Abstract
Vertebral discitis-osteomyelitis is an infection of the spine that involves the intervertebral disc and the adjacent vertebral body but may also extend into the paraspinal and epidural soft tissues. If blood cultures and other culture data fail to identify a causative microorganism, percutaneous sampling is indicated to help guide targeted antimicrobial therapy. Despite limited supporting evidence, withholding antimicrobial therapy for up to 2 weeks is recommended to maximize microbiological yield, although literature supporting this recommendation is limited. During the procedure, technical factors that may improve yield include targeting of paraspinal fluid collections or soft tissue abnormalities for sampling, acquiring multiple core samples if possible, and use of larger gauge needles when available. Repeat sampling may be indicated if initial percutaneous biopsy is negative but should be performed no sooner than 72 h after the initial percutaneous biopsy to ensure adequate time for culture results to return.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Vertebral discitis-osteomyelitis (DO) is an infection of the intervertebral disc, the adjacent vertebral bodies, and surrounding epidural and paravertebral structures. Primary risk factors for DO include intravenous drug use, endocarditis, immunosuppression, diabetes mellitus, and active malignancy [1,2,3,4]. The incidence of DO is approximately 5.4 per 100,000 people and is increasing, probably due to several factors including the opioid epidemic, increased rates of spine surgery and percutaneous intervention, increase in intravascular procedures, and greater survival in patients with chronic diseases [4,5,6,7,8].
DO is usually the result of hematogenous spread of infection with the most common causative pathogen Staphylococcus aureus [9,10,11]. Common sources include urinary tract infections, pneumonia, endocarditis, or bacteria in the oral cavity [10, 12]. The pathophysiology of DO differs between children and adults due to age-dependent changes in vascularity of the intervertebral disc and endplate. In children and young adults up to 20 years of age, blood vessels penetrate the annulus fibrosis of the intervertebral disc [13, 14]. As a result, direct hematogenous infection of the intervertebral disc via these vessels can occur in children and in young adults. In adults over the age of 20 years, these blood vessels regress, and DO is believed to occur as a result of deposition of pathogen near the vertebral endplate resulting in osteomyelitis and secondary infection into the intervertebral space [7, 15]. Another proposed route for hematogenous spread of infection is by retrograde flow of pathogens from the pelvis via the epidural venous plexus, a theory that is supported by the fact that urinary tract infections and other pelvic infectious are relatively common sources of DO in adults [16]. Approximately 14–26% of cases of DO are the result of direct infection of the intervertebral disc and adjacent structures, most common as a result of spine surgery [17, 18]. The incidence of post-operative DO ranges from 0.2 to 3.6%, although the literature on infection rates for specific procedures is poor [18].
MRI is the imaging modality of choice for identifying DO, allowing for characterization of the extent of bone and soft tissue involvement and for identification of targets that may guide percutaneous sampling [19, 20]. The reported sensitivity and specificity of MRI for pyogenic DO are high (96% and 92–94%, respectively) [12, 17, 19, 21]. The findings with the highest sensitivity are paraspinal inflammation (97.7–100%), disc enhancement (95.4%), and fluid-signal intensity replacing the disc (93.2%) [21, 22]. Other MRI findings of DO and their sensitivities include endplate erosion or destruction (84.1%), nuclear cleft effacement (83.3%), loss of intervertebral disc height (52%), epidural inflammation (40.0%), paravertebral abscess (40.0%), and decreased disc signal on T1-weighted sequences (29.5%) [21, 22]. Although paravertebral abscess and epidural inflammation have lower sensitivities, they have relatively high specificities (83.3% and 90.9%, respectively) [22]. The specificity of paraspinal inflammation specificity is lower (50.0%) and increases to 62.5% if the thickness of the inflammation is greater than 5 mm [22]. The other imaging findings have much lower specificities (4.2–45.8%) [22]. Unless otherwise contraindicated, gadolinium-based intravenous contrast agents should be used to distinguish paraspinal or epidural phlegmon from abscess [23].
