Abstract
Purpose
To compare the microbiology results and needle gauge for CT-guided biopsies of suspected discitis-osteomyelitis.
Methods
All CT-guided biopsies performed for suspected discitis-osteomyelitis at our institution between 2002 and 2019 were reviewed. Biopsy location, needle type and gauge, microbiology, pathology, and clinical and imaging follow-up were obtained through chart review. Yield, sensitivity, specificity, and accuracy were calculated. A pairwise analysis of different needle gauges was also performed with calculations of odds ratios. Naïve Bayes predictive modeling was performed.
Results
241 (age: 59 ± 18 years; 88 [35%] F, 162 [65%] M) biopsies were performed. There were 3 (1%) 11 gauge (G), and 13 (5%) 12-G biopsies; 23 (10%) 13-G biopsies; 75 (31%) 14-G biopsies; and 90 (37%) 16-G, 33 (14%) 18-G, and 4 (2%) 20 G biopsies. True disease status (presence of infection) was determined via either pathology findings (205, 86%) or clinical and imaging follow-up (36, 14%). The most common true positive pathogen was Staphylococcus aureus (31, 33%). Overall biopsy yield, sensitivity, specificity, and accuracy were 39%, 56%, 89%, and 66%, respectively. Pooled biopsy yield, sensitivity, specificity, and accuracy was 56%, 69%, 71%, and 69% for 11–13-G needles and 36%, 53%, 91%, and 65% for 14–20-G needles, respectively, with an odds ratio between the two groups of 2.29 (P = 0.021). Pooled biopsy yield, sensitivity, specificity, and accuracy was 48%, 63%, 85%, and 68% for 11–14-G needles and 32%, 49%, 91%, and 64% for 16–20-G needles, respectively, with an odds ratio between the two groups of 2.02 (P = 0.0086).
Conclusion
The use of a larger inner bore diameter/lower gauge biopsy needle may increase the likelihood of culturing the causative microorganism for CT-guided biopsies of discitis-osteomyelitis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Spinal discitis-osteomyelitis is estimated to account for 3–5% of all cases of osteomyelitis [1]. The diagnosis of discitis-osteomyelitis is typically made using a combination of patient history, clinical exam, laboratory testing for acute-phase reactants, blood culture data, and diagnostic imaging. The isolation of a causative organism is important for determining the appropriate antibiotic regimen [2,3,4,5]. Blood cultures are positive in approximately 58% of cases of discitis-osteomyelitis [6,7,8]. Current Infectious Disease Society of America guidelines recommend percutaneous biopsy in patients with suspected discitis-osteomyelitis when other culture data do not yield a causative organism [9].
Percutaneous CT-guided biopsy is widely considered a safe, accurate, and, when compared with open biopsy, economical method of acquiring tissue for microbiologic and histologic evaluation. One meta-analysis assessing diagnostic culture results for vertebral osteomyelitis following image-guided percutaneous biopsy showed a pooled yield of 48% [10], although the reported range is wide (19–91%) [11,12,13,14,15,16,17,18]. Repeat CT–guided biopsy in the setting of a negative initial biopsy has been shown to improve overall yield, although the yield from the second biopsy is variable and ranges from 14 to 60% [19, 20].
The purpose of this study was to assess the impact of the size of the CT-guided percutaneous biopsy needle on microbiologic yield in patients with suspected discitis-osteomyelitis. We hypothesize that microbiology samples acquired with lower-gauge (larger inner diameter) biopsy needles would be more likely to yield a causative organism.
Materials and methods
Patient selection
This investigation was approved by the institutional review board and was compliant with the guidelines of the Health Insurance Portability and Accountability Act (HIPAA). From a searchable database of all radiology studies performed at our institution, we retrospectively identified 241 cases of suspected spinal discitis-osteomyelitis in which CT-guided percutaneous biopsy was performed between 2002 and 2019. Biopsies done on patients who had a previous biopsy-proven or clinically proven diagnosis of discitis-osteomyelitis at the same level were not included in this analysis. Patients who did not have biopsy-proven discitis-osteomyelitis and who did not receive sufficient follow-up to establish a clinical diagnosis of osteomyelitis were also not included. Patients with a history of biopsy-proven discitis-osteomyelitis at the level of repeat biopsy were also not included.
