Abstract
Objectives
To prospectively assess the utility of transabdominal ultrasound in surveillance of known pancreatic cystic lesions (PCL) using same day MRI as reference standard.
Methods
In an IRB-approved study with written informed consent, patients with known PCL underwent pancreas US on same day as surveillance MRI. US was performed blinded to same date MRI results. Transverse (TR), antero-posterior (AP), cranio-caudal (CC), and longest any plane diameter, were measured for each PCL at US and MRI. Visualization was correlated with patient (weight, abdominal diameter, thickness of abdominal fat, sex) and cyst (location, size, internal complexity) factors.
Results
252 PCLs evaluated in 57 subjects (39 females; mean age 67 (range 39–86) yrs). Mean maximum PCL diameter 8.5 (range 2–92) mm. US identified 100% (5/5) of cysts ≥3 cm; 92% (12/13) ≥2 and <3 cm; 78% (43/55) ≥1 and <2 cm; 35% (27/78) ≥5 mm and <1 cm; and 16% (16/101) <5 mm. US visualization correlated with PCL location (<0.0001), size (p < 0.0001), patient gender (p = 0.005), participation of attending radiologist (p = 0.03); inversely with patient weight (p = 0.012) and AP abdominal diameter (p = 0.01).
Conclusion
Many PCLs are visualized and accurately measured at follow-up with transabdominal ultrasound. Visualization correlates with lesion size, location, patient sex, weight, and abdominal diameter.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Incidentally found pancreatic cystic lesions (PCLs) are commonly encountered; the prevalence of pancreatic cysts found on CT and MRI varies between 2.6% [1] to 19.6% [2]. The vast majority of incidentally found PCLs represent small side branch intraductal papillary mucinous neoplasms (IPMN) with indolent behavior [3]. However, some IPMN lesions can progress to invasive malignancy, sometimes after years of stability at observation, prompting recommendations of surveillance [2,3,4,5,6,7,8,9,10,11,12,13, 27]. The current standard of practice is to follow these cysts with MR imaging [14], but there are multiple drawbacks of MRI as a primary modality for this purpose: MR examinations are lengthy and can be uncomfortable for patients, particularly those with claustrophobia; some patients may not have access to MRI, or may not be able to undergo MRI due to contraindications. Furthermore, MR is a relatively costly method. Despite the efforts of several expert panels and clinical societies to develop guidelines [4, 5, 15,16,17,18, 27], full consensus has not yet been achieved as to the appropriate frequency of imaging surveillance of PCLS as well as overall length of surveillance required [6, 19], and at present, the cumulative imaging burden of pancreatic cyst surveillance can be substantial both for patients and for health care systems [20]. For these reasons, an alternative method of imaging follow-up for PCL would be beneficial.
Ultrasound is a widely available and relatively low cost imaging modality that does not expose patients to ionizing radiation. It offers high contrast resolution for fluid containing structures and as a result, is commonly used in clinical practice for evaluation of cysts in other organs (liver, kidney, ovaries), and has been used successfully in the evaluation of features of pancreatic cystic lesions [21]. The potential benefits of ultrasound for follow-up of pancreatic cysts could include reduced cost, easier access, and fewer limitations in patient participation such as those with MR restricted devices/hardware, claustrophobia or MR scanner body size limitations. However, it is acknowledged by practitioners of ultrasound that overlying bowel gas and reduced acoustic penetration related to patient body habitus may result in reduced visualization of portions of the gland. The prevalence of pancreatic cysts at transabdominal ultrasound in the general population has been investigated previously [22, 23]. However, to the best of our knowledge, no prior studies have prospectively evaluated the utility of transabdominal ultrasound as an alternative to MRI in follow-up imaging of pancreatic cystic lesions.
The purpose of this study is to prospectively evaluate the utility of targeted transabdominal ultrasound of the pancreas for surveillance of PCLs known to exist from prior MR imaging, using same day MRI as reference standard.
Methods
Subjects
IRB approval was obtained for this HIPAA-compliant prospective study. Prior to study initiation, a power analysis was conducted with the assumption that the detection rate for cysts on US would be 50%, which indicated that 225 cases would be needed to achieve 0.85 power for a 95% confidence interval of ±10% width. Patients scheduled for routine MRI follow-up of pancreatic cystic lesions from March 2014 through February 2015 were identified through departmental scheduling records. Exclusion criteria included known pancreatic malignancy, collections related to acute or chronic pancreatitis, post-operative status, pregnancy, non-English language proficiency, inability to consent to research due to need for sedation to facilitate MRI, and inability to contact patient prior to MRI to offer participation. Consecutive eligible patients were invited to participate in the study. Those who agreed to participate were then scheduled for ultrasound on the same date as their clinical MR examination. Written informed consent was obtained prior to all ultrasound examinations.
Study imaging protocol
Prior to the research ultrasound, the prior MRI examination prompting follow-up was reviewed by a radiologist co-investigator (either BT or CS, each with 1 year of experience in abdominal imaging). A worksheet was completed for each case which used a diagrammatic format to indicate the number of PCLs, PCL size, general morphology, and location of cysts within the pancreas on prior MRI (Fig. 1). The worksheet was provided to the person performing the research ultrasound and used as reference during the ultrasound examination. The ultrasound operator was also able to view the prior MRI before beginning the US examination in order to simulate the conditions of a follow-up examination. However, ultrasound examinations were performed in a blinded fashion with respect to images and reports of the same date MRI examination.