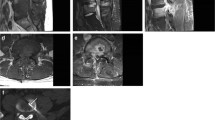
Non-pyogenic DO can be due to a variety of atypical organisms, including Mycobacterium tuberculosis, fungi, or Brucella. Tuberculous DO typically has a more indolent course than pyogenic DO and usually begins in the anterior part of spine and soft tissues and progresses posteriorly [24]. MRI findings of tuberculous DO include marrow signal abnormality and deformity of a single or multiple contiguous vertebral bodies [24, 25] with relative or complete sparing of the intervertebral disc [24,25,26]. Large paravertebral fluid collections can occur, tracking into the paraspinal soft tissues or the anterior longitudinal ligament over multiple vertebral levels in a pattern referred to as “subligamentous spread” (Fig. 1). Fluid collections typically have thin walls with smooth peripheral enhancement [24, 25, 27]. Fungal DO is more common among immunocompromised patients, persons who inject IV drugs, and patients who have had recent exposure to antibiotics [10]. As with tuberculous DO, fungal infection is typically indolent [27]. MRI findings more specific for fungal DO include partial disc and endplate involvement, and eccentric paravertebral soft tissue inflammation [27]. The intranuclear cleft is often preserved, and the disc may not demonstrate hyperintense T2 signal [25, 28]. MRI of Brucella DO typically shows relative sparing of the intervertebral disc despite surrounding marrow signal abnormality [10, 29]. Other characteristic findings include absent or mild deformity of the vertebral bodies, small paraspinal abscesses with irregular walls, facet joint involvement and sacroiliitis [25, 30]. Recognition of the features of non-pyogenic is crucial to ensure that sampled material is sent for the appropriate microbiological analysis.
a Sagittal T2-weighted MR images of the thoracic spine show large heterogeneously T2 hyperintense anterior paraspinal (white arrows) and epidural (dashed arrows) fluid collections. The T9-10 disc is relatively preserved (black arrows). b Axial fat-suppressed post-contrast images show large paraspinal (white arrows) and epidural (white asterisk) fluid collections with central non-enhancement and thick peripheral enhancement. The T10 endplate *(black asterisk) also shows enhancement. The features are compatible with tuberculous discitis with osteomyelitis. c Prone axial CT image through the lower thoracic spine during CT-guided biopsy shows the biopsy needle targeting the soft tissue/fluid collection in the surrounding paraspinal tissues (arrow). Culture grew Mycobacterium tuberculosis complex
If DO is suspected based on the clinical presentation and imaging findings, and blood cultures are negative, tissue sampling with open biopsy or percutaneous needle sampling may be needed to identify the causative pathogen and its susceptibilities in guide treatment with the appropriate antimicrobial agent [31, 32]. The two techniques for isolating the responsible microorganism are percutaneous sampling and open biopsy. A meta-analysis from 2017 shows a relatively poor yield of 48% from image-guided percutaneous sampling, compared to 76–91% for open biopsy [33, 34]. Despite a higher yield from open biopsy, percutaneous image-guided sampling is the technique of choice for identifying the causative microorganism owing to several advantages, including decreased morbidity, shorter associated hospitalization time, and lower complication rates [35, 36]. Open biopsy is often performed when open surgery is otherwise required, such as when neurological compromise would require posterior spinal decompression [33, 34].
In this paper, we will discuss clinical and technical factors that affect microbiological yield from percutaneous needle biopsy of the spine in the setting of DO.
CT-guided percutaneous biopsy
Given the complex anatomy of the spine, the presence of critical surrounding structures, and the conspicuity of abnormal tissues on CT, CT-guided biopsy is favored for percutaneous sampling of DO. At our institution, core biopsies are performed using a coaxial technique, where an introducer needle or cannula is inserted into or at the edge of the area suspicious for DO, and then a core biopsy needle is advanced coaxially through the introducer to obtain multiple core samples. In the thoracic and lumbar spine, the intervertebral disc, vertebral endplate, and adjacent paraspinal soft tissues can be accessed either by passing the needle lateral to the posterior elements (posterolateral approach) or by inserting the needle into the posterior elements and advancing it through the pedicle (transpedicular approach) (Fig. 2). Anatomy of the cervical spine permits safe access to abnormal soft tissue posteriorly and laterally but also anteriorly, where avoiding the airway, esophagus, and great vessels is necessary to prevent injury to these critical structures (Fig. 3) [37, 38].
