Abstract
Purpose
To investigate incidental pancreatic cysts (IPCs) size discrepancy in a cohort of patients receiving both computed tomography (CT) and magnetic resonance imaging (MRI) and its impact on clinical management based on the 2010 American College of Radiology (ACR) guidelines.
Methods
This was a HIPAA-compliant, retrospective, IRB-approved study. Informed consent was waived. Patients with known IPCs and at least one case-pair, consisting of an abdominal CT and MRI examination within 180 days between 05/1999 and 12/2011, were included. Maximum diameter of cysts was measured in both the CT and MR examinations. A subset of 30 patients was measured by three radiologists independently to assess inter-observer variability. Absolute difference in diameter measurements between CT and MRI was calculated. Influence of cyst size, cyst location, and patient characteristics such as weight, height, and body mass index (BMI) on variability of size measurements were evaluated. Clinical impact in terms of current ACR guidelines was assessed.
Results
Overall, 267 case-pairs of cysts in 113 patients were included in this study. 59/267 cysts were visualized on MRI but not on CT (22.1%, 95% CI 17.1%–27.1%; 32 patients, 64.6 ± 11.7 years, BMI 28.5 ± 4.8 kg/m2), with a median MRI cyst size of 7.8 mm, IQR 6.0–9.0 mm, range 2–17.8 mm. 208 case-pairs in 113 patients with a mean BMI of 26.9 ± 5.1 kg/m2 (range 16.9–39.5 kg/m2) and mean cysts size of 13.4 ± 8.1 mm (range 3–49 mm) were seen in both CT and MRI. The mean absolute size difference for IPCs measured on MRI and CT was 2.1 ± 1.8 mm (median 1.5 mm, IQR 0.9–2.9 mm, range 0–9 mm). Absolute size difference between CT and MRI measurements increased with size of the cyst (r = 0.31, p < 0.001), whereas location of the cyst did not influence the absolute difference between CT and MRI measurements (p = 0.44). Patient weight and BMI had a negative correlation with the difference in cyst size between CT and MRI (weight r = −0.17, p = 0.023; BMI r = −0.17, p = 0.027), with cyst measurements being larger on MRI in thin patients and on CT in obese patients. Inter-reader variability was excellent (ICC = 0.99). In 12/208 (5.7%, 95% CI 2.7%–9.1%), variability between CT and MRI would have changed ACR-based follow-up recommendation.
Conclusion
There was a median difference of 1.5 mm between measurements of IPCs on CT vs. MRI. If imaging modality was switched during follow-up, variability of measurements may lead to inappropriate change of follow-up regimen in 6% of all cases. A single follow-up CT for incidental IPCs does not seem sufficient due to a high number (22%) of missed IPCs on CT.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The detection of incidental pancreatic cystic (IPC) lesions has increased considerably over the last years likely due to an increasing number and higher resolution of cross-sectional abdominal imaging examinations [1–3]. IPCs have been reported in 3%–20% of all patients undergoing abdominal computed tomography (CT) or magnetic resonance imaging (MRI) [2, 4–6]. Although only a small portion of incidentally detected IPCs [1, 7], will develop into malignancy, it is currently impossible to predict which cysts will become malignant, therefore imaging follow-up is frequently recommended [8].
Different guidelines for the follow-up of IPCs have been proposed by various authorities such as the Sendai [9], the Fukuoka [10], the American College of Radiology (ACR) Incidental Findings Committee [8], and the American Gastroenterological Association [11, 12] guidelines. While these guidelines differ in follow-up intervals, all of them identify cyst diameter as a major determinant for management decisions in their follow-up charts [8–11]. For IPCs without solid components, lesion size is the critical factor to distinguish between surgical and non-surgical management and to determine the follow-up interval [8–11, 13].
