Abstract
Objective
Image-guided percutaneous bone biopsy may be requested by clinicians to guide antibiotics management decisions in patients with extremity osteomyelitis. Much of the clinical literature describes a high rate of bone biopsy culture positivity in patients with osteomyelitis, but anecdotally biopsy is felt to be fairly low yield in many musculoskeletal radiology practices. The objective of the study is to determine the culture positivity rate and clinical utility of bone biopsy in guiding the management of patients with osteomyelitis.
Materials and methods
All image-guided bone biopsy procedures of the pelvis or foot performed at a single institution were identified by a retrospective report search, and only those with a clinical suspicion for infection were included. Cases were included based on convincing imaging findings of osteomyelitis on retrospective review. Microbiology results were reviewed in the clinical chart, as were antibiotics management decisions and response to antibiotics therapy.
Results
A total of 60 bone biopsies met the inclusion criteria, 25 within the foot and 35 biopsies of the pelvis. Overall, 11 out of 60 core biopsies (18%) yielded positive cultures. Antibiotics management was altered in only 27% patients with a positive culture; thus, only 5% of patients with MRI findings of osteomyelitis undergoing biopsy had an impact on management.
Conclusion
Percutaneous bone biopsies may have a low rate of culture positivity, and even when positive, frequently do not have an impact on antibiotics choice. These data differ from much of the clinical literature, which describes a very high rate of culture positivity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Osteomyelitis poses a complex challenge for clinicians, as patients typically have multiple other systemic medical problems. The Infectious Disease Society of America (IDSA) [1] recommends a deep tissue biopsy from appropriately obtained specimens for culture before starting empiric antibiotics treatment for diabetic pedal osteomyelitis; in many situations, this will be a percutaneous biopsy performed under imaging guidance. Adherence to these clinical guidelines likely varies widely among clinicians and across institutions, with empiric antibiotics chosen by some and aggressive pursuance of biopsy by others. Surgery remains the mainstay of therapy for pelvic decubitus ulcer osteomyelitis patients, with antibiotics used to augment infection control after biopsy material is obtained as part of a surgical debridement procedure [2,3,4]. However, percutaneous biopsy is a frequently requested intervention in these patients at the authors’ institution.
There has been a recent paradigm shift in the management of pedal osteomyelitis as surgical debridement has fallen progressively out of favor with increasing use of aggressive antibiotics therapy [1, 5, 6]. This approach relies heavily on percutaneous bone biopsy to tailor antibiotics to a specific infectious agent. This recommendation is based in large part on several studies cited in the infectious disease literature that demonstrate rates of culture positivity well over 75%.
At the authors’ institution, it was felt that the culture positivity yield for percutaneous biopsies was significantly lower than that cited in the IDSA guidelines. Wu et al. found a culture positivity growth rate of only 29% in patients without intraosseous abscess [7], which suggests that a significantly lower culture positivity rate may be present in some centers for percutaneous biopsies. The clinical impact of percutaneous bone biopsy may be lower at centers where the expected culture positivity rate is significantly lower. The purpose of this investigation is to determine the culture positivity rate and clinical utility of bone biopsy in patients with osteomyelitis.
Materials and methods
Before beginning the evaluation, approval from the institutional review board was obtained. No informed consent was necessary because of the retrospective design of the study. A database search was performed to access all CT or fluoroscopically guided bone biopsy procedures performed by the institution’s musculoskeletal radiology division between 2010 and 2014 on patients with clinically suspected osteomyelitis. Patients were excluded if the biopsy site was in a location other than the foot or the pelvis. Of note, patients who underwent a percutaneous spine biopsy were excluded from the dataset on the basis that many spine biopsy cases may obtain a small volume of intervertebral disc material or paraspinal material that may potentially alter culture growth rates [7,8,9].