Spine biopsy technique
All procedures were performed using a GE LightSpeed 16 slice CT scanner. A total of 10 radiologists with 1–41 years of experience were the primary operators on the procedures. The decision regarding use of local anesthesia, conscious sedation, or general anesthesia was made after discussion with the patient, the primary patient care team, the radiology nursing staff, and the radiologist with consideration of the patient’s clinical status, patient positioning, the procedure target (disc/endplate versus disc versus soft tissue), the procedure target location, and prior patient experience with sedation. Procedural CT images were acquired at 2.5–5.0-mm slice thickness at 100–140 kVp and 40–440 mAs with a field of view ranging from 22 to 36 cm. Soft tissue kernels were applied.
The exact site of biopsy was determined by the radiologist after consideration of location of greatest abnormality, the safety of the required approach, patient comfort, and likelihood of highest diagnostic yield. All biopsies were performed using coaxial technique. Biopsies including portions of endplate bone were performed with an Arrow OnControl system (12- or 13-gauge) (Teleflex, Wayne, PA, USA), Ackerman biopsy needle (14-gauge) (Cook Medical), or a combination of a bone penetration system (14-gauge) (Bonopty, AprioMed) to access bone and a biopsy needle (15-gauge) (Ostycut, Bard Biopsy Systems) to perform the bone biopsy. For soft tissue biopsies, a Temno Evolution core biopsy needle (14-, 16-, 18-, or 20-gauge) system was used. Core samples were placed in sterile, preservative-free, non-bacteriostatic normal saline for microbiological and pathological analysis.
Biopsies reviewed were performed from January 2002 through December 2018. All reports and biopsy images were retrospectively reviewed for needle position, needle type, and biopsy level. T12-L1 was considered a thoracic level, and L5-S1 was considered a lumbar level.
Samples were sent for microbiological and pathological analysis, including Gram stain, acid-fast stain, fungal stain, and culture. Aerobic and anaerobic cultures were incubated for a 7-day period, while fungal and mycobacterial cultures were held for 28 and 56 days, respectively.
The gold standard for diagnosis of discitis-osteomyelitis was anatomic pathology, obtained either at the time of CT-guided biopsy procedure or during subsequent open surgical biopsy or debridement. If pathology was not available, then positive blood or urine culture with same organism as spine sampling was used as the gold standard. If blood culture was not available, a combination of imaging and clinical follow-up was used. Finally, if imaging was not available, clinical follow-up alone was used as the gold standard.
Imaging technique and review
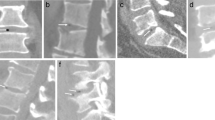
Diagnostic MRI of the spine was performed in all patients prior to aspiration. MRI was obtained in the supine position. Standard MRI protocol includes a sagittal T1, sagittal fast spine echo (FSE) T2, sagittal FSE T2 with fat suppression (FS) or sagittal STIR, and axial T2 sequences. Post-contrast T1 fat–suppressed sagittal and axial images and CT examinations with or without contrast were also available in some patients. MRI features considered diagnostic of discitis-osteomyelitis were T2 hyperintense signal of the intervertebral disc, erosive endplate changes, abnormal T1 hypointense and T2 hyperintense endplate signal, endplate or disc enhancement, and inflammation or fluid collections within the paravertebral or epidural space (Figs. 1 and 2). CT features considered diagnostic of discitis-osteomyelitis were endplate erosions, loss of intervertebral disc height, paravertebral inflammatory changes or fluid collections, and epidural inflammatory changes or fluid collections (Fig. 3).