Although both CT and MRI are considered acceptable imaging modalities for the for follow-up of pancreatic cysts [8–11], MRI’s inherent higher contrast resolution and lack of ionizing radiation makes is preferable for repeated imaging in patients with IPCs [8]. However, CT offers superior spatial resolution compared to MRI. Furthermore, both imaging modalities may be used in the same patient (e.g., IPC initially detected on CT and then followed with MRI or patient initially evaluated with MRI switches to CT due to availability or contraindication to MRI such as indwelling device or claustrophobia). Additionally, MRI capacity is still limited in many institutions, and cost and convenience may lead to follow-up CT studies. Understanding the impact of imaging modality on IPC size measurements is therefore critical to implement follow-up strategies. To our knowledge, the variability of IPC size measurements on CT and MRI has been only reported in a small cohort of patients and emphasizing its correlation with measures on endoscopic ultrasound [3]; the impact of such differences on clinical management was not evaluated.
Therefore, the goal of this study was to quantify the variability of IPC measurements in a cohort of patients receiving both CT and MRI and to evaluate the possible impact of measurement variability on follow-up management according to the current ACR guidelines.
Materials and methods
IRB
This Health Insurance Portability and Accountability Act (HIPAA) compliant, single-institution, retrospective study was approved by the local institutional review board (IRB) with a waiver of informed consent.
Data collection
A retrospective search of the institution’s clinical MRI database was performed for all patients with incidental pancreatic cysts (IPCs), who had at least one CT examination and one MRI examination within 180 days of each other between May 1999 and December 2011. Each pair of CT and MR examinations in the same patient performed within 180 days was defined as a case-pair and included in the study. Patients with known or suspected pancreatic pathology prior to the initial examination (CT or MRI) and patients who had undergone a procedure for their pancreatic cyst between both studies were excluded. Patient characteristics such as height, weight, body mass index (BMI), and outcome data of IPCs were obtained from the reports in the medical records.
The medical records were also reviewed for histopathological classification of the IPCs in cases where patients underwent endosonographic ultrasound (EUS) or surgery.
Imaging protocols
All studies were performed on 1.5 and 3.0 Tesla MRI scanners (Signa LX Excite, GE Healthcare, Milwaukee, WI, USA; Symphony and Espree, Siemens Healthcare GmbH, Erlangen, Germany). As incidental IPCs can be detected on all scans that include the upper abdomen, different scan protocols were used depending on the clinical indication of the original scan. All studies included coronal (ranges TE = 58–80 ms, TR = 911–1690 ms, slice thickness = 5 mm, pixel spacing = 1.1–1.6 mm) and axial (TE = 58–80 ms, TR = 931–1320 ms, slice thickness = 5 mm, pixel spacing = 1.1–1.7 mm) single-shot T2-weighted fast spin echo sequences which were used for the lesion measurements.
CT examinations were performed on various CT scanners with 4-320 slices (Discovery, Lightspeed and HiSpeed QX CT scanner, GE Healthcare, Milwaukee, WI, USA; Definition AS CT scanner, Siemens Healthcare GmbH, Forchheim, Germany; Aquilion ONE CT scanner, Toshiba Medical Systems, Okinawa, Japan) available at that time in our hospital. The standard institutional abdominal CT protocols were used and all examinations included a portal venous phase scan of the abdomen after contrast application (i.e., Optiray 350, Tyco Health Care, Mansfield, TX; Omnipaque 350, GE Healthcare, Milwaukee, WIS; or Visipaque 320, GE Healthcare, Milwaukee, WIS). Axial reconstructions were routinely generated with 5-mm slice thickness and pixel size range of 0.6–0.9 mm (range FOV = 300–460 mm, matrix 512 × 512). Coronal and sagittal reformations were generated with 5-mm thickness and pixel size range of 0.6–1.0 mm (range FOV = 300–480 mm, matrix 512 × 512).
Analysis of cases with cyst detected in one exam (MRI) only
Cysts detected on only one examination were further evaluated. In cases where cysts were not visible on the first of the case-pair studies, previous imaging studies were reviewed in the Picture Achieving and Communications System (PACS, GE Medical, Milwaukee, Wisconsin, US) to identify whether the cysts newly appeared between the cyst-pair studies or were not visualized due to the imaging technique. In cases where cysts were not visible on the later of both studies, follow-up examinations were reviewed to confirm the presence of the cysts. Location and size of the previously undetected cysts were further analyzed.
