Abstract
Objective
The objective was to evaluate magnetic resonance imaging (MRI) findings in patients with fibular stress injuries.
Materials and Methods
The study group consisted of 20 patients with clinically diagnosed fibular stress injuries who were evaluated with MRI. Radiographs were performed in 14 of the 20 patients. The MRI examinations and radiographs were retrospectively reviewed in consensus by two musculoskeletal radiologists.
Results
All 20 patients with clinically diagnosed fibular stress injuries had periosteal edema and bone marrow edema within the fibula on MRI. The periosteal reaction and bone marrow edema were present within the distal fibula in 14 patients, the middle fibula in 1 patient, and the proximal fibula in 5 patients. The periosteal reaction was located on the anterior cortex in 1 patient, the posterior cortex in 4 patients, the lateral cortex in 11 patients, and circumferentially distributed throughout the cortex in 4 patients. Nine patients had abnormal T1 and T2 signal intensity within the fibular cortex. Initial and follow-up radiographs showed periosteal reaction in 15% and 50% of patients with fibular stress injuries respectively.
Conclusions
The majority of fibular stress injuries involve the lateral cortex of the distal fibula.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Stress injuries represent a spectrum of osseous abnormalities that occur in response to chronic repetitive stress applied to healthy bone. Stress injuries are most commonly seen in athletes and military recruits who are involved in strenuous running activities [1–4]. Chronic repetitive stress results in an imbalance between osteoclastic and osteoblastic activity, which ultimately weakens bone. If the repetitive stress continues, repair mechanisms may be overwhelmed and a true fracture may develop. However, most stress injuries represent stress reaction and show no evidence of a break in the continuity of bone on histologic analysis [5–8]. The vast majority of stress injuries involve the tibia, followed in order of decreasing frequency by the metatarsals, fibula, navicular, femur, and pelvis [1–4].
Fibular stress injuries are relatively uncommon. In a review of multiple clinical series of stress injuries in athletes and military recruits, the fibula was noted to be involved in an average of 11.9% of cases [1–4]. Most previous studies have reported only the clinical and radiographic findings in patients with fibular stress injuries [9–11]. The magnetic resonance imaging (MRI) findings in these individuals have been previously described only in case reports [12–14]. Thus, this study was performed to evaluate the MRI findings in a larger number of patients with fibular stress injuries.
Materials and methods
Study group
This study was performed in compliance with HIPAA regulations and with approval from our Institutional Review Board. A waiver of informed consent was obtained prior to performing the study.
A musculoskeletal MRI database was used to identify 20 consecutive patients (8 males and 12 females; aged between 13 and 46 years, with an average age of 21.3 years) who were referred for an MRI examination of the lower extremity at our institution by a sports medicine specialist between 1 January 1996 and 1 January 2008 to rule out fibular stress injury. All patients were involved in sports activities that included cross country running, marathon running, basketball, volleyball, soccer, football, triathlon, decathlon, and sprinting. All patients complained of focal pain within the fibula (with duration of clinical symptoms ranging between 7 and 240 days, with an average duration of 56.0 days) that was exacerbated by physical activity. All patients had point tenderness over the fibula on physical examination. None of the patients had a history of acute trauma to the lower extremity or clinical manifestations to suggest the presence of infection or malignancy.
Radiographic examination
Fourteen of the 20 patients in the study group had a radiographic examination of the fibula performed at the time of presentation to their physician. Ten of these 14 patients had multiple follow-up radiographic examinations of the fibula performed between 3 weeks and 3 months after their initial radiographs were obtained. All radiographic examinations consisted of anteroposterior and lateral views of the fibula performed using standardized technique [15].
