxity of the native knee in extension [19]. The posterior slope of the tibial component is fine-tuned so that a caliper measurement of the anterior offset of the tibia from the femur with trial implants with the knee in 90° of flexion matches the knee at the time of exposure after compensating for medial cartilage wear [10, 11]. These two intraoperative quality assurance steps minimize the proportion of knees requiring soft-tissue releases when performing kinematic alignment [5, 6, 9, 16]. Hence, the restoration of the native joint lines and the minimal release of soft tissues may explain the low incidence of tibial component failure and notable absence of varus subsidence of the tibial baseplate after kinematic alignment [11,12,13, 24].
In summary, the low 0.3% of incidence of early tibial component failure and the mechanisms of either posterior subsidence or posterior edge wear of the insert and not varus subsidence, and kinematic alignment’s target of restoring the native joint lines should be of interest to surgeons focused on setting a limit to the varus angle of the tibial component with respect to the mechanical axis of the tibia.
References
Abdel MP, Bonadurer GF 3rd, Jennings MT, Hanssen AD (2015) Increased aseptic tibial failures in patients with a BMI ≥35 and well-aligned total knee arthroplasties. J Arthroplast 30(12):2181–2184. doi:10.1016/j.arth.2015.06.057
Berend ME, Ritter MA, Meding JB, Faris PM, Keating EM, Redelman R, Faris GW, Davis KE (2004) Tibial component failure mechanisms in total knee arthroplasty. Clinical Orthop Rel Res (428):26–34
Gunst S, Fessy MH (2015) The effect of obesity on mechanical failure after total knee arthroplasty. Ann Transl Med 3(20):310. doi:10.3978/j.issn.2305-5839.2015.10.37
Ritter MA, Davis KE, Meding JB, Pierson JL, Berend ME, Malinzak RA (2011) The effect of alignment and BMI on failure of total knee replacement. J Bone Joint Surg Am 93(17):1588–1596. doi:10.2106/JBJS.J.00772
Calliess T, Bauer K, Stukenborg-Colsman C, Windhagen H, Budde S, Ettinger M (2016) PSI kinematic versus non-PSI mechanical alignment in total knee arthroplasty: a prospective, randomized study. Knee Surg Sports Traumatol Arthrosc. doi:10.1007/s00167-016-4136-8
Waterson HB, Clement ND, Eyres KS, Mandalia VI, Toms AD (2016) The early outcome of kinematic versus mechanical alignment in total knee arthroplasty: a prospective randomised control trial. Bone Joint J 98-B(10):1360–1368. doi:10.1302/0301-620X.98B10.36862
Dossett HG, Estrada NA, Swartz GJ, LeFevre GW, Kwasman BG (2014) A randomised controlled trial of kinematically and mechanically aligned total knee replacements: two-year clinical results. Bone Joint J 96-B(7):907–913. doi:10.1302/0301-620X.96B7.32812
Nam D, Nunley RM, Barrack RL (2014) Patient dissatisfaction following total knee replacement: a growing concern? Bone Joint J 96-B(11 Supple A):96–100. doi:10.1302/0301-620X.96B11.34152
Young SW, Walker ML, Bayan A, Briant-Evans T, Pavlou P, Farrington B (2017) The Chitranjan S. Ranawat Award: no difference in 2-year functional outcomes using kinematic versus mechanical alignment in TKA: a randomized controlled clinical trial. Clin Orthop Relat Res 475(1):9–20. doi:10.1007/s11999-016-4844-x
Howell SM, Hull ML (2016) Kinematic alignment in total knee arthroplasty. In: Scott S (ed) Insall and Scott surgery of the knee. 5th edn. Elsevier, Philadelphia
Howell SM, Papadopoulos S, Kuznik KT, Hull ML (2013) Accurate alignment and high function after kinematically aligned TKA performed with generic instruments. Knee Surg Sports Traumatol Arthrosc 21(10):2271–2280. doi:10.1007/s00167-013-2621-x