Sagittal T1-weighted (a), STIR (b), and fat-suppressed post-contrast (c) MR images of the thoracic spine show low T1-signal intensity, high STIR signal intensity, and enhancement of the T5-6 intervertebral disc with erosive/destructive changes of the adjacent T5 and T6 vertebral endplates (vertical arrows), suggestive of discitis-osteomyelitis. There is also anterior (horizontal solid arrows) and posterior (horizontal dotted arrows) paraspinal low T1 signal intensity, high STIR signal intensity, and enhancing soft tissue consistent with phlegmon. d Axial fat-suppressed post-contrast MR image through the T5-6 disc shows paraspinal (white *) and anterior epidural (black *) enhancing soft tissue consistent with phlegmon. e Prone axial CT image through the T5-6 disc during CT-guided biopsy shows the biopsy needle targeting the T5-6 disc and endplates using a transpedicular approach (arrow). Culture grew Enterobacter cloacae
Sagittal T1-weighted (a), STIR (b), and fat-suppressed post-contrast (c) MR images of the cervical spine show low T1-signal intensity, high STIR signal intensity, and non-enhancement of the C6-7 intervertebral disc with erosive/destructive changes of the adjacent C6 and C7 vertebral endplates (vertical arrows), suggestive of discitis-osteomyelitis. There is also anterior paraspinal low T1 signal intensity, high STIR signal intensity, and enhancing soft tissue (horizontal arrows) consistent with phlegmon. d Axial fat-suppressed post-contrast MR image through the C6-7 disc redemonstrates the paraspinal enhancing soft tissue consistent with phlegmon (*). e Prone axial CT image through the C6-7 disc during CT-guided biopsy shows the biopsy needle targeting the C6-7 disc and endplates using a transpedicular approach (arrow). Culture grew Staphylococcus aureus
Prior antimicrobial exposure
Patients with suspected DO may receive antimicrobial therapy prior to the requested biopsy. Antimicrobial therapy may be initiated during the course of the patient’s work up before DO is clinically suspected or identified by imaging. In order to decrease the potential impact of antecedent antimicrobial therapy on microbiological yield from tissue sampling, the current Infectious Diseases Society of America (IDSA) guidelines for DO management recommend holding antimicrobial medications for 1–2 weeks prior to attempted biopsy [31]. Exceptions are made in cases of hemodynamic instability or neurological compromise, where immediate surgical intervention and treatment with empiric antimicrobial therapy is recommended.
Despite these recommendations, the literature does not show a clear relationship between antecedent antimicrobial therapy and decreased DO biopsy yield. A 2017 meta-analysis of open and percutaneous biopsy of DO showed a lower microbiological yield of biopsy in the setting of antecedent antimicrobial therapy (32% versus 43%) [33]. However, this difference was not statistically significant. One of the limitations of this study and of interpreting the literature on this topic more broadly is the variability of how antecedent antimicrobial therapy is defined. The criteria used to define antecedent antimicrobial therapy, including the type of medication and the time between cessation of therapy and biopsy, are variable. Furthermore, the studies included in this meta-analysis evaluated open biopsies, CT-guided percutaneous biopsies, fluoroscopically guided percutaneous biopsies, and fine needle aspirations. In some studies, the technique used for biopsies was not described. This meta-analysis also included pyogenic DO as well as non-pyogenic DO, including tuberculous DO. Many studies mixed acute and chronic osteomyelitis, for which the impact of antecedent antimicrobial therapy may be different. Two subsequent studies have similarly also shown no effect of antecedent antimicrobial exposure on microbiological yield [39, 40]. Therefore, although it is probably appropriate to follow IDSA guidelines and hold antimicrobial therapy for 1–2 weeks prior to percutaneous sampling, the lack of strong evidence supporting this recommendation underscores that sampling should still be performed if holding antimicrobials is not clinically advisable.
Repeat biopsy
Current ISDA guidelines recommend repeat biopsy in suspected DO if the initial biopsy was nondiagnostic, meaning that either no microbe or only skin contaminants could be isolated, and there are no other culture data to guide management [31]. These guidelines emphasize the importance of targeted antimicrobial therapy. However, the microbiological yield from a repeat biopsy is probably lower than from the initial biopsy, particularly in patients who have had antecedent antimicrobials [32, 34, 41]. A single study showed that 75% of initial biopsies that will ultimately yield an organism will detect the microorganism within 3 days of the biopsy. This suggests that it may be preferable to wait 3 days before repeating a percutaneous biopsy to ensure sufficient time to receive the initial biopsy culture results [42].
Technical factors
Target tissue
Although centered at the vertebral endplate and intervertebral disc, DO commonly involves surrounding soft tissue structures. The impact on microbiological yield of targeting different tissue types for percutaneous biopsy has not been thoroughly established.
Studies of positive culture rates from percutaneous biopsy of bone are highly variable. Studies of bone biopsy at all sites, although primarily of the foot in the setting of suspected diabetic foot osteomyelitis, show microbiological yield ranging from 18 to 99% [39, 43,44,45]. The applicability of these results to spine biopsies should be interpreted with caution, given that limited windows of access to abnormal bone and the presence of surrounding critical structures often means that a limited number of core samples of bone can be acquired.