Biopsy specimens were typically obtained under CT guidance, with fluoroscopy guidance used for a small number of foot biopsies (Fig. 1). Any overlying ulcer or infected skin was avoided. A skin nick was made for needle entry. A dorsal approach was utilized for most foot biopsies, with a transverse approach utilized in cases where the infection was in a distal phalanx underlying a nail bed. Given the superficial nature of most of the foot biopsies, the needle was held in place by a combination of skin tenting, placement of a sterile towel next to the needle, or a sterile clamp along the needle until access into the bone was secured. Biopsies were performed solely within the musculoskeletal radiology division by one of six different faculty members. Biopsy specimens for culture were submitted in a sterile tube with a small amount of saline placed in the tube to prevent the specimen drying and then promptly sent to the laboratory for testing, as per institutional protocol. Depending on operator preference, ordering physician preference, and the safety of obtaining multiple samples, an additional core biopsy specimen was at times submitted in formalin for histopathological evaluation. Biopsies were obtained with different needles as determined by operator preference using systems ranging in size from 11-gauge to 14-gauge and including Bonopty (Apriomed, Uppsala, Sweden), Laurane (Laurane Medical, Westbrook, CT, USA), Ostycut (Bard, Tempe, AZ, USA), and Core Assure (Parallax, Santa Monica, CA, USA). Any procedure-related complication was documented.
a A CT-guided biopsy image demonstrates a biopsy approach to the ischial tuberosity, which avoids a nearby ulcer and targets an area of subtle sclerosis corresponding to an area of chronic osteomyelitis seen by MRI. b A CT-guided biopsy image from a different patient demonstrates a dorsal approach to the fourth metatarsal head with the needle extending nearly to the volar surface. There is a corresponding plantar ulceration and slightly erosive change along the plantar aspect of the fourth metatarsal head. c Fluoroscopy-guided biopsy image demonstrates a needle approaching the eroded aspect of the first distal phalanx laterally, targeting the area of osteolysis
The biopsy site for each patient was documented. Patients who had multiple biopsies in separate encounters or during the same encounter were included as separate samples within the dataset. Included subjects were confirmed to have a high degree of suspicion for osteomyelitis by the referring clinician based on their physical examination and laboratory findings. Imaging from all patients was re-reviewed by a dedicated musculoskeletal radiologist with 6 years of experience. MRI criteria for determining imaging findings of osteomyelitis (Fig. 2a) included confluent infiltrative hypointense signal on T1-weighted images, hyperintense signal on STIR-weighted images, and the presence of an adjacent ulceration [10,11,12,13,14]. CT or radiographic findings of progressive cortical destruction and osteolysis in the presence of a soft-tissue ulcer was also classified as osteomyelitis and included in the data set (Fig. 2b). A low threshold was utilized for excluding patients with minimal or questionable imaging findings. Patients with potential alternate diagnoses besides osteomyelitis, including metastatic disease, arthritis, osteonecrosis, gout, or fracture, were excluded from the dataset. Biopsies that did not successfully yield specimens were still included in the data set based on intention-to-treat principles. Biopsy images from all procedures were also reviewed to determine if the biopsy needle contacted the area of T1 hypointensity (in patients who had preprocedural MRI) or active osteolysis (in patients with preprocedural X-ray or CT).
a A T1-weighted sagittal image through the calcaneus demonstrates confluent T1 hypointensity (arrow) within the posterior aspect of the calcaneus with overlying skin and soft-tissue changes corresponding to the clinically visible ulceration, all findings consistent with osteomyelitis. b A frontal foot radiograph demonstrates osteolysis of the first distal phalanx (arrow) adjacent to an area of skin and soft-tissue irregularity corresponding to a clinical ulceration that has progressed from a previous radiograph, meeting radiography/CT criteria for osteomyelitis. Both of these examinations met the inclusion criteria for the study
The data analysis included documentation of previous antibiotics treatment in the past 24 h for each selected patient in addition to microbiology and potentially histopathology results from each case. The time period 24 h was chosen, as many of the patients were new admissions from outside institutions with unclear records with regard to previous antibiotics therapy, and because it was a common practice for infectious disease doctors at our institution to temporarily withhold antibiotics at admission with the hope of increasing the rate of culture positivity. The cultured organism and the clinical antibiotics selection decisions were recorded for each case, and specifically any alteration of antibiotics choice was noted.