MRI examination
All 20 patients in the study group underwent an MRI examination of the fibula within 2 weeks of the time of presentation to their physician. All MRI examinations were performed on the same General Electric 1.5-Tesla field strength magnet (General Electric Medical Systems, Milwaukee, WI, USA) using a phased array extremity coil. All MRI examinations included an axial T1-weighted spin-echo sequence (TR/TE: 400–600 ms/15–30 ms) and a frequency selective, fat-suppressed T2-weighted fast spin-echo sequence (TR/TE: 2,000–4,000 ms/60–80 ms, with an echo train of 8). All MRI examinations also included a T1-weighted spin-echo sequence (TR/TE: 400–600 ms/15–30 ms) and either a frequency-selective, fat-suppressed T2-weighted fast spin-echo sequence (TR/TE: 2,000–4,000 ms/60–80 ms, with an echo train of 8) or short tau inversion recovery sequence (TI/TR/TE: 160 ms/3,000 ms/44 ms, with an echo train of 8) performed in the coronal and/or sagittal planes. All MRI examinations were performed with a field of view between 16 and 24 cm, a slice thickness between 3 and 7 mm, with an interslice gap between 0.4 and 3 mm, a matrix of 256 × 192 or 256 × 256, and one or two excitations. During all MRI examinations, a vitamin E capsule was placed at the site of the patient’s maximal pain to localize symptoms.
Review of radiographic examinations
All radiographs were retrospectively reviewed in consensus by two fellowship-trained musculoskeletal radiologists who had between 4 and 7 years of clinical experience. The radiologists were unaware of the clinical findings of the prior radiographic interpretation of each patient when retrospectively reviewing their radiographic examination. The radiologists determined the presence or absence of decreased cortical density, periosteal reaction, endosteal thickening, and a cortical fracture line within the fibula on each radiographic examination. When a radiographic abnormality was identified, the radiologists documented its location within the proximal, mid, and distal fibula, and within the anterior, posterior, medial, or lateral fibula.
Review of MRI examinations
All MRI examinations were retrospectively reviewed in consensus by the same two fellowship-trained musculoskeletal radiologists. The radiologists were unaware of the clinical and radiographic findings of each patient when retrospectively reviewing their MRI examination. In order to prevent recall bias, the radiologists reviewed the MRI examination of each patient at least 1 month after reviewing their radiographic examination.
The radiologists determined the presence or absence of periosteal edema, bone marrow edema, and intracortical signal abnormality with the fibula on each MRI examination. When an MRI abnormality was identified, the radiologists documented its location within the proximal, mid, and distal fibula, and within the anterior, posterior, medial, or lateral fibula. Periosteal edema was defined as a linear area of high T2 signal intensity immediately adjacent to the outer surface of the fibular cortex. Bone marrow edema was defined as a focal or ill-defined area of low T1 signal intensity and high T2 signal intensity within the intramedullary canal of the fibula. Intracortical signal abnormality was defined as globular or linear areas of intermediate T1 signal intensity and intermediate to high T2 signal intensity within the fibular cortex. The radiologists also graded the severity of the fibular stress injuries using the Fredericson MRI Classification System (Table 1) [16].
Results
Radiographic findings of fibular stress injuries
None of the patients had decreased cortical density or endosteal thickening of the fibula on initial or follow-up radiographs. Two of the 14 patients (14.3%) with initial radiographs had a periosteal reaction on the lateral cortex of the fibula (Fig. 1). Five of the 10 patients (50%) with follow-up radiographs had a periosteal reaction on the lateral cortex of the fibula (Fig. 2). The periosteal reaction was located on the distal third of the fibula in 4 patients (80%) and on the proximal third of the fibula in 1 patient (20%). The 1 patient with a periosteal reaction on the proximal third of the fibula also had a fracture line through the lateral fibular cortex. All patients with radiographic abnormalities had Fredericson grade 4 stress injuries on MRI (Figs. 1, 2).