Analysis of incidental pancreatic cysts
An IPC was defined as a cystic lesion (i.e., less than 20 HU on CT and homogenous high signal intensity on MRI T2-weighted images following the signal intensity of fluid elsewhere, such as cerebrospinal fluid) within the pancreatic parenchyma in asymptomatic patients [8]. Measurements of the pancreatic cyst dimension were performed by one of six radiologists with 1–3 years of fellowship training in abdominal CT and MRI. For standardization of measurements, all radiologists were instructed how to appropriately choose a slice and perform maximum dimension measurement to include the outer borders of the cyst as well as all cyst components. Three sample measurements under supervision of the senior author (blinded) were performed before the start of the study.
Each radiologist performed all measurements for both studies (CT and MRI) of the same patient in order to reflect the clinical routine of comparing current and previous examinations. Measurements were performed as follows: if visible, the maximal cyst dimension was chosen on the coronal MRI or CT images in the oldest included scan. The cyst was then measured on this plane for both MRI and CT in all follow-up studies. If the cyst was not visible on the coronal plane, the axial plane was used in all studies. In 73/267 (27%) cases, no coronal or sagittal MPRs were available, and measurements were performed solely on axial images. If coronal MRI images and coronal multi-planar CT reformations (MPR) were available, measurements were performed on coronal images. In the rest of the cases, measurements were performed on axial images. A fellowship-trained abdominal radiologist (ORB) with 14 years of experience reviewed all cysts on CT and on MRI for the presence of septa and mural nodes.
Measurement discrepancy analysis
The differences between maximum dimension measurements in CT and MRI were calculated to evaluate if one modality systematically leads to larger cyst diameter measurements. The absolute differences between maximum dimension measurements in CT and MRI were calculated to evaluate if variability between both methods depends on patient characteristics. Data were analyzed for different possible biases: cyst location, body weight, patient height, BMI and size of the cyst. The cysts were then assigned to one of the following groups based on their MRI size: 0–4.9, 5.0–9.9, 10.0–19.9, 20.0–29.9, ≥ 30.0 mm.
Inter- and intra-observer variability
To assess inter-observer variability, two clinical fellows and one research fellow in the department of radiology (CC with 6 years, TM with 6 years and JB with 3 years of experience in radiology) independently measured a subset of 30 examinations. The subset was chosen after consulting a statistician to include the variety of cyst sizes (mean size 16 ± 9 mm, range 4–38 mm). The maximal dimension on the axial CT images was measured. For patients with multiple IPCs, the exact location of the respective cyst was provided to all observers using an image number which included the cyst.
To assess intra-observer variability, one fellowship-trained abdominal radiologist (ORB) with 14 years of experience measured the same subset of cysts three times in a random order with 18 months of time difference between the review sessions.
Analysis of clinical impact
The 2010 ACR guidelines were used to evaluate the clinical impact of variability in cyst measurements [8, 10]. Cysts that would have been in a different follow-up category (<2, 2–3, >3 cm) due to the variability of measurements between MRI and CT were identified. In these cases, clinical follow-up, imaging follow-up, and pathology were obtained from the online medical records and outcome was evaluated.
Statistical analysis
Statistical analysis was performed using Matlab (Mathworks, Natick, MA, USA). All data are presented in mean ± standard deviation with ranges. 95% confidence intervals (95% CI) and median with interquartile ranges (IQR) are provided. Histograms, Bland–Altman plots, and boxplots were created for visual analysis of the data. Analysis of variance (ANOVA) was performed to assess the influence of cyst location on differences in measurement between CT and MRI. Correlations were calculated using Pearson correlation coefficients. Intraclass correlation (ICC) was calculated to assess inter- and intra-observer agreement. Fisher’s exact test was performed to compare frequencies. Mann–Whitney U test was used to compare the number of CT scanner slices. The level of statistical significance was set to α = 0.05.
Results
Patients
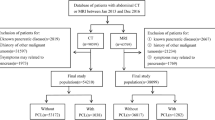
We identified 284 case-pairs of 156 patients with both CT and MRI examinations within 180 days in our database. In 6/284 (2%) case-pairs of 6 patients with more than one case-pair, no cyst was seen in either examination (but seen in other case-pairs in the same patient) so those case-pairs where the cyst was not visible were excluded from further analysis. In addition, 11/284 (4%) case-pairs in 5 patients were excluded because patients underwent a procedure (i.e., endoscopic ultrasound with cyst aspiration) between the two studies. Therefore, 267 case-pairs were finally included in the study (Fig. 1).