Two criteria were applied to determine a threshold for altered antibiotics management resulting from a positive culture. First, the cultured pathogen from the bone biopsy was required to be a different pathogen than results obtained from a blood culture or wound culture within the past month. Second, antibiotics therapy had to be tailored to the sensitivity results acquired from a positive culture. Referring clinicians at the authors’ institution typically request a biopsy procedure with the intention of providing the patient with targeted antibiotics therapy to the organism in the bone, and not just globally treating organisms in the overlying ulcer and soft tissues. Patients who were administered an antibiotics regimen based specifically on the bone biopsy data were considered to have had a significant management alteration as a result of their percutaneous procedure. The clinical suspicion of potential skin contamination as the cause of the positive culture by the infectious disease specialist was noted.
Histopathology results were recorded for those in whom a sample was sent. Histology was deemed to be positive for osteomyelitis if there was acute or chronic inflammation. Pathologically normal bone was classified as histologically negative, and other nonspecific pathological findings were deemed indeterminate.
Patient response to antibiotics therapy was recorded as positive or negative for those who were not lost to follow-up. A positive response was determined based on decreased osseous signal changes on MRI, clinical examination improvement, and/or improvement in laboratory inflammatory markers (C-reactive protein and erythrocyte sedimentation rate) documented within 4 months of biopsy. Patients who had no change, clinical/imaging worsening, or went on to debridement/amputation were deemed to have a negative response to antibiotics therapy.
Results
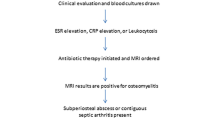
A total of 85 nonspine biopsies were performed by the musculoskeletal radiology division for potential osteomyelitis at the authors’ institution during the specific time period, of which 60 met the inclusion criteria (Fig. 3). These consisted of 25 biopsies of the foot/toe and 35 biopsies of the pelvis and femur. All foot osteomyelitis patients had an overlying ulcer. All pelvic biopsy patients had an adjacent decubitus ulceration, with the exception of one patient who had a rectal abscess adjacent to the area of osteomyelitis. All patients underwent preprocedural imaging, which demonstrated findings of osteomyelitis; in 56 of the biopsies the patient had undergone preprocedural MRI, whereas 2 had only previous CT and 2 had only previous radiographs. The area of T1 marrow hypointensity or active osteolysis was targeted in all biopsy images.
Positive cultures were obtained in 11 out of 60 biopsies overall (18%), including 6 out of 35 pelvic biopsies (17%) and 5 out of 25 foot/toe biopsies (20%). Two of the positive pelvic biopsies were obtained from different areas of the same patient on the same day. Among the foot biopsies, 2 of these cultures were felt likely to be contaminants by the infectious disease specialists, as documented on the clinical chart, including coagulase-negative staphylococci and coryneform Gram-positive rods. None of the cases of positive culture in the pelvic biopsy cases were felt to be due to contamination by the referring service. Polymicrobial infection was seen in only one patient, a pelvic biopsy patient who grew Proteus mirabilis and Gram-positive anaerobic cocci. There was no statistical difference between the number of culture-positive foot/toe and pelvis/proximal femur bone biopsies.
Antibiotics had been given within 24 h before the biopsy in 18 out of 60 procedures (30%), including 7 out of 25 foot/toe biopsies (28%) and 11 out of 35 pelvic biopsies (31%). Among biopsies with culture-positive biopsies, 2 out of 11 (18%) received antibiotics in the 24 h before the procedure. Among patients who had recently received antibiotics, 0 out of 7 foot (0%) and 2 out of 11 pelvic biopsies (18%) were culture-positive.
In 2 patients an inadequate sample was sent. One of these the samples was lost in transit and a repeat biopsy was performed. The other was a case in which the patient experienced a pulseless electrical activity (PEA) arrest of unknown etiology during the procedure such that the procedure was terminated after a sample was obtained for histology, but before a sample could be obtained for culture. Most specimens were not sent for histopathology, and among those that were sent, the histopathology findings were consistent with osteomyelitis in 1 out of 8 foot biopsies (13%) and 9 out of 14 pelvis biopsies (64%). All 10 of the samples positive for osteomyelitis at histopathology had no growth on culture.