Pancreatic cystic lesion (PCL) worksheet. Worksheets were created for each case in order to simulate as closely as possible the follow-up conditions available in MRI (where PCLs are directly compared with prior MR examinations on PACS) for the US environment. PCL size on prior MRI was provided and location and general morphology were indicated by drawing on the diagram for each PCL
Ultrasound imaging was performed with standard clinical ultrasound machines (IU22, Philips Healthcare, Best, Netherlands (n = 36); Epiq, Philips Healthcare, Best, Netherlands (n = 20), and Voluson, GE, Waukesha, WI (n = 1). Initial scanning was performed with curvilinear array 5-1 MHz transducers on IU-22 units, and with C9-2 transducers on EPIQ units; additional linear array (L9-3 and L12-5) and sector (S4-1) transducers, could be subsequently utilized per operator discretion. In IU-22 examinations, there were 4 instances of additional transducer use, including linear array (L9-3, n = 2 and L12-5, n = 1) and sector (S4-1, n = 1) transducers, and in EPIQ examinations there was one instance of additional use of a C5-1 transducer. The single examination performed using a Voluson ultrasound machine utilized a 2–8 MHz curvilinear array transducer. US unit selection was based upon the site of service and availability of machines. In order to reflect general practice, examinations were performed by available staff, inclusive of sonographers, advanced practice sonographers (APS), radiology fellows, and radiologist attending co-investigators (MS, with 7 years of experience in abdominal ultrasound, and RK, with 39 years of experience in abdominal ultrasound). Order of scan operator was determined according to general practice at our institution, i.e., initial scanning was routinely performed by the sonographer or APS with additional scanning by radiology trainee and/or attending obtained in cases in which lesions were not visualized at initial imaging; in some cases, only a radiology fellow or attending scanned the patient due to staff availability. Ultrasound imaging included grayscale and color and/or spectral Doppler images of the pancreas in transverse, sagittal and oblique planes. Color Doppler images were provided of all cysts to ensure that blood vessels were not inadvertently mistaken for cysts. Measurements of all visible cysts were obtained and recorded from transverse and sagittal images in transverse (TR), antero-posterior (AP), and cranio-caudal (CC) dimensions. In addition, oblique images were obtained by angling the transducer to obtain the longest diameter for each cyst in any dimension. Examinations were performed initially in the supine position and supplemented with position changes (i.e. decubitus or seated upright positioning) as necessary to displace bowel gas. If the pancreas remained partially obscured following repositioning, patients were asked to drink a small quantity (4–8 oz) of water and imaging was repeated. Specific training in performance of pancreatic US for study purposes was not provided. Duration of research US examination was recorded as time interval between time stamps on first image and last image.
MRI was performed for clinical purposes according to standard department protocol for follow-up of pancreatic cystic lesions, at 1.5 T (GE, Waukesha, WI; Siemens, Malvern, PA) or 3 T (GE, Waukesha, WI) including the following sequences: 3 plane localizer, coronal SSFSE /HASTE, axial SSFSE/HASTE, coronal thick slab SSFSE/HASTE with 5 radially oriented prescriptions, and respiratory-triggered coronal 3D MRCP with maximum intensity projection reconstruction (sequence parameters listed in Appendix 1). No intravenous contrast was administered according to departmental protocol for follow-up of known pancreatic cystic lesions, but patients received oral preparation with 1.0 mL gadobutrol (Bayer, Whippany, NJ) mixed with 50 mL water 15 min prior to scanning.
Clinical data analysis
Patient gender, age, height, and weight were recorded; patient weight was recorded for both the date of the research US/MRI, as well as on the date of the prior MRI prompting follow-up.
Image analysis
Image analysis was performed by two readers in consensus (MS, with 7 years, and CS, with 1 year of experience in abdominal imaging) using PACS (GE, Centricity, Boston, MA). For both the MRI examination on the date of the research US as well as the prior MRI examination, AP abdominal diameter, and AP thickness of abdominal fat were measured using the axial SSFSE/HASTE image obtained at the level of the splenic and superior mesenteric vein confluence. Thickness of fat was obtained by placing calipers on the subcutaneous fat perpendicular to the abdominal wall at the midline using the linea alba as reference. Abdominal diameter was obtained by placing calipers from the anterior skin surface at midline, to the intersection with a line drawn across the most posterior skin surfaces of the back (Fig. 2).
Method of measurement of AP abdominal diameter and thickness of subcutaneous fat. Measurements were made at the level of the confluence of the SMV and splenic vein. AP diameter was measured by placing a line across the most posterior surface of the patient’s back, and measuring along the length of a line drawn perpendicular to this to the anterior skin surface at the midline. Subcutaneous fat thickness was measured by drawing a line parallel to this from the skin surface to the interface of subcutaneous fat and abdominal muscle fascia (short line)
PCL measurements in TR, AP, and CC dimensions and the longest diameter obtainable in any plane were recorded for each PCL in the pancreas, for both US and MRI. US dimensions were recorded from measurements made at time of scanning, while MR measurements were made on PACS in a separate session with consensus to ensure same-cyst comparisons. In patients with numerous PCLs, a maximum of 10 PCLs were measured. Presence or absence of (pseudo)septations, defined as thin linear features within a PCL (echogenic linear foci at US; foci of linear hypointensity with respect to PCL fluid contents at T2WI at MRI), presence or absence of internal vascularity (color signal at Doppler imaging at US) and presence or absence of nodularity, defined as rounded or irregular solid components within a PCL, were recorded for each PCL.