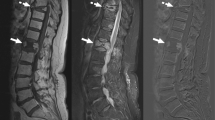
A 22-year-old man with history of intravenous drug use presents with acute on chronic back pain. a Sagittal T1, b sagittal STIR, c sagittal T1 fat suppressed post contrast, d axial T1, and e axial T1 fat suppressed post-contrast images demonstrate fluid within the L4-L5 intervertebral disc, with surrounding endplate destruction and low T1 endplate marrow signal. There is also increased T2 signal and enhancement in the epidural space, without focal fluid collection, consistent with phlegmon. The disc/endplate abnormality is slightly eccentric to the left, and this area was targeted on the biopsy. f Axial noncontrast CT image from the biopsy 3 days after the MRI demonstrates the tip of a 12-gauge core needle biopsy device (arrow) sampling the disc and endplate. The cultures grew Methicillin-sensitive Staphylococcus aureus (MSSA), a true positive result
A 36-year-old man with history of intravenous drug use presents with 4 months of progressive back pain, and acute fevers, chills, and diaphoresis. a Sagittal T1, b sagittal STIR, c sagittal T1 fat suppressed post contrast, d axial T1, and e axial T1 fat suppressed post contrast images demonstrate destruction of the L4-L5 intervertebral disc, which is also peripherally enhancing. There is also endplate destruction and low T1 endplate marrow signal. There is pronounced surrounding soft tissue enhancement, eccentric to the right, but no abscess or drainable fluid collection. f Axial noncontrast CT image from the biopsy 2 days after the MRI demonstrates the tip of a 18-gauge core needle biopsy device (arrow) sampling the disc. The cultures did not grow any organism, a false negative result
A 84-year-old female with acute back pain. a Sagittal noncontrast CT of the lumbar spine demonstrates endplate erosions at L4-L5 (arrows). b Axial noncontrast CT image from the biopsy 1 day after the diagnostic CT demonstrates the tip of a 16-gauge core needle biopsy device (arrows) sampling the disc. The cultures did not grow any organism, a false negative result. c Sagittal noncontrast CT of the lumbar spine 5 weeks later demonstrates progressive L4-L5 endplate destructive changes, confirming the diagnosis of discitis-osteomyelitis (arrows)
On follow-up CT or MRI between 1 and 12 months after initial imaging, the following findings were diagnostic for discitis-osteomyelitis: worsening erosive endplate changes, loss of vertebral body height, and persistent or increased paravertebral and epidural fluid collections and enhancement. If the CT or MRI was more than 12 months after the initial imaging, osseous fusion across the disc space was diagnostic for healed discitis-osteomyelitis. A positive culture was categorized as a false positive if the cultured species was determined to be a contaminant by the care team and/or Infectious Disease consultant and the patient clinically improved without the use of antibiotics.
Clinical review
The patient age, gender, and anatomic pathology results were recorded. The date of the biopsy was recorded. Microbiological studies, including culture, and sensitivity data from urine, blood, or repeat percutaneous biopsy or surgical biopsy samples, were also reviewed.
The medical record of each of these patients was reviewed, including clinical notes, laboratory results (CBC, ESR, CRP), radiology reports, microbiology reports, and pathology reports. Pathology results showing acute osteomyelitis or acute discitis were considered positive for discitis-osteomyelitis. Clinical features considered supportive of a diagnosis of discitis-osteomyelitis in conjunction with imaging findings included the presence of fever (> 38.0 °C), elevated erythrocyte sedimentation rate (ESR, ≥ 30 mm/h) or elevated C-reactive protein (CRP, ≥ 8.0 mg/L), leukocytosis (≥ 10 × 103 μL), and back pain or radiculopathy. An appropriate treatment response was used to confirm infection. These findings included resolution or improvement in fever and other symptoms, improvement or normalization of laboratory values (i.e., leukocytosis, elevated inflammatory markers). Clinical notes from Infectious Disease specialists were used in all cases to support or not support a diagnosis of discitis-osteomyelitis. This diagnosis was made by Infectious Disease specialists in consideration of imaging findings, laboratory results, microbiology results, and response to treatment.