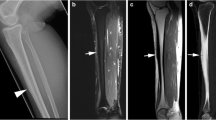
A 13-year-old male basketball player with a 2-week history of proximal fibular pain. a Initial lateral radiograph of the fibula shows faint periosteal reaction (arrows) on the posterior cortex of the proximal fibular diaphysis. b Follow-up lateral radiograph of the fibula 1 month later shows consolidation of the periosteal reaction (arrows). c Corresponding sagittal fat-suppressed T2-weighted fast spin-echo MRI of the fibula performed 1 day after the initial radiographic examination shows periosteal edema (arrows) on the posterior fibular cortex with adjacent intramedullary bone marrow edema (arrowhead). d Corresponding axial fat-suppressed T2-weighted fast spin-echo MRI of the fibula performed 1 day after the initial radiographic examination shows periosteal edema (arrows) on the posterior and lateral fibular cortex with adjacent intramedullary bone marrow edema (small arrowhead). Also note the globular intracortical signal abnormality (large arrowhead) within the posterior fibular cortex
A 23-year-old male football player with a 4-week history of distal fibular pain. a Initial anteroposterior radiograph of the fibula shows no abnormality. b Follow-up anteroposterior radiograph of the fibula 3 weeks later shows periosteal reaction (arrow) on the lateral cortex of the distal fibular diaphysis. c Corresponding coronal fat-suppressed T2-weighted fast spin-echo MRI of the fibula performed 2 weeks after the initial radiographic examination shows periosteal edema (arrows) on the lateral fibular cortex with adjacent intramedullary bone marrow edema (small arrowhead). Also note the globular intracortical signal abnormality (large arrowhead) within the lateral fibular cortex. d Corresponding axial fat-suppressed T2-weighted fast spin-echo MRI of the fibula and e axial T1-weighted spin-echo MRI of the fibula performed 2 weeks after the initial radiographic examination shows periosteal edema (arrows) circumferentially distributed throughout the fibular cortex with adjacent intramedullary bone marrow edema (small arrowhead). Also note the globular intracortical signal abnormality (large arrowhead) within the lateral fibular cortex
MRI findings of fibular stress injuries
The MRI characteristics of the fibular stress injury in each patient are summarized in Table 2. All 20 patients with fibular stress injury had a periosteal reaction and bone marrow edema within the fibula on MRI (Fig. 3). The periosteal reaction and bone marrow edema was present within the distal fibula in 14 patients (70.0%), the middle fibula in 1 patient (5.0%), and the proximal fibula in 5 patients (25.0%). The periosteal reaction was located on the anterior fibular cortex in 1 patient (5.0%), the posterior fibular cortex in 4 patients (20.0%), the lateral fibular cortex in 11 patients (55.0%), and circumferentially distributed throughout the fibular cortex in 4 patients (20.0%). According to the Fredericson MRI Classification System, 5 patients (25.0%) had grade 2 stress injuries, 6 patients (30.0%) had grade 3 stress injuries, and 9 patients (45.0%) had grade 4 stress injuries. Of the 9 patients with Fredericson grade 4 stress injuries, the intracortical signal abnormality was located in the lateral cortex in 7 patients (78%) and in the posterior cortex in 2 patients (22%; Fig. 4). The intracortical signal abnormality had a linear configuration (i.e., fracture line) in 1 patient and a globular configuration in the remaining 8 patients. Five of the 20 patients with fibular stress injuries had associated tibial stress injuries on MRI.
A 43-year-old female marathon runner with a 2-week history of proximal fibular pain. a Axial fat-suppressed T2-weighted fast spin-echo MRI of the fibula shows periosteal edema (arrow) on the posterior cortex of the proximal fibula with adjacent intramedullary bone marrow edema (arrowhead). b Corresponding axial T1-weighted spin-echo MRI of the fibula shows intramedullary bone marrow edema (arrowhead)
A 20-year-old male decathlete with a 4-week history of distal fibular pain. a Coronal fat-suppressed T2-weighted fast spin-echo MRI of the fibula shows periosteal edema (arrows) on the lateral cortex of the distal fibula with adjacent intramedullary bone marrow edema (small arrowhead). b Corresponding coronal T1-weighted spin-echo MRI of the fibula shows intramedullary bone marrow edema (small arrowhead). c Corresponding axial fat-suppressed T2-weighted fast spin-echo MRI of the fibula shows periosteal edema (arrows) on the lateral fibular cortex with adjacent intramedullary bone marrow edema (small arrowhead). Also note the globular intracortical signal abnormality (large arrowhead) within the lateral fibular cortex
Discussion
Stress injuries of the proximal fibula were first described in 1907 in German infantrymen as a result of repetitive jumping activities [9]. However, our study has shown that most fibular stress injuries occur in the distal third of the fibula in athletes involved in strenuous running activities. Our findings are similar to those of Devas and Sweetnam, who published a case series of 40 patients with radiographically diagnosed fibular stress injuries. In their study, 70% of the fibular stress injuries were located in the distal fibula, and all stress injuries occurred in athletes and military recruits who ran long distances on hard surfaces [10].