Recommendation for change in management (defined as recommendation for consideration of endoscopic ultrasound (EUS) and/or surgical consultation, or recommendation for shortening follow-up interval for imaging evaluation) was recorded from clinical MRI report generated without knowledge of the US findings. For cases in which change in management was recommended by MRI, a determination was made as to whether the same recommendation would have been made from the US findings using the following criteria: either increase in size (defined as increase in maximum diameter of the lesion of ≥10 mm or ≥50%), or new nodularity within the PCL [24].
Statistical analysis
Descriptive statistics were calculated. Rate of PCL visualization was calculated for cyst size on a per-cyst basis according to 5 groups: cysts ≥3 cm; ≥2 and <3 cm; ≥1 and <2 cm; ≥5 mm and < 1 cm; and <5 mm. In addition, rate of visualization of all cysts in the pancreas on a per patient basis was calculated for the same 5 groups. MRI measurements were used as reference standard for PCL size. Frequency of PCL visualization at US was correlated with PCL location in the pancreas (pancreatic head, neck, body, uncinate process, and tail). Correlation of PCL visualization at US with patient gender, cyst location, presence or absence of (pseudo)septations, and US operator, were evaluated using chi-square test. Correlations of PCL visualization at US with patient age, PCL size, thickness of abdominal subcutaneous fat, patient weight, abdominal AP diameter, were assessed by rank sum test. Correlation coefficients were generated for interaction of patient weight, BMI and AP abdominal diameter. Correlation of cyst size and location as well as PCL size and presence of (pseudo)septations, were assessed by Kruskal Wallis test, along with correlation of PCL septations with PCL location using chi-square test. Logistic regression predictive models were constructed using PCL size alone, and using PCL size, patient AP abdominal diameter, PCL location, and presence of (pseudo)septations; ROC analyses were performed for each model. PCL location was coded as an ordinal variable in the order of increasing percentage of visualized cysts. In order to determine whether patient body habitus factors, which would be used to predict future suitability for US surveillance in the 4-factor model, changed between the prior and follow-up examinations, patient weight, AP abdominal diameter, and thickness of fat measurements obtained on the dates of prior MRI and follow-up MRI were compared for each patient using t-test. The analysis was performed using Matlab (Mathworks, Natick, MA). The level of significance was set at α = 0.05.
Results
PCL and patients
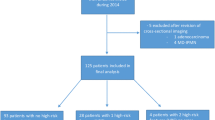
252 PCL were evaluated in 57 patients [39 (68%) females, 18 (32%) males, mean age 67 ± 10 (range 39–86) years] (Fig. 3). The mean maximum cyst diameter was 8.5 ± 9.0 mm (range 2–92 mm), median 6 mm, IQR 3–11 mm. Median number of cysts per patient was 4 (IQR 2–6). Mean interval between prior MRI examination and follow-up US/MRI examinations was 15 ± 10 (range 3– 57) months. Average duration of ultrasound examinations was 14.3 min (range 3–30 min).
Frequency of visualization of cysts and factors that correlated with cyst visualization at US
PCL were identified at ultrasound in 100% (5/5) of cysts ≥3 cm; 92.3% (12/13) ≥2 and <3 cm; 78.2% (43/55) ≥1 and <2 cm; 34.6% (27/78) ≥5 mm and <1 cm; and 15.8% (16/101) <5 mm (Figs. 4, 5, 6). Percentage of cysts visualized at US according to cyst features and location are shown in Table 1.
A, B 63-year-old man with 3 mm pancreatic cystic lesion at US and MRI. Sagittal gray scale US image (A) shows same pancreatic cystic lesion (arrow) and adjacent pancreatic duct in cross section (PD, pancreatic duct). Axial HASTE MR image (B) demonstrates the PCL (arrow) measuring 3 mm in maximum diameter with adjacent pancreatic duct. PD, pancreatic duct; SV, splenic vein
There were significant correlations between PCL visualization at US and maximum cyst size (p < 0.001), patient weight (p = 0.012), and AP abdominal diameter (p = 0.011). Patient gender was significantly correlated with cyst visualization, with PCLs seen more commonly in females (79/168; 47.0%) than males (24/84; 28.6%), p = 0.005. PCLs with internal (pseudo)septations (35/51; 68%) were more commonly visualized at US than those without (pseudo)septations (68/201; 33.8%) (p < 0.0001). No significant correlation was observed between thickness of subcutaneous abdominal fat and PCL visualization at ultrasound (p = 0.39) or between patient age and PCL visualization (p = 0.35). The participation of an attending radiologist in ultrasound scanning was significantly correlated with PCL visualization (p = 0.03).
