Abstract
Purpose
To determine the number of days to positive CT-guided biopsy sample culture in patients with discitis-osteomyelitis.
Methods
Our study was IRB approved and HIPAA compliant. All CT-guided biopsies performed for acute discitis-osteomyelitis with positive microbiology between 2002 and 2018 were reviewed. Microbiological organism and days to positive biopsy were documented. Mean, median, skew, and standard deviation were calculated. The proportion of positive cultures that become positive after each day has elapsed was also calculated.
Results
There were 96 true positive cultures, with 64 (67%) male and 32 (33%) female, ages 57 ± 18 (range 19–87) years. Overall, including all culture results, the mean number of days to positive culture was 2.9 ± 3.5 days. The median number of days was 2, with a positive skew of 2.9. At days 1, 2, 3, 4, and 5, 48%, 68%, 78%, 85%, and 89%, respectively, of biopsy samples had a positive microbiology culture.
Conclusion
Approximately three-quarters of discitis-osteomyelitis pathogens will be identified by biopsy sample culture by 3 days after CT-guided biopsy. This finding should be considered if planning for a repeat biopsy in the setting of a negative microbiology culture.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
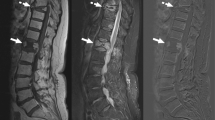
Percutaneous CT-guided biopsy is currently the first-line procedure for sampling discitis-osteomyelitis in the setting of negative blood cultures to determine the causative microbiological organism, because it is safe, accurate, and minimally invasive [1, 2]. A meta-analysis from 2017 estimates diagnostic yield for percutaneous biopsy at 48% [3, 4]. When a biopsy does not yield a positive microbiology result, a second biopsy can improve the yield, but the reported yield in the second biopsy has been as low as 14% and as high as 60% [5, 6]. Nevertheless, even if the potential yield is low, the biopsy may be warranted because of the potential impact on patient management, and therefore, a second biopsy is also recommended in the most recent (2015) Infectious Disease Society of America (IDSA) clinical practice guidelines for discitis-osteomyelitis [7,8,9,10]. Although routine cultures for bacterial organisms are held for 7 days, most positive cultures likely present in a shorter period of time. While diagnostic yield for surgical biopsy is approximately 76% and has been reported to be as high as 91%, procedure and hospitalization time, complication rate, and patient morbidity are also higher for surgical biopsies [1,2,3,4].
The appropriate number of days to wait for cultures to turn positive after an initial biopsy and before repeating the biopsy is not known. If the waiting period is too short, many unnecessary repeat biopsies would be performed. If the waiting period is too long, the patient may suffer delays before the initiation of appropriate treatment. Therefore, the purpose of this study was to determine the percutaneous CT-guided spine biopsy culture time to positivity in patients with discitis-osteomyelitis, in order to determine the number of days to wait for culture results before repeating a biopsy.
Materials and methods
Patient selection
This investigation was approved by the institutional review board and was compliant with the guidelines of the Health Insurance Portability and Accountability Act (HIPAA). From a searchable database of all radiology studies performed at our institution between 2002 and 2018, we retrospectively identified 250 cases of suspected discitis-osteomyelitis, which were percutaneously biopsied using CT guidance. Of these, 104/250 (42%) had positive microbiology results.
Spine biopsy technique
All procedures were performed using a GE Lightspeed 16 slice CT scanner. A total of 10 radiologists with 1–41 years of experience were the primary operators on the procedures. Procedural CT images were acquired with 100–140 kVp and 40–440 mAs, 2.5–5.0 mm slice thickness, and 22–36 cm field of view. Soft tissue kernel was applied.
All biopsies were performed using coaxial technique. Biopsies including portions of endplate bone were performed with an Arrow OnControl system (12 or 13 gauge) (Teleflex Medical, Wayne, PA), Ackerman biopsy needle (14 gauge) (Cook Medical, Bloomington, IN), or Ostycut biopsy needle (16 gauge) (Bard Biopsy, Tempe, AZ) to perform the bone biopsy. For soft tissue biopsies, a Temno Evolution core biopsy needle system (14, 16, 18, or 20 gauge) (Merit Medical, Jordan, UT) was used. Core samples were placed in sterile, preservative-free, non-bacteriostatic normal saline.