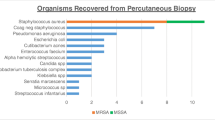
Two studies specifically comparing the microbiological yield of DO biopsy of different target tissues have yielded conflicting results. In a study of 111 biopsies, there was no significant difference in microbiological yield when paravertebral soft tissue, disc, or combined disc and vertebral endplate were sampled [46]. Other authors, in a study of 136 percutaneous spine biopsies, showed a higher microbiological yield from soft tissues, including disc and abscess, than from bone [47]. A study of osteomyelitis not limited to the spine showed aspiration of 2 mL or more of pus is associated with higher microbiological yield than biopsy of bone (Fig. 4) [48].
Sagittal T1-weighted (a), STIR (b), and fat-suppressed post-contrast (c) MR images of the lumbar spine show low T1-signal intensity, high STIR signal intensity, and non-enhancement of the L5–S1 intervertebral disc with erosive/destructive changes of the adjacent L5 and S1 vertebral endplates (vertical arrows), suggestive of discitis-osteomyelitis. There are also anterior (horizontal solid arrows) and posterior (horizontal dotted arrows) paraspinal low T1 signal intensity, high STIR signal intensity, and peripherally enhancing collections suggestive of abscesses. d Axial fat-suppressed post-contrast MR image through the L5–S1 disc shows peripherally enhancing collections adjacent to the vertebral body (white *) and the left L5-S1 facet joint (black *) suggestive of abscesses. e Prone axial CT image at the level of the S1 superior endplate during CT-guided biopsy shows the biopsy needle targeting the collection adjacent to the left L5–S1 facet joint (arrow), which yielded 3 mL of purulent material. Culture grew Mycobacterium tuberculosis
Some groups have suggested the value of submitting tissue for histopathological evaluation, which can provide diagnostic evidence of DO even if the culture results of aspiration are negative [49,50,51]. Given that the causative microorganism is frequently not isolated through percutaneous tissue sampling and that the imaging findings of DO can overlap with other noninfectious conditions, confirmation of DO through histopathology may be helpful for guiding management and potentially avoiding treating a patient without spine infection with a long course of empiric broad-spectrum antimicrobials. It should be noted, however, that the diagnostic criteria for the histopathological diagnosis of osteomyelitis are not clearly defined and, as a result, interobserver variability amongst pathologists is high [52, 53].
Needle gauge
Several needle sizes are available for percutaneous biopsy, and needle selection is dictated by the imaging approach and the target tissue. The impact of needle gauge on yield has not been definitively established. A study of 241 patients with DO showed that core needle biopsies using 14-gauge and larger needles were associated with a higher microbiological yield. However, two studies have also shown no association between needle gauge and yield, although one study did not include larger gauge (12- and 13-gauge) needles, and the majority of the biopsies in the other were performed with 14- and 15-gauge needles [46, 48]. Despite limited evidence, a larger bore needle should be considered for tissue sampling if safe access to abnormal tissue permits.
If the target of sampling is fluid, the use of a 20- gauge or larger needle can increase the likelihood that thick fluid can be successfully aspirated. As an alternative to aspiration with a needle, a biopsy needle introducer can be advanced into a suspected fluid collection and used for aspiration. If the operator is unable to aspirate fluid, a core biopsy needle can be advanced through the introducer in order to take core samples.
Number of core samples
As a result of the presence of critical structures around the spine, percutaneous access to abnormal tissue in DO is often limited to certain anatomic windows. This impacts both the approach the operator must take as well as the depth to which the needle can be advanced safely to acquire tissue samples. If care is not taken, structures distal to the tip of the needle, such as the great vessels, bowel, pleura, mediastinum, esophagus, and airway could be injured. As a result, there is often a limited number of core samples that can be obtained without repositioning the introducer needle, which would result in increased procedure time and further risk of injury to adjacent critical structures. The assumption that a larger amount of tissue acquired improves the microbiological yield is supported by the fact that open biopsies, where more tissue is acquired and submitted for culture, have a higher yield than percutaneous biopsies [54]. A study in which the number of core samples from DO biopsies was recorded showed no statistically significant difference in microbiological yield when one core was acquired compared to when two or more cores were acquired [46]. One study of 142 biopsies of bones not limited to the spine showed no association between microbiological yield and the number of core samples submitted [39]. A study of 203 bone biopsies of non-vertebral osteomyelitis also showed no impact of the number of core samples on microbiological yield [45]. None of these studies, however, specified the lengths of the cores acquired, a parameter which presumably reflects the total amount of tissue sampled. Furthermore, these studies did not specify whether core samples were taken from a single needle trajectory, or whether multiple separate sites of abnormal tissue were targeted.