Comparison of US size measurements with MR size measurements
The maximum diameter at US was on average smaller than the measured maximum diameter at MRI by a mean of 0.7 mm (range −9 to +4 mm). Mean matched-plane difference in cyst diameters between MRI and US were 0.9, 1.6, and 1.4 mm for TR, AP, and CC measurements, respectively. Under-measurement was slightly more common than over-measurement for US: cysts were under-measured by US in 46% and over-measured in 31% of maximum diameter measurements, respectively. Maximum diameter PCL measurements at US were within 1 mm of corresponding MR measurements in 60/103 (58.3%), within 2 mm in 79/103 (76.7%), within 3 mm in 88/103 (85.4%), within 4 mm in 95/103 (92.2%), and within 5 mm in 98/103 (95.1%).
Comparison of US feature analysis with MRI feature analysis
On MRI, 51/252 (20.2%) of PCLs contained internal (pseudo)septations and 2/252 (0.8%) PCL contained internal nodularity. In the group of lesions with (pseudo)septations at MRI that were visualized at US (35/51), 97% (34/35) of the (pseudo)septations were appreciated at US. In one case (1/103 total PCLs visualized with US; 1.0%), US reported internal (pseudo)septations which were not appreciable at MRI. There were no findings of intracystic nodularity at US. In one case, a calcification was demonstrated within a cyst at US but was not visible at MRI; the presence of the calcification was confirmed on a CT examination performed for other purposes. Nodularity was reported at MRI in two cases that are described in more detail in the analysis of change in management.
Correlations between PCL features and patient features
There was a significant correlation between PCL location and size (p < 0.0001), with cysts in the pancreatic tail noted to be smaller in size, with average maximum diameters of PCLs as follows: head, 12.4 ± 15.9 (range 2–92) mm; uncinate, 10.9 ± 7.2 mm (range 3–31) mm; neck, 13.3 ± 8.2 (range 5–28) mm; body, 7.7 ± 5.4 (range 2–28) mm; tail, 5.5 ± 3.7 (range 2–18) mm. Similarly, PCLs with and without internal (pseudo)septations differed in size, with septated cysts showing larger maximum diameters: nonseptated cysts, mean diameter 5.9 ± 4.0 mm; septated cysts, mean diameter 18.7 ± 14.1 mm (p < 0.0001).
Patient factors related to body size were clustered, with high correlation between AP abdominal diameter, weight, and BMI. Correlation coefficients ranged from 0.87 to 0.88, reflecting a high degree of interaction, as follows: weight and BMI, 0.88; weight and AP abdominal diameter, 0.87; BMI and abdominal diameter, 0.87. In addition, a significant correlation between AP abdominal diameter and patient gender was observed, with mean AP diameter larger for males (248 ± 23 mm) than for females (223 ± 41 mm) (p = 0.01).
Proportion of patients with all PCLs visualized by US
US was able to identify all PCLs in the pancreas in 13/57 (22.8%) of patients. All PCLs measuring ≥5 mm were seen in 24/51 (47.1%) patients, ≥1 cm were seen in 32/42 (76.2%) patients, and all PCLs measuring ≥2 cm were seen in 15/16 (93.8%) of patients.
Prediction of cyst visualization
AUC obtained using cyst size alone was 0.83, consistent with good ability of the model to predict visualization of PCL on US. The AUC obtained when using the four-factor model factors (cyst size, cyst location, patient AP diameter, and presence of internal (pseudo)septations) was 0.88 (Fig. 7). There was a slight but statistically significant decrease in all three measures of patient size in our study group over time as follows: weight decreased by 1.5 pounds (CI 0.57–2.37; p = 0.002); AP abdominal diameter decreased by 2.6 mm (CI 0.63–4.5; p = 0.009), and thickness of fat decreased by 0.9 mm (CI 0.54–1.33; p < 0.005).
ROC analysis for predictive modeling of PCL visualization at US. Predictive model utilizing PCL size alone is plotted in blue and predictive model utilizing four PCL and patient features (PCL size, abdominal AP diameter, PCL location, and presence or absence of internal (pseudo)septations) is plotted in red. Outcome variable for both models is whether the PCL will be visible at US. Utilizing the 4-feature model (red ROC curve) for prediction of PCL visualization at US, at prescribed sensitivities (i.e., percentage of all visible cysts correctly predicted to be visible) of 80%, 85%, and 90%, specificity (i.e., percentage of all non-visible cysts correctly predicted to be non-visible) was 82%, 65%, and 42%, respectively
Change in PCL during follow-up interval
A change in recommendation from routine screening was made at clinical MRI interpretation for 2/252 PCLs. In one case, MRI reported new possible nodularity within a PCL as well as change in maximum diameter from 14 to 20 mm, and recommended EUS. The maximum diameter obtained at US measurement for this PCL was 19 mm and no nodularity was appreciated at US; both US and MRI also noted stable internal (pseudo)septations within the lesion. Thus, US would not have resulted in identical recommendations for alteration of surveillance routine in this patient due to insufficient growth of <10 mm or 50% diameter, and lack of demonstrable nodularity. Follow-up EUS showed no nodular component within the PCL; cyst aspiration was nondiagnostic. Review of the case at multidisciplinary pancreas tumor board concluded that the PCL did not show specifically worrisome features and resulted in a recommendation to return to surveillance imaging in 6 months. Following that interval, the patient and surgeon elected to proceed with pancreatic resection due to ongoing symptoms of abdominal discomfort, anticipation of a prolonged surveillance period, and preexisting pancreatic insufficiency reducing the additional morbidity of pancreatic resection, with pathologic diagnosis of mucinous cystic neoplasm with low grade dysplasia and no invasive carcinoma. In the second case, MR showed an area of new nodularity or debris within a PCL. The PCL was not seen at US (Fig. 8). Subsequent EUS-guided FNA was nondiagnostic; the patient underwent resection with pathologic diagnosis of combined main and side branch duct type non-invasive IPMN with low to high grade dysplasia with gastric/foveolar phenotype, and no evidence of ductal adenocarcinoma.