Case-pairs with cysts visualized in one examination only (MRI)
In 59/267 case-pairs (22.1%, 95% CI 17.1%–27.1%; 32 patients), cysts were seen on MRI but not on CT (Fig. 2). Mean patient age was 64.6 ± 11.7 years (range 46.6–84.4 years old), with mean patient weigh of 74.8 ± 15.0 kg (range 48.5–115.7 kg). There were no case-pairs with a cyst seen on CT but not on MRI. Median cyst size in these patients was 7.8 mm (IQR 6.0–9.0 mm). Median absolute time interval between CT and MRI was 58 days (IQR 23–138 days). The number of cysts visualized on MRI but not seen on CT decreased with increasing cyst size—from 50% for cysts smaller than 5 mm to 3% for cysts larger than 15 mm (Table 1). Analyzed by cysts, the location of cysts not visualized on CT but seen on MRI included the head in 8/40 (20%), the tail in 6/40 (15%), the body in 22/40 (55%), and the uncinate process in 4/40 (10%) of the pancreas, (Table 2 shows the number of case-pairs with missed cysts, in 19 cases a cyst was not visible on CT in more than one case-pair). In 22/59 (37.2%) case-pairs, a previous MRI study was available and confirmed that the cyst had not newly appeared between CT and MRI.
In case-pairs where cysts were not visualized on CT, coronal or sagittal MPRs were available less frequently than in case-pairs where cysts were visualized on both scans (27/59, 45.7% vs. 167/208, 80%; p < 0.0001).
Patients with cysts visualized on CT and MRI examinations
In 208/267 case-pairs (77.9%, 95% CI 72.9%–82.9%, 113 patient), with a mean age of 65.5 ± 11.3 years (range 38.3–88.4 years), mean weight of 74.4 ± 17.7 kg (range 44.5–146.5 kg), mean height of 165 ± 9 cm (range 147–193 cm), and mean BMI of 26.9 ± 5.1 kg/m2 (range 16.9–39.5 kg/m2), cysts were seen in both imaging examinations. The median absolute time interval between the MRI and CT was 46 days (IQR 15–103 days, range 0–179 days). The median number of CT slices was lower in case-pairs where IPCs were not visible on CT (median 8, IQR 1–52 vs. median 16, IQR 8–64; p < 0.001).
CT and MRI Characteristics of Incidental Pancreatic Cysts
The median number of IPCs per patient was 1 (IQR 1–1, range 1–6). The mean size of IPCs on MRI was 13.4 ± 8.1 mm (range 3–49 mm, Fig. 3), whereas the mean size on CT was 12.8 ± 7.9 mm (range 2.0–52.2 mm). Cysts measured 0–4.9 mm in 8/208 (3.9%), 5.0–9.9 mm in 80/208 (38.5%), 10.0–19.9 mm in 87/208 (41.8%), 20.0–29.9 mm in 21/208 (10.1%), and ≥30 mm in 12/208 (5.8%) of the case-pairs on MRI. The mean absolute size difference between IPCs measured on MRI and CT was 2.1 ± 1.8 mm (median 1.5 mm, IQR 0.9–2.9 mm, range 0–9 mm) (Table 3; Figs. 4, 5). Using a threshold of two standard deviations, a size difference of up to 6 mm may be caused by the variability between the measurement on CT and MRI.
On MRI, 68/208 (33%) cysts showed septa, while only in 24/208 (12%) cases septa was seen on CT (p < 0.001) (Fig. 6). CT did not detect septa in cases where MRI failed to demonstrate septa. The average size of the pancreatic cystic lesion with septa seen on MRI was 18.1 ± 10.3 mm, while on CT it was 23.8 ± 10.2 mm (p = 0.02). Mural nodule was seen on MRI in 8/208 (3.8%) cases, and in 9/208 (4.3%) on CT (p = 0.8), with only 3 cases where both MRI and CT demonstrated mural nodules in the same case-pair.