Statistical analysis
Yield (the total number of true-positive findings divided by the total number of biopsy specimens obtained multiplied by 100%), sensitivity, specificity, and accuracy were calculated. A pairwise analysis of different needle gauges was performed with calculations of odds ratios. A pairwise analysis of the first 121 biopsies in the cohort compared with the following 120 biopsies in the cohort was performed with calculations of odds ratios.
Naïve Bayes predictive modeling was performed for the observations needle size, type of tissue biopsied, whether the biopsy was one of the first 121 biopsies performed or one of the last 120 biopsies performed, and the gender of the patient. A normal distribution was assumed for the priors. All predictors were considered independent uniform inputs. The maximum and minimum observed values were used to define a uniform distribution. From this, Monte Carlo samples are drawn for each factor, and the importance of each variable as a predictor is assessed and reported as a “Total Effect,” which weighs a combination of the relative contribution of the factor alone as well as with other factors.
All statistics were performed using JMP Pro version 14.0.0 (SAS Institute Inc., Cary, NC).
Results
Patient cohort
The study group included 241 patients (83 [35%] female patients, 158 [65%] male patients; mean age 59 ± 18 years; range, 4–99). There were 69/241 (29%) thoracic and 172/241 (71%) lumbar biopsies. There were 103/241 (42%) were of disc/endplate, 109/241 (44%) were of disc only, and 29/241 (13%) were of paravertebral soft tissue. Pathology was available for 205/241 (85%) patients. The true positive microbiological results are detailed in Table 1. The positive cultures deemed to be false positives by the clinical teams were Propionibacterium acnes (4/8, 50%) and coagulase-negative Staphylococcus (4/8, 50%).
Spine biopsy
The needle distribution and the disease/test categorization are detailed in Table 2. The pooled needle gauge analyses, including odds ratios, are detailed in Tables 3, 4, and 5.
In patients under the age of 50, the microbiological yield was 44% (31/71). In patients aged 50 and older, the microbiological yield was 37% (64/170). The odds ratio of a true positive microbiology result comparing biopsies done in patients under the age of 50 and patients aged 50 and older is 0.78 (95% confidence interval 0.44–1.37, P = 0.39). For the first 121 biopsies performed in this cohort, the microbiological yield was 35% (42/121). For the following 120 biopsies performed in this cohort, the microbiological yield was 44% (53/120). The odds ratio of a true positive microbiology result comparing the first 121 biopsies compared with the last 120 biopsies was 1.49 (95% confidence interval 0.89–2.50, P = 0.15). No needles of 13 gauge or larger were used in the group consisting of the first 121 biopsies performed. During the subsequent half of the biopsies performed, 13 gauge or larger needles were used in 32% (38/120) of cases. The needle gauges used for biopsies in the first and second halves of the cohort are shown in Table 6.
The odds ratio of a true positive microbiology result comparing needles 13-gauge or larger versus 14-gauge or smaller was 2.3, and this comparison was statistically significant (95% confidence interval 1.14–4.58, P = 0.02). The odds ratio of a true positive microbiology result comparing needles 14-gauge or larger versus 16-gauge or smaller was 2.0, a comparison that was also statistically significant (95% confidence interval 1.20–3.43, P = 0.008). The remainder of the comparisons demonstrated an odds ratio greater than 1 with the larger inner diameter/lower gauge needles being more likely to result in true positive microbiology results. However, these comparisons were not statistically significant.
The odds ratio of a true positive microbiology result comparing 13 gauge needles to 14 gauge needles was 0.7, and this comparison was not statistically significant (95% confident interval 0.280–1.84, P = 0.63).
Naïve Bayes predictive modeling
Using the naïve Bayesian predictive model with the five clinical predictors, the misclassification rate was 0.36. There were 66/95 (69%) misclassifications in the positive microbiological yield group and 21/146 (14%) in the negative microbiological yield group. A larger needle gauge was a more important predictor of a positive microbiological yield than the type of tissue biopsied, whether the biopsies were chronologically in the first half or second half of the cohort, and patient gender (total effect 0.78, 0.12, 0.088, 0.002, respectively).