A, B 63-year-old female with 2.4 cm known pancreatic cystic lesion not seen at US. Transverse T2W HASTE image (A) shows the 2.4 cm uncinate process PCL (arrow) with a small dependent nodule. Transverse and sagittal US images (B) show a separate PCL in the pancreatic neck also known to exist from prior MRI (long arrows), but the area of the known larger uncinate process PCL (asterisk) is obscured by bowel gas (short arrows) and cannot be assessed. SMV, superior mesenteric vein
A total of two new PCLs were seen at MRI in two patients, measuring 3 and 7 mm, and located in the pancreatic head and neck, respectively, without nodularity or internal (pseudo)septations. Neither new PCL was seen by US. In neither case did this finding result in a recommendation for departure from routine surveillance at MRI.
Discussion
In this study, we sought to determine the utility of targeted transabdominal ultrasound of the pancreas for surveillance of known PCLs. We found that the majority of PCLs could be visualized with US, particularly those ≥1 cm in size: on a per-cyst basis, 82.2% of PCLs ≥1 cm could be visualized with US, while all PCLs ≥1 cm in diameter known to exist in the pancreas can be seen in 76.2% of patients, and all PCLs ≥2 cm are seen in 93.8% of patients.
PCL visualization was strongly correlated with the size of the cyst as well as cyst location within the pancreas. Not surprisingly, larger PCLs were more likely to be visualized with ultrasound, with visualization rates varying from 100% for cysts ≥3 cm, to a low rate of 15.8% of cysts less than 5 mm in diameter.
PCL location also correlated with visualization at US, with PCLs most frequently visualized when located in the pancreatic neck (83%), followed by the pancreatic head and body (53% in both cases). This distribution of cyst visualization is similar to that reported in a recent study of the prevalence of pancreatic cysts in the general population as observed by ultrasound, in which cysts were distributed in the pancreatic head (33.6 %), uncinate (10.7 %), corpus (47.3 %), and tail (5.4 %) [22]. These findings are understandable in the context of general ultrasound practice, since the best visualized portions of the pancreas are often caudal to the costal margin, allowing direct transducer pressure to displace interposed gas. That cysts located in the pancreatic tail were least likely to be visualized (18%) is also in keeping with previously published literature [25]. This can also be explained in part by anatomic constraints, as the pancreatic tail typically resides posterior to the gastric body and transverse colon (reducing acoustic access), and above the costal margin (reducing accessibility to transducer pressure). Indeed, Sumi et al. used Global Positioning System (GPS)-like fusion technology to show that the mean real unobservable portion of the pancreas, corresponding to the portion of the pancreatic tail not accessible by ultrasound, resulted in approximately 25% of the pancreas not being visualized [25]. However, reduced cyst visualization in the pancreatic tail as we have noted in our series is likely multifactorial, since we also observed that pancreatic tail cysts were smaller in size than those occurring in other portions of the pancreas. Thus, both anatomic factors and size criteria may have contributed to reduced visualization of cysts in the tail of the pancreas. The fact that a slight preponderance of PCL were located in the tail of the pancreas in our series makes this an important area for targeted inspection.
Patient size criteria, including AP abdominal diameter, weight, and BMI, as well as patient gender, correlated with PCL visualization at US. All three measures of patient size showed high correlation with each other, suggesting that any one measure may be used for predictive purposes for cyst visualization. That cysts were seen more frequently in females than in males may be attributed in part to the statistically larger abdominal diameter of males in our study. Other factors that could be hypothesized as potential contributing features might include differences in abdominal wall compliance or proportion of abdominal fat between the sexes, although these were not specifically evaluated in this study. Sumi et al., in contrast to our results, found no difference between males and females in either length of the unobservable length of the pancreas or ratio of unobservable length to total pancreatic length [25]. However, in their study, no difference was found in abdominal circumference between genders and thus differences in patient population could relate to this difference in result. The thickness of the abdominal subcutaneous fat was not found to correlate with cyst visualization, suggesting that this will not serve as a helpful predictor of patient suitability for US. In our study, patients’ weight and body habitus indicators changed only slightly between prior (prompting) MR or CT and the follow-up examination. Thus a practice of predicting suitability for US on the basis of an initial prompting study at the time of cyst discovery would be supported as appropriate by our data.