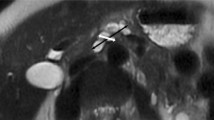
Sixty-five-year-old patient who underwent CT (A) as part of the pre liver transplantation workup. Two simple cysts (14 and 11 mm) were found in the body and tail of the pancreas (white arrows). MRI (B) was performed one day later and confirmed the two cysts. However, cyst size was larger (19 and 15 mm) and septa within both cysts were detected (bold arrows)
Impact of cyst size and location
There was a linear correlation between cyst size measured on CT and MRI (Figs. 4, 5).
The absolute size difference between CT and MRI measurements increased with the size of the cyst (r = 0.31, p < 0.001) (Fig. 7). Cysts were most frequently found in the pancreatic head (72/208, 34.6%) and least frequently in the uncinate process (25/208, 12.0%). Location of the cyst did not influence the absolute difference between CT and MRI measurements (p = 0.44).
Histopathological classification
25/113 (22%) patients underwent EUS. The cytology was non-diagnostic in 16/25 (64%), showed benign findings without mucin in 6/25 (24%), benign with mucin in 3/25 (12%) patients, and atypical findings without mucin in 1/25 (4%) patients.
4/113 (4%) underwent pancreatic surgery, which showed Pancreatic Intraepithelial Neoplasia, type 1 in 3/4 (75%) patients, low-grade IPMN in 2/4 (50%) patients, and moderate-grade IPMN in 1/4 (25%) patient (multiple diagnoses were present in the same patient).
Impact of patient size
Patient weight and BMI had a negative correlation with the signed difference in cyst size between CT and MRI (weight r = −0.17, p = 0.023; BMI r = −0.17, p = 0.027). IPC measurements were larger on MRI vs. CT in thin patients, while they were larger on CT vs. MRI in obese patients (Fig. 8).
The absolute difference in cyst size between CT and MRI was not influenced by the weight (r = 0.04, p = 0.59) or BMI (r = −0.013, p = 0.87) of the patient meaning that the absolute variability of IPC measurements between CT and MRI does not depend on patient size.
Impact on clinical management
ACR guidelines for IPCs [8] recommend surgical procedure for cysts larger than 3 cm and a single imaging follow-up for cysts smaller than 2 cm. There were 12/208 (5.8%) cysts that were larger than 3 cm and 175/208 (84.1%) cysts that were smaller than 2 cm.
In 6/208 case-pairs (2.9%, CI 0.6%–5.5%, 4 patients), cysts were larger than 3 cm in one imaging modality but smaller in the other. In 5/208 case-pairs (2.9%), cysts were larger on MRI, and in 1/208 (0.5%), the cyst was larger on CT. Mean absolute difference in size between the modalities across these 6 cyst-pairs was 4.1 ± 1.9 mm (range 2.0–6.5 mm). MRI was always the later of the two methods (median interval 52 days, IQR 12–96 days), meaning that follow-up management according to the ACR guidelines would have been changed from follow-up imaging to surgery in 5/208 (2.9%) case-pairs and from surgery to follow-up imaging in 1/208 (0.5%) case-pairs. Mean follow-up time was 5.9 ± 3.3 years (range 1.4–9.8 years), and follow-up imaging was available in all case-pairs. Final diagnosis from pathology was available in 5/208 (2.9%) patients, and by clinical follow-up and follow-up imaging in one patient and included IPMN in 5/208 (2.9%) patients, and low-grade pancreatic intraepithelial neoplasia in 1/208 (0.5%) of the case-pairs.
In 6/208 (2.9%, 95% CI 0.6%–5.2%, 5 patients) cases, cysts were larger than 2 cm in one imaging modality but smaller in the other. In 4/208 case-pairs (1.9%), cysts were larger on MRI, and in 2/208 case-pairs (1.0%), cysts were larger on CT. MRI was the later imaging study in all case-pairs (median interval 41 days, IQR 33–102 days) meaning that in 4/208 case-pairs, (1.9%) the second follow-up scan would have changed the clinical management from single follow-up to at least yearly follow-up. Mean absolute difference in size was 5.8 ± 2.7 mm (range 2.7–9 mm). Mean follow-up time was 9.0 ± 3.9 years (range 2.6–12.3 years) and follow-up imaging was available in all case-pairs. Final diagnosis was made by clinical follow-up and follow-up imaging in all patients and included IPMN in 3/208 (1.4%), serous cystadenoma in 1/208 (0.5%), and a simple cyst in 2/208 (1.0%) of the case-pairs.