Samples were sent for microbiological and pathological analysis, including Gram stain, acid-fast stain, fungal stain, and culture. Aerobic and anaerobic cultures were incubated for a 7-day period, unless otherwise requested, while fungal and mycobacterial cultures were held for 29 and 56 days, respectively.
Microbiological analysis
Microbiology and pathology medical results of all positive aerobic, anaerobic, mycobacterial, and fungal cultures from the CT-guided procedures were evaluated. All cases were evaluated by an infectious disease consultant. True positives and false positives were distinguished through a combination of pathology obtained at the time of biopsy, imaging (MRI, CT) prior to the biopsy, imaging 1 week to 1 year after the biopsy, clinical presentation and follow-up, response to treatment, and laboratory values (CBC, ESR, CRP, blood culture). The number of days to a positive microbiology result was recorded. “Day 0” was the day the CT-guided biopsy was performed and the culture incubation began. The earliest the cultures were checked was day 1 (i.e., 1 day after the biopsy was performed). The number of days to positive culture was defined as the initial day from day 0 that any growth was identified in the culture, even if the specific organism was not identified until a later date. The mean, median, skew, standard deviation, and proportion of biopsies positive on each day up to 7 days were calculated. The causative microbiology organism was also recorded.
Results
The true positive group included 96 patients. There were 64 (67%) males and 32 (33%) females. The mean age was 57 ± 18 (range 19–87) years. Within this group, 30 (31%) had antibiotics within 1 week prior to the biopsy, 21 (22%) had a history of intravenous drug use, and 39 (41%) were immunocompromised (diabetes, cirrhosis, cancer on treatment, end-stage renal disease, inflammatory disease (rheumatoid arthritis, psoriasis) on treatment, and liver transplant on immunosuppression).
The microbiology results are detailed in Table 1. The majority (84/98, 86%) of causative organisms were bacteria. There were 10 (10%) bone only, 30 (31%) bone and disc, 48 (50%) disc only, and 10 (10%) paravertebral soft tissue biopsies.
The number of days to positive biopsy is detailed in Tables 2, 3, and 4. Overall, including all culture results, the mean number of days to positive culture was 2.7 ± 3.4 days. The median number of days was 1.5, with a positive skew of 3.1. By days 2, 3, and 4, 69%, 80%, and 87% of biopsy samples had a positive microbiology culture.
The mean number of days to positive bacterial culture was slightly shorter, 1.9 ± 2.0 days, with a median of 1 day and positive skew of 2.4 days. The mean number of days to positive fungal culture was similar to the overall culture results, 2.9 ± 0.83 days, with a median of 3 days and positive skew of 0.83 days. The mean number of days to positive mycobacterial culture was longer, 12.8 ± 5.8 days, with a median of 13.5 days and negative skew of − 0.63 days.
The number of days to positive culture was similar among the biopsy tissue types (Table 3). The mean number of days to positive culture appears slightly longer for the paravertebral soft tissue samples. Of note, the paravertebral soft tissue group includes 2 Mycobacterium tuberculosis patients, whose cultures were positive on days 14 and 21.
The false-positive group included 8 patients. There were 7 (88%) males and 1 (12%) female. The mean age was 59 ± 16 (range 31–84) years. Within this group, no patients had antibiotics within 1 week prior to the biopsy, 2 (25%) had a history of intravenous drug use, and 6 (75%) were immunocompromised (diabetes, end-stage renal disease, and diabetes). The cultured organisms were Staphylococcus (4/8, 50%) epidermidis and Propionibacter acnes (4/8, 50%). The false-positive microbiology cultures turned positive on days 3, 4, 5, 5, 5, 6, 6, and 7. The mean (± SD), median, and skew were 5.1 (± 1.2), 5, and − 0.3 days.