PCL measurements at US were highly accurate in comparison with measurements at MRI, with mean difference in maximum diameter measurement of 0.7 mm. The slight tendency of US to underestimate cyst diameter may potentially be explained by differences in cyst depiction across modalities: at US, the anechoic lumen of the cyst is measured and the outer cyst wall is often imperceptible, while MRI measurements typically include the wall of the PCL. An additional factor which could contribute to differences in measurement is the known variability in PCL measurement between individuals [26], which relates to the irregular shape of many PCLs. Development of a program of PCL surveillance at a particular practice environment should include education of interpreting radiologists and sonographers as to consistent methods of caliper placement to ensure appropriate inter-study comparisons.
In our study, in 23% of patients, all cysts in the pancreas were visualized with US. If confirmed in future practice, this would suggest that nearly a quarter of patients should be able to undergo US surveillance of known PCLs as a replacement for MRI evaluation for at least a portion of their surveillance routine. Such patients could be identified through the use of predictive models and ultrasound attempted. If any known cysts were not visualized, MR could then be performed for subsequent examinations.
A less conservative proposal for the potential role of US hinges upon our observations that the PCLs not seen by US were most commonly small, measuring <1 cm in diameter. Indeed, when analyzing ultrasound’s performance on a per patient basis, US was able to visualize all cysts measuring ≥1 cm in diameter in 76.2% of patients, and visualized all PCLs ≥2 cm in diameter in 93.8% of patients. Furthermore, we have shown that the success of US can be predicted using cyst and patient factors, which suggests that the performance of US would be further improved if applied in a more targeted fashion to suitable patients according to the results of predictive modeling. The difference in performance of US for PCLs according to size is important because some current consensus recommendations utilize PCL size, in patients without other worrisome features (i.e. pancreatitis, thickened enhancing PCL walls, nonenhancing mural nodule, main pancreatic duct dilation, or duct cutoff with gland atrophy), to determine appropriate surveillance regimen [4]. According to the Fukuoko criteria of 2012, PCLs of <1 cm in diameter meeting these criteria may be safely followed up at intervals of 2–3 years [4]. If such an approach is widely adopted, then the reduced visualization of subcentimeter cysts at US may not represent a contraindication to intermittent US surveillance, if MR is performed at suitable intervals to allow intermittent reassessment of these smaller PCLs. Though not the focus of our study, future research may expand upon this question by investigating whether lack of visualization of a PCL known to be small previously, is predictive of a lack of adverse change in that PCL. It is important to note that lack of visualization of a known cyst of 1 cm or greater would still serve as an indicator of failure of US to achieve adequate surveillance, and should prompt a return to MR surveillance under this model. This is exemplified by the patient observed in our study in whom a known cyst which was ultimately managed with resection, could not be seen due to poor visualization of the pancreas with US. It is also noted that the recently-published Whitepaper of the ACR Committee on Incidental Findings regarding pancreatic cysts, differs from the Fukuoko criteria in recommending follow up of cysts <1.5 cm in diameter for 5 years, with specific recommendations depending on patient age [27]. However, this document also suggests that <5 mm (“white dot”) cysts can be managed with a single follow up at 2 years, and that such lesions may not be reported by some radiologists in patients 75–80 years or older. The role of US in surveillance will need to be placed in context with evolving recommendations and guidelines as knowledge accumulates regarding the natural history and management of pancreatic cystic lesions.
The possibility of development of new PCLs in the interval since prior MRI, is a separate consideration of interest. In our study, we noted that two cysts that were new in the interval were not detected with US. This is potentially explainable by the study design, as US operators were instructed specifically to follow known PCLs and not to evaluate the pancreas for new development of PCLs. Neither new cyst resulted in a change in management in clinical MR reports and thus the ultimate recommendation of the clinical MR examination and that which would have been provided following the research US examination, would not have differed in these cases. Nevertheless, the clinical importance of new PCLs developing during a follow-up interval and the performance of US for detecting such new lesions, or potential need for intermittent MRI to capture lesions developing in these intervals, are questions that may be addressed in future research.
Although more data are needed for a full understanding of the association, it has been reported that PCLs themselves may serve as biomarkers for an increased risk of pancreatic malignancy, with associations observed between PCLs and development of solid pancreatic masses elsewhere in the pancreas [28,29,30,31,32]. Further understanding of the importance of this association has the potential to change the nature of surveillance programs for patients with PCLs in general. The utility of US for detection of early solid pancreatic malignancies developing separately from existing PCLs was not the focus of this study and can be investigated in future work.
The presence of an attending radiologist showed significant correlation with cyst visualization in our study, which likely reflects greater familiarity with pancreatic anatomy as well as the ability to interpret the preceding MR examination. The focused nature of the examination as well as unfamiliarity with the anatomic details of the pancreas may render pancreas US difficult for some sonographers, and all staff performing pancreas sonography may benefit from initial training by attending sonologists during the initial phase of a pancreas ultrasound surveillance program. In addition, future directions may include the use of GPS-like image fusion technology to aid in appropriate localization of PCLs, as has been employed previously in pancreatic ultrasound [25, 33]. In such a strategy, prior MR imaging could be used as a map for PCL location, which could theoretically decrease the impact of unfamiliarity with anatomic landmarks for less experienced operators.