Only 3 out of 11 biopsies with culture positive results (27%) had antibiotics management altered based on susceptibility laboratory analysis, as determined by the criteria set forth, or 3 out of 60 biopsies performed (5%). One of these patients had not previously been on antibiotics, but was started on targeted antibiotics therapy after the biopsy. The other 2 had their previous antibiotics regimen altered based on the culture results. Three patients did not meet the first criteria requiring that bone biopsy results yield a different pathogen when compared with another site in the same clinical scenario, including 2 pelvic biopsy patients with methicillin-susceptible Staphylococcus aureus on blood culture and 1 foot biopsy patient with Serratia marcescens in a wound culture. Two pelvic biopsy patients did not have any change in antibiotics management after the biopsy compared with pre-biopsy, 1 of whom had concern for polymicrobial infection as the stated reason for not treating with targeted antibiotics therapy. There was concern for contamination in 2 of the positive foot biopsy cultures; 1 had no change in antibiotics therapy and the other had changes in their antibiotics regimen that were coincidental and not an attempt to target the culture results.
Among patients who had negative biopsy cultures, most (71%) were maintained on the same antibiotics regimen as they had been on either before the procedure or while culture data were pending (Table 1). A small number of patients had a change in antibiotics management, were started on broad-spectrum antibiotics after culture results were known, or were discharged without antibiotics. Most patients (58%) had a positive response to antibiotics therapy within the first 4 months (Table 2), regardless of whether culture results were positive or negative.
Discussion
The results of this retrospective analysis suggest that there was limited utility for CT- or fluoroscopy-guided percutaneous bone needle biopsy in our patient population, as cultures were usually negative and management was seldom altered based on the pathogen sensitivity data. Only 11 out of 60 postprocedural samples (18%) were found to be culture-positive, and antibiotics management was altered in only 3 out of 60 biopsies performed (5%). This represents a significant discrepancy when compared with much of the literature, which has consistently reported culture positivity rates of 75% and above.
There are several potential explanations for this discrepancy with the previous literature. Many of the studies on bone biopsies focus on surgical as opposed to percutaneous specimens [1, 5, 6, 15,16,17]. Surgical specimens are typically much larger than those obtained percutaneously, and as such, the high success rates from open surgical biopsy cannot be assumed to be the same as for percutaneous biopsy [18]. Other articles have combined extremity and spine biopsies into one data set [19, 20]; however, the culture positivity rate of spine biopsies is typically considered to be higher than for the extremities [7], which may result in an elevation of the culture positivity rate. Multiple articles on bone biopsy culture come from a single group, which has reported consistently high culture positivity rates ranging from 68 to 86% [21,22,23,24]. The authors from that group describe a percutaneous approach similar to that used in our study; however, it may be difficult to generalize the high rate of culture positivity from that single group to other institutions. In support of our low rate of culture positivity, Wu et al. found a growth rate of 29% in patients without an intraosseous abscess [7], which is similar to our finding of 18% culture positivity.
Alternatively, there may be slight variations in the procedure or technique, or differences in the microbiological analysis of the sample once the bone biopsy is complete. The infectious disease literature has suggested that a frequent cause of false-negative cultures is that the biopsy misses the area of active osteomyelitis [1]; however, this seems unlikely in our series, as all biopsies were targeting the area of marrow T1 hypointensity or active osteolysis upon review of the biopsy images. Recent antibiotics use has also been suggested as a potential cause of lower culture positivity rate [1, 7], although there is some controversy in the literature, as recent publications have shown no significant difference in culture rate in patients on antibiotics undergoing spinal biopsy [25,26,27]. Antibiotics did not appear to be an issue in the current series; the percentage of positive biopsies where the patient had had recent antibiotics use (18%) was similar to the rate of antibiotics usage in the biopsy population as a whole (30%).
In contrast to our finding that percutaneous biopsy rarely alters clinical management, there are several articles in the infectious disease literature that do illustrate an impact on antibiotics management and subsequent patient outcomes [15, 22]. However, 75–85% of patients had positive cultures in those studies; thus, there were more patients with positive cultures for the potential change in antibiotics management to have an impact. At an institution where the expected culture positivity rate is lower, the impact on clinical management may also be low.
The possibility of polymicrobial infection was a cited concern among clinicians in our chart review, as the reason for not tailoring the antibiotics regimen in cases with positive bone culture. Polymicrobial infection is in fact quite common in diabetic foot infection [23, 28]. Polymicrobial infection is also present in virtually all cases of pelvic osteomyelitis associated with decubitus ulceration, with an average of four bacterial isolates per case [2]. With only one case showing polymicrobial positive culture, our rate of polymicrobial infection seems lower than in the published literature, particularly for the decubitus ulceration cases where polymicrobial infection is the norm.