Inter- and intra-observer variability
Mean size of the 30 cases that were measured to assess inter- and intra-observer variability was 16.2 ± 9.2 mm (median 12.4 mm, IQR 8.9–23.3 mm, range 4.2–37.5 mm). There was an excellent inter-observer agreement with ICC = 0.99 and a mean standard deviation on CT for each cyst across all readers of 0.92 ± 0.08 mm (95% CI 0.89–0.95 mm within- subject). The within-subject mean standard deviation of the difference between CT and MRI for each cyst across all readers was 1.95 ± 0.09 mm, 95% CI 1.92–1.99.
There was an excellent intra-observer agreement with ICC = 0.99 and intra-observer mean standard deviation on CT for each cyst of 0.03 ± 1.61 mm.
Discussion
In this study, we found a median absolute difference in IPCs diameter measurements between CT and MRI of 1.5 mm. All IPCs were visible on MRI; however, in 22% of case-pairs, IPCs could not be visualized on CT. Cyst size, patient weight, and BMI, but not cyst location, influenced variability between CT and MRI measurements. Variability between imaging modalities would have changed clinical management according to the ACR guidelines in 6% of all case-pairs studied.
We found an excellent linear correlation between diameter measurements on CT and MRI with a median absolute difference of 1.5 mm (Fig. 4). This is smaller compared to a 3 mm difference previously reported by Maimone et al. [3]. The range of the absolute difference in our cohort (0–9 mm) was also smaller compared to a previous study (2–20 mm) [3]. The smaller differences found in our study may be caused using a standardized measurement method by specially trained radiologists in our study, which has been reported to decrease measurement variability [14]. Although the largest diameter of each cyst was also used for comparison between CT and MRI measurements in the previous study by Maimone et al. [3], the authors did not describe in detail how measurements were performed, and therefore, a direct comparison between our studies is challenging.
In 22% of all case-pairs in our study, a cyst visible on MRI could not be visualized on the corresponding CT study, while no cyst was visualized on CT but not on MRI. The size of cysts that were not visible on CT ranged from 2 to 18 mm with 37% of cysts less than 1 cm not visualized on CT. As expected, our results confirmed higher diagnostic yield of MRI compared to CT for IPCs. Additionally, our results indicate that MPRs were available less frequently in case-pairs where IPCs were not visualized on CT and the median number of CT scanner slices was lower, indicating that multi-slice CT scanner is desirable when imaging IPCs. Although MPRs are currently performed in all cases in our department, MPRs were not available in all CT exams in the study, as some studies were old. Therefore, our results support the current guidelines that recommend the addition of multi-planar reformations to thin-slice contrast-enhanced CT examinations [8]. However, our results also indicate that MRI is more sensitive for pancreatic cysts than a CT, even when multi-planar reformations are used, likely due to its superior soft tissue contrast.
Additionally, more septa were detected with MRI and septated cysts were smaller on MRI compared with CT. This likely reflects the fact that it is easier to detect thin septa in the larger lesions on CT, while MRI’s superior contrast resolution accentuates contrast between the septa and cyst contents, and thus enables septa detection in relatively small lesions. Of note, we found no difference in the detection rate of mural nodularity; however, CT and MR agreed on presence of mural nodules in only 30% of the cases. The mural nodularity on CT might include false positive cases due to partial volume effect, rather than true mural nodule. Thus, MRI is the preferred method for follow-up of pancreatic cysts, also given the lack of ionizing radiation [8, 15]. Further research is necessary to address the role of other non-ionizing radiation techniques such as ultrasound in the follow-up of pancreatic cysts.
The variability of cyst measurements between CT and MRI in our study increased with the size of the IPC, indicating that in larger IPCs a lower absolute precision should be expected. The location of the IPC within the pancreas did not influence the differences in size measurements. These data may be helpful in deciding if an increase in cyst size is likely due to variability of measurement or a true increase with potential changes in follow-up management and may be considered when developing management guidelines in patients with IPCs.