Discussion
Our findings show that most discitis-osteomyelitis pathogens will be identified by 3 days after CT-guided biopsy, suggesting that a repeat biopsy should be considered as soon as 3 days after the initial biopsy, if the microbiology culture remains negative.
Percutaneous CT-guided biopsy has an important role in discitis-osteomyelitis. The diagnosis of discitis-osteomyelitis is made through a combination of clinical features, laboratory studies, and MRI. However, determining the causative organism is important for tailoring antibiotic treatment [5, 11,12,13]. Although dependent on the offending pathogen, positive blood cultures are present in only approximately one-half of patients with discitis-osteomyelitis [14,15,16]. If the blood culture is negative, image-guided biopsy is currently the first-line procedure for sampling discitis-osteomyelitis to determine the causative microbiological organism, because it is safe, accurate, and minimally invasive [1, 2]. The number of days to wait before repeating the biopsy is unknown. The bacterial cultures are routinely held for 7 days in order to minimize the number of false-negative cases. Nevertheless, even if the potential yield is low, the biopsy may be warranted because of the potential impact on patient management [7,8,9]. Our study shows that the mean and median days to positive culture are 2.9 and 2 days, respectively. By day 3, 78% of cultures had shown microbiological growth. These findings suggest that, if the culture remains negative on day 3, a repeat biopsy, if warranted, could be considered. If a repeat biopsy was considered too early, then many of those biopsy would have been unnecessary. If the biopsy is delayed for a long period of time, appropriate treatment would be delayed [4, 7, 17,18,19].
Current literature regarding time to positive culture time focuses on blood cultures obtained in patients with systemic inflammation reaction syndrome and suspicious for bacteremia. Shorter time to positive blood culture may be a proxy for burden of infection and associated with poorer outcomes including increased mortality [20, 21]. The offending pathogen may also affect the time to positive blood culture, with Candida showing the longest time to positive blood culture [22]. In our study, the sample size of nonbacterial pathogens was not large enough to detect differences in time to culture positivity, also a limitation of this study.
The distribution of the type of bacteria in this study cohort is also interesting. The typical causative bacterial organisms are Staphylococcus aureus, streptococcal species, enteric bacteria, and other Gram-negative rods [10]. In our study, the most common organism in this study was Staphylococcus aureus (22/98, 22%). However, in a 2009 review of the literature, Mylona et al. surveyed 8 studies, and in this cohort, the percentages of Staphylococcus aureus cases were 24, 34, 40, 40, 42, 52, and 60%, all higher than the percentage seen in our cohort [16]. In more recent studies, Ziu et al. found 60/164 (37%) of their cohort had Staphylococcus aureus infections, and Hopkinson and Patel found 9/23 (39%) of their cohort had Staphylococcus aureus infections [23, 24]. The second most common organism in our study was Coagulase-negative Staphylococcus, an organism which colonizes the skin. Furthermore, Salmonella species were more common in our cohort than Escherichia coli. Our cohort only includes the patients which had negative blood cultures, and it is possible that this group has more atypical bacteria and/or bacteria that is more difficult to detect in the patient’s blood. Alternatively, the organisms that were cultured may be present in the tissues in higher concentrations. Certainly there is some unidentified factor, which is selecting for a unique group of micro-organisms.
The effect of antibiotics on culture and time to positive culture are unknown. Some studies have shown a decreased microbiological yield. Presumably, the effects are dependent on multiple factors, including (1) causative microbiological organism; (2) type of antibiotics; (3) antibiotic dose; (4) sensitivity of the organism to the antibiotics administered; (5) number of doses administered; and (6) time gap between the most recent dose of antibiotic and the biopsy. The IDSA guidelines recommend holding antibiotics for 1–2 weeks pending a microbiological culture with sensitivities when possible [10, 25]. Unfortunately, we have found in our institution that many patients receive at least one dose of broad spectrum antibiotics prior to the input of an infectious disease consult. Although we looked at antibiotic administration prior to biopsy, we found that our population was too heterogeneous regarding the type, dose, number of doses, and time between dose and biopsy to draw any meaningful conclusions, another limitation of this retrospective study. In an ideal situation, these factors could be carefully controlled and studied.