The limitations of this study include its sample size; future research can expand upon the results of this pilot data to further evaluate the utility of US surveillance of pancreatic cysts in a larger population of patients. It is acknowledged that the all-operators design of our study may have resulted in a lower success rate of ultrasound in visualizing PCLs than could be achievable using only expert operators. However, this study design was pursued intentionally in order to ensure applicability of our results to general practice, in which US operators of various levels of experience may participate in these examinations. Having observed these results, we suggest that in order to achieve best performance in future clinical practice or research investigations, it may be prudent to include the involvement of an attending radiologist or expert advanced practice sonographer. Our study is not supported by correlation with a histologic reference standard in the vast majority of cases, since surgery and/or biopsy were generally not indicated in this surveillance population. The frequency with which US operators viewed the prior MRI prior to scanning was not recorded, although the use of the PCL worksheet, intended to serve as a portable method of bringing prior MR information into the US scan room, was universal. Another area of future study could incorporate the use of contrast-enhanced US (CEUS) since CEUS has been shown to further enhance the ability to US to characterize internal architecture of pancreatic cystic lesions [34] and to improve the US diagnosis of pancreatic lesions in general [35, 36].
In conclusion, targeted ultrasound of the pancreas performed for evaluation of known PCLs allows visualization and accurate measurement of the majority of PCLs, including 82.2% of PCLs ≥1 cm; visualization correlates strongly with PCL size, location, patient sex, weight, and abdominal diameter. The mean difference in maximum diameter measurements obtained by US with measurements obtained from same date MR examinations was less than 1 mm. Involvement of attending radiologist correlates with increased likelihood of PCL visualization. Our findings suggest that US may be considered an adjunct to MR surveillance of PCLs, and may be used in alternation with MRI for appropriately selected patients. Future research should include investigations into the utility of US for detection of new PCLs or pancreatic masses developing separately from known PCLs.
References
Laffan TA, Horton KM, Klein AP, et al. (2008) Prevalence of unsuspected pancreatic cysts on MDCT. AJR Am J Roentgenol 191(3):802–807
Lee KS, Sekhar A, Rofsky NM, Pedrosa I (2010) Prevalence of incidental pancreatic cysts in the adult population on MR imaging. Am J Gastroenterol 105(9):2079–2084
Ahn DW, Lee SH, Kim J, et al. (2012) Long-term outcome of cystic lesions in the pancreas: a retrospective cohort study. Gut Liver 6(4):493–500
Tanaka M, Fernandez-del Castillo C, Adsay V, et al. (2012) International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology 12(3):183–197
Tanaka M, Chari S, Adsay V, et al. (2006) International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology 6(1–2):17–32
Abbott DE, Ahmad SA (2014) Comparison of the Sendai and Fukuoka consensus guidelines for the management of mucinous cystic lesions of the pancreas: are we making progress? Ann Surg Oncol 21(6):1770–1772
Lee SH, Shin CM, Park JK, et al. (2007) Outcomes of cystic lesions in the pancreas after extended follow-up. Dig Dis Sci 52(10):2653–2659
Maguchi H, Tanno S, Mizuno N, et al. (2011) Natural history of branch duct intraductal papillary mucinous neoplasms of the pancreas: a multicenter study in Japan. Pancreas 40(3):364–370
Khannoussi W, Vullierme MP, Rebours V, et al. (2012) The long term risk of malignancy in patients with branch duct intraductal papillary mucinous neoplasms of the pancreas. Pancreatology 12(3):198–202
Handrich SJ, Hough DM, Fletcher JG, Sarr MG (2005) The natural history of the incidentally discovered small simple pancreatic cyst: long-term follow-up and clinical implications. AJR Am J Roentgenol 184(1):20–23
Lee CJ, Scheiman J, Anderson MA, et al. (2008) Risk of malignancy in resected cystic tumors of the pancreas < or =3 cm in size: is it safe to observe asymptomatic patients? A multi-institutional report. J Gastrointest Surg 12(2):234–242
Wu BU, Sampath K, Berberian CE, et al. (2014) Prediction of malignancy in cystic neoplasms of the pancreas: a population-based cohort study. Am J Gastroenterol 109(1):121–129; quiz 30.