The difference in cyst size measurement between CT and MRI showed a weak but significant correlation with patient weight (r = −0.17) and BMI (r = −0.17) However, the effect was very weak and the absolute difference was not influenced by patient characteristics. Our results are in accordance to a previous study that found no difference in variability between CT and MRI cyst size measurements when comparing different size groups (<10, 10–20, 20–30 and ≥30 mm) [3].
The inter- and intra-observer agreement for measurements of IPC size in our study was excellent. To our knowledge, inter- and intra-observer variability of multi-detector CT measurements for IPCs has not been previously reported. The variability reported here is, however, well within the range of previously reported coefficients of diameter measurement variation in other lesions measured on multi-detector CT ranging from 1% to 10% [16, 17].
According to the ACR guidelines, IPCs with a maximal diameter larger than 30 mm should be resected, whereas cysts between 20 and 30 mm should be further evaluated with MRI/MRCP and followed with intervals that differ depending on their imaging characteristics. Lesions below 20 mm only require a single follow-up imaging evaluation which preferably should be performed with MRI [8]. The variability between CT and MRI measurements in our study would have led to a change in the follow-up management in 6% of all case-pairs, where the IPC was visible on both scans. Although this variability is relatively low, a change in management in 6% of patients could influence costs (i.e., number/frequency of follow-up imaging studies) and potential morbidity (i.e., related to surgery). Thus, follow-up imaging with the same modality seems preferable.
Our results indicate that variations in the measurements of IPCs’ maximum dimension between CT and MR have to be expected to be as high as 6 mm. This needs to be taken into consideration for those patients in whom the follow-up imaging modality is switched from CT to MRI or vice versa. While a change in cyst size ≥6 mm likely represents a true change in the maximum dimension of the cyst, those changes of <6 mm can be potentially explained by inter-modality variability.
A large number of cysts (22%) were visible on MRI but were not apparent on CT. According to current the ACR guidelines, a single follow-up, preferably with MRI, is recommended for cysts smaller than 2 cm. However, the single follow-up may also be performed on CT as MRI is not defined as mandatory. Our results indicate that a cyst not seen on CT may not have resolved but may just have been missed in a considerable number of cases. This is especially relevant for a cyst size below 20 mm as no additional follow-up is currently recommended for these cysts, if they do not exhibit growth in the 1 year follow-up. Moreover, delayed growth of IPCs smaller than 20 mm can occur in up to 27% of all IPC [13]. Thus, our results seem to indicate that lack of visualization of an IPC previously identified on MRI on a single follow-up CT is not sufficient for the follow-up of these lesions, especially in IPCs smaller than 20 mm.
There are limitations to our study. A time difference of 180 days between two consecutive studies was used, which is higher than in previous studies [3]. This time difference of 180 days was chosen as recent studies reported that the vast majority of IPCs are stable over time [13]. However, this might have influenced our results. Our study included patients imaged over a long period of time, beginning in 1999. The spatial resolution of CT improved during the last decade. Therefore, measurement variability may be smaller with modern multi-detector CT scanners. Additionally, different MR protocols were used over time, which reflects clinical routine. In case-pairs where IPCs were not visualized on CT, an MRI was used to confirm that the cysts were truly missed, but the MRI was only available in 37% of those cases. Importantly, patients with IPCs are also at risk for the development of pancreatic carcinoma remotely from the cyst, anywhere in the gland which has been described as the concept of “field defect” [13, 18, 19]. We did not assess the impact of imaging modality in overall patient oncologic outcome.
In conclusion, we have shown that on average, there is a small variability of IPC size measurements between CT and MRI. However, change of imaging modality may cause a bias of up to 6 mm with resultant change in follow-up management in 6% of all cases, when following the current recommendations from the ACR. Furthermore, a follow-up CT for incidental IPCs failed to demonstrate 22% of these lesions overall and 37% of IPCs smaller than 1 cm. Our data would indicate that MRI is preferred to follow-up cysts smaller than 1 cm. Further research is necessary to clarify the impact of follow-up imaging and to detect size changes in these very small cysts vs. the development of neoplastic lesions in the rest of the pancreas and ultimately in patients’ outcomes.