The mean days to positive biopsy were similar for all biopsied tissue types, except for paravertebral soft tissue, where the mean was 5.5 days, compared with 2.7 days for all samples. The paravertebral soft tissue biopsy group only included 10 cases, two of which were Mycobacterium tuberculosum (MTB). MTB has an atypical presentation with a disproportionately large soft tissue mass and relative sparing of the discs [26, 27]. Therefore, it makes sense in these situations to target the paravertebral soft tissues and may be a confounding factor in the observed differences.
The average number of days to positive culture was longer for the false-positive group (5.1 days) compared with the true positive group 2.9 days. Although meaningful statistics cannot be performed given the small number of false-positive cases, and the imbalance between the size of the true positive and false positive groups, this observation is interesting and should be further studied.
The main limitation of this study is its retrospective study design. Because the protocol for checking the microbiology growth in the cultures is not performed in a controlled fashion, and because the time of sample collection is not precisely recorded, the time point granularity of this study is restricted to “days.” Ideally, the study would be performed prospectively, with the exact time of sample collection recorded, immediate delivery of the sample to microbiology, immediate processing of the biopsy sample by the microbiology lab, and hourly evaluation of the cultures for growth.
In conclusion, if a microbiology culture remains negative after 3 days after initial CT-guided biopsy for discitis-osteomyelitis, a second biopsy, if warranted, may be considered.
References
Huang AJ, Kattapuram SV. Musculoskeletal neoplasms: biopsy and intervention. Radiol Clin N Am. 2011;49:1287–305 vii.
Skrzynski MC, Biermann JS, Montag A, Simon MA. Diagnostic accuracy and charge-savings of outpatient core needle biopsy compared with open biopsy of musculoskeletal tumors. J Bone Joint Surg Am. 1996;78:644–9.
McNamara AL, Dickerson EC, Gomez-Hassan DM, Cinti SK, Srinivasan A. Yield of image-guided needle biopsy for infectious discitis: a systematic review and meta-analysis. AJNR Am J Neuroradiol. 2017;38:2021–7.
Marschall J, Bhavan KP, Olsen MA, Fraser VJ, Wright NM, Warren DK. The impact of prebiopsy antibiotics on pathogen recovery in hematogenous vertebral osteomyelitis. Clin Infect Dis. 2011;52:867–72.
Czuczman GJ, Marrero DE, Huang AJ, Mandell JC, Ghazikhanian V, Simeone FJ. Diagnostic yield of repeat CT-guided biopsy for suspected infectious spondylodiscitis. Skelet Radiol. 2018;47:1403–10.
Terreaux W, Geoffroy M, Ohl X, Job L, Cart P, Eschard J-P, et al. Diagnostic contribution of a second percutaneous needle biopsy in patients with spontaneous diskitis and negative blood cultures and first biopsy. Joint Bone Spine. 2016;83:715–9.
Zimmerli W. Clinical practice. Vertebral osteomyelitis. N Engl J Med. 2010;362:1022–9.
Devkota P, Krishnakumar R, Renjith KJ. Surgical management of pyogenic discitis of lumbar region. Asian Spine J. 2014;8:177–82.
Perronne C, Saba J, Behloul Z, Salmon-Céron D, Leport C, Vildé JL, et al. Pyogenic and tuberculous spondylodiskitis (vertebral osteomyelitis) in 80 adult patients. Clin Infect Dis. 1994;19:746–50.
Berbari EF, Kanj SS, Kowalski TJ, Darouiche RO, Widmer AF, Schmitt SK, et al. 2015 Infectious Diseases Society of America (IDSA) clinical practice guidelines for the diagnosis and treatment of native vertebral osteomyelitis in adults. Clin Infect Dis. 2015;61:e26–46.