Given the sparse evidence on the optimal number of core samples, operators should consider maximizing the number of core samples that can be acquired safely following a single placement of the introducer needle to provide the highest volume of tissue for culture. There is currently no evidence to support repositioning the introducer needle to acquire additional samples, given the implications of this on lengthening the procedure and increasing the risk of injury to adjacent structures.
16S rRNA
Polymerase chain reaction (PCR) targeting of 16S ribosomal RNA (rRNA) is a technique that has become increasingly available and may be a valuable tool to supplement culture data. PCR is used to amplify and sequence the bacterial rRNA gene, which is present in all bacteria and which contains regions that are conserved across many species, to determine the species of a bacterial pathogen [55]. This tool may be of particular utility for pathogens that are difficult to grow in laboratory culture media or that have been killed by antecedent antimicrobial therapy [56,57,58].
To our knowledge, the utility of this technique in identifying the causative pathogen in DO has not been thoroughly evaluated. In a study of 104 biopsy or aspirate samples, 83 of which were orthopedic samples, authors reported a high diagnostic sensitivity and specificity of PCR (88.5% and 83.5%, respectively) [59]. A study of samples in 71 patients showed that PCR results changed management in 18 patients (25%). A study of 132 clinical samples that included tissues of all types showed that 16S PCR findings, when interpreted in light of other culture information, resulted in treatment change in only 6 patients (4.5%) [56]. In a study including 391 musculoskeletal specimens of various types, the PCR positivity rate in infected cases was 30%, and the PCR data only led to a change of management in 6% [60]. The majority of these samples were joint fluid or soft tissue; only 4% of the specimens were of bone. More evidence is needed to understand the value of routine 16S rRNA PCR in DO.
Conclusion
Percutaneous sampling of DO is often indicated in order to obtain culture and sensitivity information to guide targeted antimicrobial therapy. However, the microbiological yield from percutaneous sampling is less than 50%. Per ISDA guidelines, antimicrobial therapy should be held for 1–2 weeks prior to percutaneous sampling [31]. However, given weak evidence supporting this recommendation, sampling can still be considered if delaying biopsy until antimicrobial therapy has been withheld is not clinically advisable. When performing percutaneous sampling, paraspinal fluid collections should be targeted if present, as this may be associated with higher microbiological yield. Other soft tissue targets such as disc material or paravertebral phlegmon may have higher microbiological yield than bone. Submitting tissue samples for histopathological analysis can be helpful if histopathological confirmation of infection is needed to guide management, although histopathological definitions of osteomyelitis result in substantial interobserver variability amongst pathologists. Larger gauge needles (12- and 13-gauge) should be favored when technically feasible. More work is needed to better understand how these and other clinical and technical factors impact microbiological yield from percutaneous sampling of DO.
References
Lu Y-A, Hsu H-H, Kao H-K, Lee C-H, Lee S-Y, Chen G-H, Hung C-C, Tian Y-C. Infective spondylodiscitis in patients on maintenance hemodialysis: a case series. Ren Fail. 2017;39:179–86.
Wang Z, Lenehan B, Itshayek E, Boyd M, Dvorak M, Fisher C, Kwon B, Paquette S, Street J. Primary pyogenic infection of the spine in intravenous drug users: a prospective observational study. Spine. 2012;37:685–92.
Pigrau C, Almirante B, Flores X, Falco V, Rodríguez D, Gasser I, Villanueva C, Pahissa A. Spontaneous pyogenic vertebral osteomyelitis and endocarditis: incidence, risk factors, and outcome. Am J Med. 2005;118:1287.
Mylona E, Samarkos M, Kakalou E, Fanourgiakis P, Skoutelis A. Pyogenic vertebral osteomyelitis: a systematic review of clinical characteristics. Semin Arthritis Rheum. 2009;39:10–7.
Skaf GS, Domloj NT, Fehlings MG, Bouclaous CH, Sabbagh AS, Kanafani ZA, Kanj SS. Pyogenic spondylodiscitis: an overview. J Infect Public Health. 2010;3:5–16.
Torda AJ, Gottlieb T, Bradbury R. Pyogenic Vertebral osteomyelitis: analysis of 20 cases and review. Clin Infect Dis. 1995;20:320–8.