References
Zaheer A, Pokharel SS, Wolfgang C, Fishman EK, Horton KM (2013) Incidentally detected cystic lesions of the pancreas on CT: review of literature and management suggestions. Abdom Imaging 38(2):331–341
Laffan TA, Horton KM, Klein AP, et al. (2008) Prevalence of unsuspected pancreatic cysts on MDCT. AJR Am J Roentgenol 191(3):802–807
Maimone S, Agrawal D, Pollack MJ, et al. (2010) Variability in measurements of pancreatic cyst size among EUS, CT, and magnetic resonance imaging modalities. Gastrointest Endosc 71(6):945–950
Lee KS, Sekhar A, Rofsky NM, Pedrosa I (2010) Prevalence of incidental pancreatic cysts in the adult population on MR imaging. Am J Gastroenterol 105(9):2079–2084
Zhang X-M, Mitchell DG, Dohke M, Holland GA, Parker L (2002) Pancreatic cysts: depiction on single-shot fast spin-echo MR images. Radiology 223(2):547–553
Ip IK, Mortele KJ, Prevedello LM, Khorasani R (2011) Focal cystic pancreatic lesions: assessing variation in radiologists’ management recommendations. Radiology 259(1):136–141
Dewhurst CE, Mortele KJ (2012) Cystic tumors of the pancreas: imaging and management. Radiol Clin North Am 50(3):467–486
Berland LL, Silverman SG, Gore RM, et al. (2010) Managing incidental findings on abdominal CT: white paper of the ACR incidental findings committee. J Am Coll Radiol JACR 7(10):754–773
Tanaka M, Chari S, Adsay V, et al. (2006) International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatol Off J Int Assoc Pancreatol IAP Al 6(1–2):17–32
Tanaka M, Fernández-del Castillo C, Adsay V, et al. (2012) International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatol Off J Int Assoc Pancreatol IAP Al 12(3):183–197
Vege SS, Ziring B, Jain R, Moayyedi P (2015) Clinical guidelines committee, American gastroenterology association. American gastroenterological association institute guideline on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology 148(4):819–822
Sahani DV, Kambadakone A, Macari M, et al. (2013) Diagnosis and management of cystic pancreatic lesions. Am J Roentgenol 200(2):343–354
Brook OR, Beddy P, Pahade J, et al. (2016) Delayed growth in incidental pancreatic cysts: are the current American college of radiology recommendations for follow-up appropriate? Radiology 278(3):752–761
Dunn DP, Brook OR, Brook A, et al. (2016) Measurement of pancreatic cystic lesions on magnetic resonance imaging: efficacy of standards in reducing inter-observer variability. Abdom Radiol 41(3):500–507
Waters JA, Schmidt CM, Pinchot JW, et al. (2008) CT vs. MRCP: optimal classification of IPMN type and extent. J Gastrointest Surg Off J Soc Surg Aliment Tract 12(1):101–109
Gupta S, Wagner-Bartak N, Jensen CT, et al. (2016) Dual-energy CT of pancreatic adenocarcinoma: reproducibility of primary tumor measurements and assessment of tumor conspicuity and margin sharpness. Abdom Radiol N Y:1–8
McErlean A, Panicek DM, Zabor EC, et al. (2013) Intra- and interobserver variability in CT measurements in oncology. Radiology 269(2):451–459
Remotti HE, Winner M, Saif MW (2012) Intraductal papillary mucinous neoplasms of the pancreas: clinical surveillance and malignant progression, multifocality and implications of a field-defect. JOP J Pancreas 13(2):135–138
Uehara H, Nakaizumi A, Ishikawa O, et al. (2008) Development of ductal carcinoma of the pancreas during follow-up of branch duct intraductal papillary mucinous neoplasm of the pancreas. Gut 57(11):1561–1565
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they do not have conflict of interest pertaining to this study.
Ethical approval
This study was approved by institutional IRB with waiver of informed consent.
Rights and permissions
About this article
Cite this article
Boos, J., Brook, A., Chingkoe, C.M. et al. MDCT vs. MRI for incidental pancreatic cysts: measurement variability and impact on clinical management. Abdom Radiol 42, 521–530 (2017). https://doi.org/10.1007/s00261-016-0883-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-016-0883-8