Carragee EJ, Kim D, van der Vlugt T, Vittum D. The clinical use of erythrocyte sedimentation rate in pyogenic vertebral osteomyelitis. Spine. 1997;22:2089–93.
Conrad DA. Acute hematogenous osteomyelitis. Pediatr Rev. 2010;31:464–71.
Ledermann HP, Schweitzer ME, Morrison WB, Carrino JA. MR imaging findings in spinal infections: rules or myths? Radiology. 2003;228:506–14.
Corrah TW, Enoch DA, Aliyu SH, Lever AM. Bacteraemia and subsequent vertebral osteomyelitis: a retrospective review of 125 patients. QJM. 2011;104:201–7.
Sobottke R, Seifert H, Fätkenheuer G, Schmidt M, Gossmann A, Eysel P. Current diagnosis and treatment of spondylodiscitis. Dtsch Arztebl Int. 2008;105:181–7.
Mylona E, Samarkos M, Kakalou E, Fanourgiakis P, Skoutelis A. Pyogenic vertebral osteomyelitis: a systematic review of clinical characteristics. Semin Arthritis Rheum. 2009;39:10–7.
An HS, Seldomridge JA. Spinal infections: diagnostic tests and imaging studies. Clin Orthop Relat Res. 2006;444:27–33.
Rankine JJ, Barron DA, Robinson P, Millner PA, Dickson RA. Therapeutic impact of percutaneous spinal biopsy in spinal infection. Postgrad Med J. 2004;80:607–9.
Sehn JK, Gilula LA. Percutaneous needle biopsy in diagnosis and identification of causative organisms in cases of suspected vertebral osteomyelitis. Eur J Radiol. 2012;81:940–6.
Marra AR, Edmond MB, Forbes BA, Wenzel RP, Bearman GML. Time to blood culture positivity as a predictor of clinical outcome of Staphylococcus aureus bloodstream infection. J Clin Microbiol. 2006;44:1342–6.
Siméon S, Le Moing V, Tubiana S, Duval X, Fournier D, Lavigne J-P, et al. Time to blood culture positivity: an independent predictor of infective endocarditis and mortality in patients with Staphylococcus aureus bacteraemia. Clin Microbiol Infect. 2019;25:481–8.
Ning Y, Hu R, Yao G, Bo S. Time to positivity of blood culture and its prognostic value in bloodstream infection. Eur J Clin Microbiol Infect Dis. 2016;35:619–24.
Ziu M, Dengler B, Cordell D, Bartanusz V. Diagnosis and management of primary pyogenic spinal infections in intravenous recreational drug users. Neurosurg Focus. 2014;37:E3.
Hopkinson N, Patel K. Clinical features of septic discitis in the UK: a retrospective case ascertainment study and review of management recommendations. Rheumatol Int. 2016;36:1319–26.
Trampuz A, Piper KE, Jacobson MJ, Hanssen AD, Unni KK, Osmon DR, et al. Sonication of removed hip and knee prostheses for diagnosis of infection. N Engl J Med. 2007;357:654–63.
Kumar K. Spinal tuberculosis, natural history of disease, classifications and principles of management with historical perspective. Eur J Orthop Surg Traumatol. 2016;26:551–8.
Sharma A, Chhabra HS, Chabra T, Mahajan R, Batra S, Sangondimath G. Demographics of tuberculosis of spine and factors affecting neurological improvement in patients suffering from tuberculosis of spine: a retrospective analysis of 312 cases. Spinal Cord. 2017;55:59–63.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was waived for individual participants included in the study. The study was approved by the local Institutional Review Board (IRB) and HIPAA compliant.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yeh, K.J., Husseini, J.S., Hemke, R. et al. CT-guided discitis-osteomyelitis biopsies with negative microbiology: how many days should we wait before repeating the biopsy?. Skeletal Radiol 49, 619–623 (2020). https://doi.org/10.1007/s00256-019-03344-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-019-03344-4