Digby JM, Kersley JB. Pyogenic non-tuberculous spinal infection: an analysis of thirty cases. J Bone Joint Surg Br. 1979;61:47–55.
Beronius M, Bergman B, Andersson R. Vertebral osteomyelitis in Göteborg, Sweden: a retrospective study of patients during 1990–95. Scand J Infect Dis. 2001;33:527–32.
Reihsaus E, Waldbaur H, Seeling W. Spinal epidural abscess: a meta-analysis of 915 patients. Neurosurg Rev. 2000;23:175–204 (discussion 205).
Gouliouris T, Aliyu SH, Brown NM. Spondylodiscitis: update on diagnosis and management. J Antimicrob Chemother. 2010;65(Suppl 3):iii11-24.
Corrah TW, Enoch DA, Aliyu SH, Lever AM. Bacteraemia and subsequent vertebral osteomyelitis: a retrospective review of 125 patients. QJM Mon J Assoc Physicians. 2011;104:201–7.
An HS, Seldomridge JA. Spinal infections: diagnostic tests and imaging studies. Clin Orthop. 2006;444:27–33.
The intrinsic vasculature of developing vertebral end plates and its nutritive significance to the intervertebral discs. - Abstract - Europe PMC. https://europepmc.org/article/med/4019751. Accessed 16 May 2020.
Rudert M, Tillmann B. Lymph and blood supply of the human intervertebral disc. Cadaver study of correlations to discitis. Acta Orthop Scand. 1993;64:37–40.
Cheung WY, Luk KDK. Pyogenic spondylitis. Int Orthop. 2012;36:397–404.
Mader J, Calhoun J Osteomyelitis. In: Mandell G, Douglas R, Bennett JJ (eds) Princ. Pract. Infect. Dis. Churchill Livingstone, p 1039; 1995.
Govender S. Spinal infections. J Bone Joint Surg Br-B. 2005;87:1454–8.
Section 19, Chapter 5: Postoperative spondylodiscitis – International Society for the Study of the Lumbar Spine.
Modic MT, Feiglin DH, Piraino DW, Boumphrey F, Weinstein MA, Duchesneau PM, Rehm S. Vertebral osteomyelitis: assessment using MR. Radiology. 1985;157:157–66.
Rehm J, Veith S, Akbar M, Kauczor H, Weber M. CT-Guided percutaneous spine biopsy in suspected infection or malignancy: a study of 214 patients. RöFo - Fortschritte Auf Dem Geb Röntgenstrahlen Bildgeb Verfahr. 2016;188:1156–62.
Ledermann HP, Schweitzer ME, Morrison WB, Carrino JA. MR imaging findings in spinal infections: rules or myths? Radiology. 2003;228:506–14.
Spira D, Germann T, Lehner B, Hemmer S, Akbar M, Jesser J, Weber M-A, Rehnitz C. CT-guided biopsy in suspected spondylodiscitis–the association of paravertebral inflammation with microbial pathogen detection. PLoS ONE. 2016;11: e0146399.
Kumar Y, Gupta N, Chhabra A, Fukuda T, Soni N, Hayashi D. Magnetic resonance imaging of bacterial and tuberculous spondylodiscitis with associated complications and non-infectious spinal pathology mimicking infections: a pictorial review. BMC Musculoskelet Disord. 2017. https://doi.org/10.1186/s12891-017-1608-z.
Hong SH, Choi J-Y, Lee JW, Kim NR, Choi J-A, Kang HS. MR imaging assessment of the spine: infection or an Imitation? Radiographics. 2009;29:599–612.
Li T, Liu T, Jiang Z, Cui X, Sun J. Diagnosing pyogenic, brucella and tuberculous spondylitis using histopathology and MRI: a retrospective study. Exp Ther Med. 2016;12:2069–77.
Sharif HS. Role of MR imaging in the management of spinal infections. AJR Am J Roentgenol. 1992;158:1333–45.
Simeone FJ, Husseini JS, Yeh KJ, Lozano-Calderon S, Nelson SB, Chang CY. MRI and clinical features of acute fungal discitis/osteomyelitis. Eur Radiol. 2020. https://doi.org/10.1007/s00330-019-06603-z.
Williams RL, Fukui MB, Meltzer CC, Swarnkar A, Johnson DW, Welch W. Fungal spinal osteomyelitis in the immunocompromised patient: MR findings in three cases. AJNR Am J Neuroradiol. 1999;20:381–5.
Sharif HS, Aideyan OA, Clark DC, Madkour MM, Aabed MY, Mattsson TA, al-Deeb SM, Moutaery KR,. Brucellar and tuberculous spondylitis: comparative imaging features. Radiology. 1989;171:419–25.