Discussion
Our data suggest that a larger needle size is associated with improved microbiological yield from percutaneous biopsy of discitis-osteomyelitis. Specifically, using 13- and 14-gauge or larger inner diameter/lower gauge needles may result in a higher biopsy yield. A larger needle gauge may be a more important predictor of a positive biopsy yield than the type of tissue sampled.
Spondylodiscitis and vertebral osteomyelitis are potentially life-threatening conditions. Delay in treatment may result in sepsis, epidural abscess, neurologic complications including paralysis, and death [6, 8, 21, 22]. Clinical history, physical exam, laboratory tests, and imaging studies are used to establish the diagnosis of discitis-osteomyelitis [2,3,4,5]. The role of biopsy is to determine the causative organism and therefore to tailor antibiotic therapy [9]. Open surgical biopsy remains the gold standard for tissue sampling, for which the yield is approximately 76% but has been reported to be as high as 91% [10, 12]. However, percutaneous CT-guided biopsy is often performed and is less invasive and less expensive and results in shorter hospitalization than open surgical biopsy [23, 24].
Overall microbiology yields from a single percutaneous CT-guided biopsy is relatively low. A meta-analysis of 88 articles demonstrated a yield of 48%, which is similar to the rate of positive blood cultures (42%) [6,7,8, 10]. These studies use pooled needle sizes. In our study, biopsy yield was as high as 69% for the 12-gauge needles. Although the biopsy yield for 11-gauge needles was lower, there were only three 11-gauge biopsies. Studies evaluating the yield of a repeat bone biopsy in the setting of a negative initial biopsy have been shown to improve the overall microbiology yield, although results are variable, with reported yield in the second biopsy being as low as 14% and as high as 60% [19, 20].
A previous analysis of 41 cases of osteomyelitis did not find an association between needle gauge and biopsy yield. As in this study, 11–18-gauge biopsy needles were used. However, the study looked at cases of osteomyelitis outside of the spine and therefore all the biopsies were bone biopsies. The majority (83%) of samples were taken with 14- or 15-gauge biopsy needles. The similarity in needle sizes and the relative lack in larger or smaller gauge needles may have limited the ability of this study to detect differences in biopsy yield with needle gauge [25]. A separate series consisting of 111 spine biopsies for discitis-osteomyelitis also showed no difference in yield with different needle sizes. However, only 14–20-gauge needles were used [26]. As seen in this cohort, the largest needle gauges, particularly the 12- and 13-gauge needles, had the highest biopsy yields.
Our study includes 241 biopsies performed over a period of 17 years. In order to attempt to account for changes in both biopsy technique and potential changes in microbiological laboratory technique, the results from the first 121 biopsies performed were compared with the results from the second 120 biopsies. Although microbiological yield during the second half of the cohort was greater than during the first half, this was not statistically significant. This increased yield may in part be due to the adoption of 13-gauge and larger needles by our group during the time period of the second half of the cohort.
The limitations of this study arise from its retrospective design. As a result, we could not control for factors such as variations in operator preference, difficulty of the biopsy, location of the biopsy in the spine, which may incline the operator to use a smaller inner diameter/higher gauge needle (e.g., thoracic versus lumbar spine), and type of tissue biopsied (i.e., bone/disc versus soft tissue). This study would be best performed as a prospective study, where the same location was biopsied using multiple different gauge needles, and clinical factors such as antecedent antibiotic use could be controlled. However, such a study design could not be justified in clinical practice.