Howell SM, Howell SJ, Kuznik KT, Cohen J, Hull ML (2013) Does a kinematically aligned total knee arthroplasty restore function without failure regardless of alignment category? Clin Orthop Relat Res 471(3):1000–1007. doi:10.1007/s11999-012-2613-z
Howell SM, Papadopoulos S, Kuznik K, Ghaly LR, Hull ML (2015) Does varus alignment adversely affect implant survival and function six years after kinematically aligned total knee arthroplasty? Int Orthop 39(11):2117–2124. doi:10.1007/s00264-015-2743-5
Klatt BA, Goyal N, Austin MS, Hozack WJ (2008) Custom-fit total knee arthroplasty (OtisKnee) results in malalignment. J Arthroplast 23(1):26–29. doi:10.1016/j.arth.2007.10.001
Oussedik S, Abdel MP, Cross MB, Haddad FS (2015) Alignment and fixation in total knee arthroplasty: changing paradigms. Bone Joint J 97-B(10 Suppl A):16–19. doi:10.1302/0301-620X.97B10.36499
Dossett HG, Swartz GJ, Estrada NA, LeFevre GW, Kwasman BG (2012) Kinematically versus mechanically aligned total knee arthroplasty. Orthopedics 35(2):e160–e169. doi:10.3928/01477447-20120123-04
Nedopil AJ, Howell SM, Rudert M, Roth J, Hull ML (2013) How frequent is rotational mismatch within 0 degrees ±10 degrees in kinematically aligned total knee arthroplasty? Orthopedics 36(12):e1515–e1520
Nedopil AJ, Howell SM, Hull ML (2016) Does malrotation of the tibial and femoral components compromise function in kinematically aligned total knee arthroplasty? Orthop Clin North Am 47(1):41–50. doi:10.1016/j.ocl.2015.08.006
Roth JD, Howell SM, Hull ML (2015) Native knee laxities at 0 degrees, 45 degrees, and 90 degrees of flexion and their relationship to the goal of the gap-balancing alignment method of total knee arthroplasty. J Bone Joint Surg Am 97(20):1678–1684. doi:10.2106/JBJS.N.01256
Howell SM, Kuznik K, Hull ML, Siston RA (2010) Longitudinal shapes of the tibia and femur are unrelated and variable. Clin Orthop Relat Res 468(4):1142–1148. doi:10.1007/s11999-009-0984-6
Nedopil AJ, Howell SM, Hull ML (2017) What clinical characteristics and radiographic parameters are associated with patellofemoral instability after kinematically aligned total knee arthroplasty? Int Orthop 41(2):283–291. doi:10.1007/s00264-016-3287-z
Indrayan A (2013) Methods of clinical epidemiology. In: SARDaGMW (ed) Springer Series on Epidemiology and Public Health. Springer-Verlag Berlin Heidelberg, p 24. doi:10.1007/978-3-642-37131-8_2
Feldman DS, Henderson ER, Levine HB, Schrank PL, Koval KJ, Patel RJ, Spencer DB, Sala DA, Egol KA (2007) Interobserver and intraobserver reliability in lower-limb deformity correction measurements. J Pediatr Orthop 27(2):204–208. doi:10.1097/01.bpb.0000242441.96434.6f
Gu Y, Roth JD, Howell SM, Hull ML (2014) How frequently do four methods for mechanically aligning a Total knee arthroplasty cause collateral ligament imbalance and change alignment from normal in white patients? J Bone Joint Surg Am 96(12):e101
Acknowledgements
We wish to thank Robert Harper, MD for making the radiographic measurements used to compute the intraclass correlation coefficients.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors have not received grant support or research funding and do not have any proprietary interests in the materials described in the article.
Conflict of interest
The authors declare no competing interests.
Funding
There is no funding source.
Ethical approval
An institutional review board approved this study (IRB 813565–1). For this type of study formal consent is not required.
Rights and permissions
About this article
Cite this article
Nedopil, A.J., Howell, S.M. & Hull, M.L. What mechanisms are associated with tibial component failure after kinematically-aligned total knee arthroplasty?. International Orthopaedics (SICOT) 41, 1561–1569 (2017). https://doi.org/10.1007/s00264-017-3490-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-017-3490-6