Priest JR, Low D, Wang C, Bush T. Brucellosis and sacroiliitis: a common presentation of an uncommon pathogen. J Am Board Fam Med JABFM. 2008;21:158–61.
Berbari EF, Kanj SS, Kowalski TJ, et al. 2015 Infectious Diseases Society of America (IDSA) Clinical Practice Guidelines for the diagnosis and treatment of native vertebral osteomyelitis in adults. Clin Infect Dis Off Publ Infect Dis Soc Am. 2015;61:e26-46.
Gras G, Buzele R, Parienti JJ, et al. Microbiological diagnosis of vertebral osteomyelitis: relevance of second percutaneous biopsy following initial negative biopsy and limited yield of post-biopsy blood cultures. Eur J Clin Microbiol Infect Dis Off Publ Eur Soc Clin Microbiol. 2014;33:371–5.
McNamara AL, Dickerson EC, Gomez-Hassan DM, Cinti SK, Srinivasan A. Yield of image-guided needle biopsy for infectious discitis: a systematic review and meta-analysis. Am J Neuroradiol. 2017;38:2021–7.
Marschall J, Bhavan KP, Olsen MA, Fraser VJ, Wright NM, Warren DK. The impact of prebiopsy antibiotics on pathogen recovery in hematogenous vertebral osteomyelitis. Clin Infect Dis Off Publ Infect Dis Soc Am. 2011;52:867–72.
Huang AJ, Kattapuram SV. Musculoskeletal neoplasms: biopsy and intervention. Radiol Clin North Am. 2011;49(1287–1305):vii.
Skrzynski MC, Biermann JS, Montag A, Simon MA. Diagnostic accuracy and charge-savings of outpatient core needle biopsy compared with open biopsy of musculoskeletal tumors. J Bone Joint Surg Am. 1996;78:644–9.
Cox M, Pukenas B, Poplawski M, Bress A, Deely D, Flanders A. CT-guided cervical bone biopsy in 43 patients: diagnostic yield and safety at two large tertiary care hospitals. Acad Radiol. 2016;23:1372–5.
Kattapuram SV, Rosenthal DI. Percutaneous biopsy of the cervical spine using CT guidance. AJR Am J Roentgenol. 1987;149:539–41.
Schirò S, Foreman SC, Bucknor M, Chin CT, Joseph GB, Link TM. Diagnostic performance of CT-guided bone biopsies in patients with suspected osteomyelitis of the appendicular and axial skeleton with a focus on clinical and technical factors associated with positive microbiology culture results. J Vasc Interv Radiol JVIR. 2020;31:464–72.
Hoang D, Fisher S, Oz OK, La Fontaine J, Chhabra A. Percutaneous CT guided bone biopsy for suspected osteomyelitis: Diagnostic yield and impact on patient’s treatment change and recovery. Eur J Radiol. 2019;114:85–91.
Czuczman GJ, Marrero DE, Huang AJ, Mandell JC, Ghazikhanian V, Simeone FJ. Diagnostic yield of repeat CT-guided biopsy for suspected infectious spondylodiscitis. Skeletal Radiol. 2018;47:1403–10.
Yeh KJ, Husseini JS, Hemke R, Nelson SB, Chang CY. CT-guided discitis-osteomyelitis biopsies with negative microbiology: how many days should we wait before repeating the biopsy? Skeletal Radiol. 2020;49:619–23.
Schechter MC, Ali MK, Risk BB, Singer AD, Santamarina G, Rogers HK, Rajani RR, Umpierrez G, Fayfman M, Kempker RR. Percutaneous bone biopsy for diabetic foot osteomyelitis: a systematic review and meta-analysis. Open Forum Infect Dis. 2020;7:ofaa393.
Said N, Chalian M, Fox MG, Nacey NC. Percutaneous image-guided bone biopsy of osteomyelitis in the foot and pelvis has a low impact on guiding antibiotics management: a retrospective analysis of 60 bone biopsies. Skeletal Radiol. 2019;48:1385–91.
Hirschfeld CB, Kapadia SN, Bryan J, Jannat-Khah DP, May B, Vielemeyer O, Esquivel EL. Impact of diagnostic bone biopsies on the management of non-vertebral osteomyelitis: A retrospective cohort study. Medicine (Baltimore). 2019;98: e16954.