Brook OR, Beddy P, Pahade J, et al. (2015) Delayed growth in incidental pancreatic cysts: are the current American College of Radiology recommendations for follow-up appropriate? Radiology 278(3):752–761
Pinho DF, Rofsky NM, Pedrosa I (2014) Incidental pancreatic cysts: role of magnetic resonance imaging. Top Magn Reson Imaging 23(2):117–128
Del Chiaro M, Verbeke C, Salvia R, et al. (2013) European experts consensus statement on cystic tumours of the pancreas. Dig Liver Dis 45(9):703–711
Hruban RH, Takaori K, Klimstra DS, et al. (2004) An illustrated consensus on the classification of pancreatic intraepithelial neoplasia and intraductal papillary mucinous neoplasms. Am J Surg Pathol 28(8):977–987
Italian Association of Hospital G, Endoscopists, Italian Association for the Study of the Pancreas, et al. (2014) Italian consensus guidelines for the diagnostic work-up and follow-up of cystic pancreatic neoplasms. Dig Liver Dis 46(6):479–493
Society for Surgery of the Alimentary Tract. SSAT Patient Care Guidelines (2007) Cystic neoplasms of the pancreas. J Gastrointest Surg 11(9):1225–1227
Falconi M, Crippa S, Chari S, et al. (2015) Quality assessment of the guidelines on cystic neoplasms of the pancreas. Pancreatology 15(5):463–469
Budde C, Beyer G, Kuhn JP, Lerch MM, Mayerle J (2015) The clinical and socio-economic relevance of increased IPMN detection rates and management choices. Viszeralmedizin 31(1):47–52
Yu MH, Lee JY, Kim JH, Han JK, Choi BI (2013) Value of near-isovoxel ultrasound for evaluation of ductal communications with pancreatic cystic lesions: correlation with magnetic resonance cholangiopancreatography. Ultrasound Med Biol 39(12):2279–2284
Soroida Y, Sato M, Hikita H, et al. (2016) Pancreatic cysts in general population on ultrasonography: Prevalence and development of risk score. J Gastroenterol 51(12):1133–1140
Ikeda M, Sato T, Morozumi A, et al. (1994) Morphologic changes in the pancreas detected by screening ultrasonography in a mass survey, with special reference to main duct dilatation, cyst formation, and calcification. Pancreas 9(4):508–512
Das A, Wells CD, Nguyen CC (2008) Incidental cystic neoplasms of pancreas: what is the optimal interval of imaging surveillance? Am J Gastroenterol 103(7):1657–1662
Sumi H, Itoh A, Kawashima H, et al. (2014) Preliminary study on evaluation of the pancreatic tail observable limit of transabdominal ultrasonography using a position sensor and CT-fusion image. Eur J Radiol 83(8):1324–1331
Dunn D, Brook O, Brook A, et al. (2016) Measurement of pancreatic cystic lesions on magnetic resonance imaging: efficacy of standards in reducing inter-observer variability. Abdom Radiol 41(3):500–507
Megibow AJ, Baker ME, Morgan DE, et al. (2017) Management of incidental pancreatic cysts: a white paper of the ACR incidental findings committee. J Am Coll Radiol 14(7):911–923
Yamaguchi K, Ohuchida J, Ohtsuka T, Nakano K, Tanaka M (2002) Intraductal papillary-mucinous tumor of the pancreas concomitant with ductal carcinoma of the pancreas. Pancreatology 2(5):484–490
Yamaguchi K, Kanemitsu S, Hatori T, et al. (2011) Pancreatic ductal adenocarcinoma derived from IPMN and pancreatic ductal adenocarcinoma concomitant with IPMN. Pancreas 40(4):571–580
Tanno S, Nakano Y, Koizumi K, et al. (2010) Pancreatic ductal adenocarcinomas in long-term follow-up patients with branch duct intraductal papillary mucinous neoplasms. Pancreas 39(1):36–40
Tanaka M (2011) Controversies in the management of pancreatic IPMN. Nat Rev Gastroenterol Hepatol 8(1):56–60
Tanno S, Nakano Y, Sugiyama Y, et al. (2010) Incidence of synchronous and metachronous pancreatic carcinoma in 168 patients with branch duct intraductal papillary mucinous neoplasm. Pancreatology 10(2–3):173–178
Sofuni A, Itoi T, Itokawa F, et al. (2013) Real-time virtual sonography visualization and its clinical application in biliopancreatic disease. World J Gastroenterol 19(42):7419–7425
Fan Z, Yan K, Wang Y, et al. (2015) Application of contrast-enhanced ultrasound in cystic pancreatic lesions using a simplified classification diagnostic criterion. Biomed Res Int 2015:974621
D’Onofrio M, Biagioli E, Gerardi C, et al. (2014) Diagnostic performance of contrast-enhanced ultrasound (CEUS) and contrast-enhanced endoscopic ultrasound (ECEUS) for the differentiation of pancreatic lesions: a systematic review and meta-analysis. Ultraschall Med 35(6):515–521
Xu M, Xie XY, Liu GJ, et al. (2012) The application value of contrast-enhanced ultrasound in the differential diagnosis of pancreatic solid-cystic lesions. Eur J Radiol 81(7):1432–1437
Acknowledgements
The authors would like to acknowledge Laurie Sammons, RDMS and Lisa Napolitano, RDMS, for assistance with ultrasound examinations, as well as Lauren O’Loughlin, Bridget Giarusso, Amy Callahan, and Kelly Roth, for administrative support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No grants or funding were associated with this study.
Conflict of interest
There are no disclosures relevant to the content of this manuscript.
Appendices
Appendix 1
See Fig. 9.
US machines and transducers used. Initial scanning was performed with curvilinear array 5-1 MHz transducers on IU-22 units, and with C9-2 transducers on EPIQ units; additional linear array (L9-3 and L12-5) and sector (S4-1) transducers, could be subsequently utilized per operator discretion. US unit selection was based upon the site of service and availability of machines
Appendix 2
See Table 2.
Rights and permissions
About this article
Cite this article
Sun, M.R.M., Strickland, C.D., Tamjeedi, B. et al. Utility of transabdominal ultrasound for surveillance of known pancreatic cystic lesions: prospective evaluation with MRI as reference standard. Abdom Radiol 43, 1180–1192 (2018). https://doi.org/10.1007/s00261-017-1269-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-017-1269-2