An additional limitation of this study is the lack of information regarding the number of core samples acquired and the length of acquired core samples. These parameters were not available in the procedural notes or the microbiological records. These factors affect the total volume of sampled tissue and may affect the microbiological yield of percutaneous biopsy. In a study of diagnostic yield from bone and soft tissue lesions tumors, Wu et al. suggested a minimum of 3 specimens of bone and 4 specimens of soft tissue to maximize yield [27]. Given that this study used benign and malignant tumors, it is difficult to know whether recommendations are applicable to biopsies of infection. A fundamental technical challenge with spine biopsies arises from the limited means of accessing abnormal tissue given the location of the spinal cord, the thecal sac bony structures, and the exiting nerve roots. Limited access, in conjunction with challenges with patient positioning, means multiple passes with a coaxial needle system may be difficult. Furthermore, the presence of critical structures such as the great vessels, retroperitoneal organs, mediastinal structures, the pleura, and the lungs beyond to the biopsy device may limit the number of cores taken from a single needle position. These factors underscore the importance of using a larger-gauge needle to maximize the size of the tissue samples acquired from a single needle position. Given that the benefit of larger-gauge needles likely relates to the fact that a larger sample of tissue is acquired, we believe that multiple core samples should be considered if a smaller gauge needle is being used.
In conclusion, use of a larger-gauge needle, particularly 13- and 14-gauge and larger–inner diameter/lower-gauge needles, in percutaneous CT-guided biopsy procedures, may increase the likelihood of culturing the causative microorganism.
References
Sobottke R, Seifert H, Fätkenheuer G, Schmidt M, Gossmann A, Eysel P. Current diagnosis and treatment of spondylodiscitis. Dtsch Arztebl Int. 2008;105(10):181–7.
Carragee EJ, Kim D, van der Vlugt T, Vittum D. The clinical use of erythrocyte sedimentation rate in pyogenic vertebral osteomyelitis. Spine. 1997;22(18):2089–93.
Khan MH, Smith PN, Rao N, Donaldson WF. Serum C-reactive protein levels correlate with clinical response in patients treated with antibiotics for wound infections after spinal surgery. Spine J. 2006;6(3):311–5.
Conrad DA. Acute hematogenous osteomyelitis. Pediatr Rev. 2010;31(11):464–71.
Ledermann HP, Schweitzer ME, Morrison WB, Carrino JA. MR imaging findings in spinal infections: rules or myths? Radiology. 2003;228(2):506–14.
Zimmerli W. Clinical practice. Vertebral osteomyelitis N Engl J Med. 2010;362(11):1022–9.
Corrah TW, Enoch DA, Aliyu SH, Lever AM. Bacteraemia and subsequent vertebral osteomyelitis: a retrospective review of 125 patients. QJM. 2011;104(3):201–7.
Mylona E, Samarkos M, Kakalou E, Fanourgiakis P, Skoutelis A. Pyogenic vertebral osteomyelitis: a systematic review of clinical characteristics. Semin Arthritis Rheum. 2009;39(1):10–7.
Berbari EF, Kanj SS, Kowalski TJ, Darouiche RO, Widmer AF, Schmitt SK, et al. 2015 Infectious Diseases Society of America (IDSA) clinical practice guidelines for the diagnosis and treatment of native vertebral osteomyelitis in adults. Clin Infect Dis. 2015;61(6):e26–46.
McNamara AL, Dickerson EC, Gomez-Hassan DM, Cinti SK, Srinivasan A. Yield of image-guided needle biopsy for infectious discitis: a systematic review and meta-analysis. AJNR Am J Neuroradiol. 2017;38(10):2021–7.
Michel SCA, Pfirrmann CWA, Boos N, Hodler J. CT-guided core biopsy of subchondral bone and intervertebral space in suspected spondylodiskitis. AJR Am J Roentgenol. 2006;186(4):977–80.
Marschall J, Bhavan KP, Olsen MA, Fraser VJ, Wright NM, Warren DK. The impact of prebiopsy antibiotics on pathogen recovery in hematogenous vertebral osteomyelitis. Clin Infect Dis. 2011;52(7):867–72.