Chang CY, Simeone FJ, Nelson SB, Taneja AK, Huang AJ. Is Biopsying the paravertebral soft tissue as effective as biopsying the disk or vertebral endplate? 10-year retrospective review of CT-guided biopsy of diskitis-osteomyelitis. AJR Am J Roentgenol. 2015;205:123–9.
Kim C-J, Kang S-J, Choe PG, et al. Which tissues are best for microbiological diagnosis in patients with pyogenic vertebral osteomyelitis undergoing needle biopsy? Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis. 2015;21:931–5.
Wu JS, Gorbachova T, Morrison WB, Haims AH. Imaging-guided bone biopsy for osteomyelitis: are there factors associated with positive or negative cultures? Am J Roentgenol. 2007;188:1529–34.
Michel SCA, Pfirrmann CWA, Boos N, Hodler J. Ct-guided core biopsy of subchondral bone and intervertebral space in suspected spondylodiskitis. AJR Am J Roentgenol. 2006;186:977–80.
Howard CB, Einhorn M, Dagan R, Yagupski P, Porat S. Fine-needle bone biopsy to diagnose osteomyelitis. J Bone Joint Surg Br. 1994;76:311–4.
White LM, Schweitzer ME, Deely DM, Gannon F. Study of osteomyelitis: utility of combined histologic and microbiologic evaluation of percutaneous biopsy samples. Radiology. 1995;197:840–2.
Sybenga AB, Jupiter DC, Speights VO, Rao A. Diagnosing osteomyelitis: a histology guide for pathologists. J Foot Ankle Surg Off Publ Am Coll Foot Ankle Surg. 2020;59:75–85.
Meyr AJ, Singh S, Zhang X, Khilko N, Mukherjee A, Sheridan MJ, Khurana JS. Statistical reliability of bone biopsy for the diagnosis of diabetic foot osteomyelitis. J Foot Ankle Surg Off Publ Am Coll Foot Ankle Surg. 2011;50:663–7.
Yang S-C, Fu T-S, Chen L-H, Chen W-J, Tu Y-K. Identifying pathogens of spondylodiscitis: percutaneous endoscopy or CT-guided biopsy. Clin Orthop. 2008;466:3086–92.
Reller LB, Weinstein MP, Petti CA. Detection and identification of microorganisms by gene amplification and sequencing. Clin Infect Dis. 2007;44:1108–14.
Bador J, Nicolas B, Chapuis A, Varin V, Dullier-Taillefumier N, de Curraize C, Amoureux L, Putot A, Neuwirth C. 16S rRNA PCR on clinical specimens: Impact on diagnosis and therapeutic management. Med Mal Infect. 2020;50:63–73.
Larsen LH, Khalid V, Xu Y, Thomsen TR, Schønheyder HC, the PRIS Study Group. Differential contributions of specimen types, culturing, and 16S rRNA Sequencing in diagnosis of prosthetic joint infections. J Clin Microbiol. 2018;56:e01351-e1417.
Mishra D, Satpathy G, Wig N, Fazal F, Ahmed NH, Panda SK. Evaluation of 16S rRNA broad range PCR assay for microbial detection in serum specimens in sepsis patients. J Infect Public Health. 2020;13:998–1002.
Grif K, Heller I, Prodinger WM, Lechleitner K, Lass-Flörl C, Orth D. Improvement of detection of bacterial pathogens in normally sterile body sites with a focus on orthopedic samples by use of a commercial 16S rRNA broad-range PCR and sequence analysis. J Clin Microbiol. 2012;50:2250–4.
Fida M, Khalil S, Abu Saleh O, Challener DW, Sohail MR, Yang JN, Pritt BS, Schuetz AN, Patel R. Diagnostic value of 16S ribosomal RNA gene polymerase chain reaction/sanger sequencing in clinical practice. Clin Infect Dis Off Publ Infect Dis Soc Am. 2021;73:961–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key points
• Yield from biopsy of vertebral discitis-osteomyelitis is low.
• Technical factors that may improve yield include targeting paraspinal fluid and soft tissue abnormalities, collecting multiple core samples if feasible, and use of larger needle gauge.
• Although guidelines suggest holding antimicrobial therapy prior to biopsy, biopsy should be considered if cessation of antimicrobial treatment is not clinically reasonable.
• Repeat sampling can be considered in the setting of a negative initial biopsy but should be performed no sooner than 72 h from the initial biopsy.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Husseini, J.S., Huang, A.J. Discitis-osteomyelitis: optimizing results of percutaneous sampling. Skeletal Radiol 52, 1815–1823 (2023). https://doi.org/10.1007/s00256-022-04151-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-022-04151-0