Although complications are rare with image-guided biopsies, they can happen in any attempted procedure, and as such, all procedures should have a reasonable probability of providing useful clinical information when undertaken. Patients undergoing biopsy for osteomyelitis typically have comorbidities that may place them at a higher risk for a complication. There is also potential concern about creating a new wound with a tract leading to bone as a component of the biopsy procedure, given that these patients are already susceptible to poor wound healing [28]. In spite of the risk of percutaneous biopsy being quite low, this risk remains present and needs to be balanced against the low potential benefit to the patient.
The current study has several limitations. One is that the extremely low rate of culture positivity limits statistical analysis between different biopsy sites or the use of analytical statistics; therefore, purely observational statistics have been used. Furthermore, during the data gathering phase of the retrospective review, ordering physician tendencies were observed, introducing a component of selection bias, as some clinicians routinely requested bone biopsies based on IDSA guidelines, whereas others rarely did. Chart follow-up was only done for the first 4 months following the biopsy, as many of these chronically ill patients had delayed recurrences making it difficult to discern treatment success in the long term. Antibiotics duration could be reliably obtained only for the 24 h preceding the biopsy, as many patients were transfers from outside institutions. Although it would have been ideal for this period to be longer, it is the same as the cut-off utilized in other studies [7]. Some patients may also have had infections at other body locations requiring antibiotics.
In conclusion, there was limited utility in our study population for percutaneous bone biopsy in the management of patients with imaging findings of osteomyelitis, as culture-positive rates were low and management was rarely altered based on the biopsy results. Aggressively pursuing percutaneous biopsy may be less applicable at institutions with a low rate of culture positivity for percutaneous image-guided biopsies. Further research is needed to address the range of culture positivity rates across multiple institutions, specifically for percutaneous image-guided biopsies without inclusion of surgical or spine biopsies in the data set. Individual institutions may want to audit their own culture positivity rate before embarking on a policy of aggressively pursuing biopsy in patients with osteomyelitis.
References
Lipsky BA, Berendt AR, Cornia PB, Pile JC, Peters EJG, Armstrong DG, et al. 2012 infectious diseases society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2012;54:132–73.
Livesley NJ, Chow AW. Infected pressure ulcers in elderly individuals. Clin Infect Dis. 2002;35:1390–6.
Larson JD, Altman AM, Bentz ML, Larson DL. Pressure ulcers and perineal reconstruction. Plast Reconstr Surg. 2014;133:39e–48e.
Larson DL, Hudak KA, Waring WP, Orr MR, Simonelic K. Protocol management of late-stage pressure ulcers. Plast Reconstr Surg. 2012;129:897–904.
Game FL. Osteomyelitis in the diabetic foot: diagnosis and management. Med Clin N Am. 2013;97:947–56.
Jeffcoate WJ, Lipsky BA. Controversies in diagnosing and managing osteomyelitis of the foot in diabetes. Clin Infect Dis. 2004;39(Suppl 2):S115–22.
Wu JS, Gorbachova T, Morrison WB, Haims AH. Imaging-guided bone biopsy for osteomyelitis: are there factors associated with positive or negative cultures? Am J Roentgenol. 2007;188:1529–34.
Rankine JJ, Barron DA, Robinson P, Millner PA, Dickson RA. Therapeutic impact of percutaneous spinal biopsy in spinal infection. Postgrad Med J. 2004;80:607–9.
Chew FS, Kline MJ. Diagnostic yield of CT-guided percutaneous aspiration procedures in suspected spontaneous infectious diskitis. Radiology. 2001;218:211–4.
Collins MS, Schaar MM, Wenger DE, Mandrekar JN. T1 weighted MRI characteristics of pedal osteomyelitis. Am J Roentgenol. 2005;185:386–93.
Donovan A, Schweitzer ME. Use of MR imaging in diagnosing diabetes-related pedal osteomyelitis. Radiographics. 2010;30:723–36.
Donovan A, Schweitzer ME. Current concepts in imaging diabetic pedal osteomyelitis. Radiol Clin N Am. 2008;46:1105–24.
Morrison WB, Schweitzer ME, Batte WG, Radack DP, Russel KM. Osteomyelitis of the foot: relative importance of primary and secondary MR imaging signs. Radiology. 1998;207:625–32.