Enoch DA, Cargill JS, Laing R, Herbert S, Corrah TW, Brown NM. Value of CT-guided biopsy in the diagnosis of septic discitis. J Clin Pathol. 2008;61(6):750–3.
Chew FS, Kline MJ. Diagnostic yield of CT-guided percutaneous aspiration procedures in suspected spontaneous infectious diskitis. Radiology. 2001;218(1):211–4.
Rankine JJ, Barron DA, Robinson P, Millner PA, Dickson RA. Therapeutic impact of percutaneous spinal biopsy in spinal infection. Postgrad Med J. 2004;80(948):607–9.
D’Agostino C, Scorzolini L, Massetti AP, Carnevalini M, d’Ettorre G, Venditti M, et al. A seven-year prospective study on spondylodiscitis: epidemiological and microbiological features. Infection. 2010;38(2):102–7.
Joo E-J, Yeom J-S, Ha YE, Park SY, Lee C-S, Kim E-S, et al. Diagnostic yield of computed tomography-guided bone biopsy and clinical outcomes of tuberculous and pyogenic spondylitis. Korean J Intern Med. 2016;31(4):762–71.
Garg V, Kosmas C, Young PC, Togaru UK, Robbin MR. Computed tomography-guided percutaneous biopsy for vertebral osteomyelitis: a department’s experience. Neurosurg Focus. 2014;37(2):E10.
Czuczman GJ, Marrero DE, Huang AJ, Mandell JC, Ghazikhanian V, Simeone FJ. Diagnostic yield of repeat CT-guided biopsy for suspected infectious spondylodiscitis. Skelet Radiol. 2018;47(10):1403–10.
Terreaux W, Geoffroy M, Ohl X, Job L, Cart P, Eschard J-P, et al. Diagnostic contribution of a second percutaneous needle biopsy in patients with spontaneous diskitis and negative blood cultures and first biopsy. Joint Bone Spine. 2016;83(6):715–9.
McHenry MC, Easley KA, Locker GA. Vertebral osteomyelitis: long-term outcome for 253 patients from 7 Cleveland-area hospitals. Clin Infect Dis. 2002;34(10):1342–50.
Pigrau C, Almirante B, Flores X, Falco V, Rodríguez D, Gasser I, et al. Spontaneous pyogenic vertebral osteomyelitis and endocarditis: incidence, risk factors, and outcome. Am J Med. 2005;118(11):1287.
Huang AJ, Kattapuram SV. Musculoskeletal neoplasms: biopsy and intervention. Radiol Clin N Am. 2011;49(6):1287–305 vii.
Skrzynski MC, Biermann JS, Montag A, Simon MA. Diagnostic accuracy and charge-savings of outpatient core needle biopsy compared with open biopsy of musculoskeletal tumors. J Bone Joint Surg Am. 1996;78(5):644–9.
Wu JS, Gorbachova T, Morrison WB, Haims AH, Js W, Gorbachova T, et al. Imaging-guided bone biopsy for osteomyelitis: are there factors associated with positive or negative cultures? AJR. 2007;188:1529–34.
Chang CY, Simeone FJ, Nelson SB, Taneja AK, Huang AJ. Is Biopsying the paravertebral soft tissue as effective as biopsying the disk or vertebral endplate? 10-year retrospective review of CT-guided biopsy of diskitis-osteomyelitis. AJR Am J Roentgenol. 2015;205(1):123–9.
Wu JS, Goldsmith JD, Horwich PJ, Shetty SK, Hochman MG. Bone and soft-tissue lesions: what factors affect diagnostic yield of image-guided core-needle biopsy? Radiology. 2008;248(3):962–70.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was waived for individual participants included in the study. The study was approved by the local Institutional Review Board (IRB) and HIPAA compliant.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Husseini, J.S., Simeone, F.J., Nelson, S.B. et al. CT-guided discitis-osteomyelitis biopsies: needle gauge and microbiology results. Skeletal Radiol 49, 1431–1439 (2020). https://doi.org/10.1007/s00256-020-03439-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-020-03439-3