Yuh WT, Corson JD, Baraniewski HM, Rezai K, Shamma AR, Kathol MH, et al. Osteomyelitis of the foot in diabetic patients: evaluation with plain film, 99mTc-MDP bone scintigraphy, and MR imaging. AJR Am J Roentgenol. 1989;152:795–800.
Khatri G, Wagner DK, Sohnle PG. Effect of bone biopsy in guiding antimicrobial therapy for osteomyelitis complicating open wounds. Am J Med Sci. 2001;321:367–71.
Ertugrul MB, Baktiroglu S, Salman S, Unal S, Aksoy M, Berberoglu K, et al. The diagnosis of osteomyelitis of the foot in diabetes: microbiological examination vs. magnetic resonance imaging and labelled leucocyte scanning. Diabet Med. 2006;23:649–53.
Elamurugan TP, Jagdish S, Kate V, Chandra Parija S. Role of bone biopsy specimen culture in the management of diabetic foot osteomyelitis. Int J Surg. 2011;9:214–6.
Marschall J, Bhavan KP, Olsen MA, Fraser VJ, Wright NM, Warren DK. The impact of prebiopsy antibiotics on pathogen recovery in hematogenous vertebral osteomyelitis. Clin Infect Dis. 2011;52:867–72.
Schweitzer ME, Deely DM, Beavis K, Gannon F. Does the use of lidocaine affect the culture of percutaneous bone biopsy specimens obtained to diagnose osteomyelitis?: an in vitro and in vivo study. Am J Roentgenol. 1995;164:1201–3.
White M, Schweitzer E, Deely M, Gannon F. Study of osteomyelitis: utility of combined histologic and microbiologic evaluation of percutaneous biopsy samples. Radiology. 1995;197:840–2.
Senneville E, Lombart A, Beltrand E, Valette M, Legout L, Cazubiel M, et al. Outcome of diabetic foot osteomyelitis treated nonsurgically. Diabetes Care. 2008;31:637–42.
Senneville E, Gaworowska D, Topolinski H, Devemy F, Nguyen S, Singer B, et al. Outcome of patients with diabetes with negative percutaneous bone biopsy performed for suspicion of osteomyelitis of the foot. Diabet Med. 2012;29:56–61.
Senneville E, Melliez H, Beltrand E, Legout L, Valette M, Cazaubiel M, et al. Culture of percutaneous bone biopsy specimens for diagnosis of diabetic foot osteomyelitis: concordance with ulcer swab cultures. Clin Infect Dis. 2006;42:57–62.
Senneville E, Morant H, Descamps D, Dekeyser S, Beltrand E, Singer B, et al. Needle puncture and transcutaneous bone biopsy cultures are inconsistent in patients with diabetes and suspected osteomyelitis of the foot. Clin Infect Dis. 2009;48:888–93.
McNamara AL, Dickerson EC, Gomez-Hassan DM, Cinti SK, Srinivasan A. Yield of image-guided needle biopsy for infectious discitis: a systematic review and meta-analysis. AJNR Am J Neuroradiol. 2017;38:2021–7.
Agarwal V, Wo S, Lagemann GM, Tsay J, Delfyett WT. Image-guided percutaneous disc sampling: impact of antecedent antibiotics on yield. Clin Radiol. 2016;71:228–34.
Saravolatz LD, Labalo V, Fishbain J, Szpunar S, Johnson LB. Lack of effect of antibiotics on biopsy culture results in vertebral osteomyelitis. Diagn Microbiol Infect Dis. 2018;91(3):273-4. Elsevier Inc.
Lesens O, Desbiez F, Vidal M, Robin F, Descamps S, Beytout J, et al. Culture of per-wound bone specimens: a simplified approach for the medical management of diabetic foot osteomyelitis. Clin Microbiol Infect. 2011;17:285–91.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Said, N., Chalian, M., Fox, M.G. et al. Percutaneous image-guided bone biopsy of osteomyelitis in the foot and pelvis has a low impact on guiding antibiotics management: a retrospective analysis of 60 bone biopsies. Skeletal Radiol 48, 1385–1391 (2019). https://doi.org/10.1007/s00256-019-3152-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-019-3152-4