In our study, distal fibular stress injuries most commonly involved the lateral fibular cortex. Devas and Sweetnam also reported that the periosteal reaction and fracture line seen on radiographs in their patients with distal fibular stress injuries involved the lateral fibular cortex. The authors postulated that the dynamic motion of the distal tibiofibular joint is the mechanism responsible for distal fibular stress injuries. The toe strike phase of runners is marked by eccentric contraction of the plantar flexor muscle groups to decelerate the forward movement of the stance leg over the foot [11]. Contraction of the plantar flexor muscles pulls the fibula distally and medially against the tibia with resultant stress concentration at the distal tibiofibular joint [10]. Axial loading of the lower extremity results in compressive forces on the anterior and lateral fibular cortex and tensile forces on the posterior fibular cortex [17]. Since bone exposed to repetitive compressive forces is most susceptible to stress injury, it is not surprising that most distal fibular stress injuries involve the lateral cortex [18].
In our study, the second most common location of fibular stress injuries was in the proximal third of the fibula. Unlike distal fibular stress injuries, which most commonly involved the lateral fibular cortex, most proximal fibular stress injuries involved the posterior fibular cortex. Symeonides published a case series of 48 military recruits who developed proximal fibula stress injuries and attributed them to an exercise where recruits would jump for long distances while maintaining a squatting position [11]. Hopfenfärtner also described proximal fibular stress injuries in military recruits secondary to repetitive jumping activities [9]. Neither study described the exact location of the stress injuries on the proximal fibular cortex. The etiology of proximal fibular stress fractures is unknown. However, biomechanical studies have shown that ankle dorsiflexion during running and jumping increases load distribution through the fibula and causes torsional forces that are dissipated via external rotation at the proximal tibiofibular joint [19, 20].
Radiographs are the initial imaging study obtained at most institutions when evaluating patients with suspected fibular stress injuries. However, initial and follow-up radiographs in our study had a sensitivity of only 15 and 50% respectively for detecting fibular stress injuries. No previous study has documented the low sensitivity of radiographs for detecting fibular stress injuries. However, our findings correlate well with those of previous studies in which radiographs had a sensitivity for detecting tibial stress injuries ranging between 10 and 29% on initial examination and between 40 and 54% on follow-up examination [1, 21–25].
Although radiographs are insensitive at detecting fibular stress injuries, the presence of radiographic abnormalities may offer important prognostic information regarding the severity of the stress injury. In our study, all 5 patients who developed radiographic signs of fibular stress injury had Fredericson grade 4 injuries on MRI. There have been no previous studies correlating radiographic findings with MRI findings in patients with fibular stress injuries. However, Kijowski and associates compared radiographic and MRI findings in 80 patients with tibial stress injuries. In their study, 11 of the 13 patients with radiographic findings of tibial stress injury had Fredericson grade 4 injuries on MRI, while the remaining 2 patients had Fredericson grade 3 stress injuries [25]. This study supports our findings that radiographic abnormalities are much more common in patients with high-grade stress injuries.
In our study, the MRI findings of patients with fibular stress injuries included periosteal edema, bone marrow edema, and intracortical signal abnormality within the fibula. Similar findings have been described in the few previously published case reports on patients with fibular stress injuries who were evaluated with MRI [12–14]. The MRI findings of fibular stress injury are similar to those of other disease processes such as infection and neoplasm. For this reason, when periosteal edema, bone marrow edema, and intracortical signal abnormality are seen within the fibula on MRI, it is important to correlate the MRI findings with clinical findings before a diagnosis of fibular stress injury can be confidently made [26].
Magnetic resonance imaging was highly accurate at confirming the clinical diagnosis of fibular stress injury in all patients evaluated in our study. MRI also provided excellent assessment of the exact location and extent of injury in all patients. However, the clinical significance of using MRI to evaluate patients with fibular stress injuries remains unknown. Additional studies are needed to determine whether MRI findings such as the grade of stress injury, the extent of periosteal edema and bone marrow edema, and the pattern of intracortical signal abnormality can be used as prognostic factors to determine how long patients with fibular stress injuries must refrain from physical activity to be adequately healed.
There are several limitations to our study. One limitation was the relatively small size of our patient population. However, fibular stress injuries are relatively uncommon, and our study is by far the largest series to describe the MRI findings in patients with fibular stress injuries. A second limitation of our study was the presence of selection bias. Not all patients with fibular stress injuries at our institution were included in the study. It is likely that some patients with clinical manifestations of fibular stress injuries who had a periosteal reaction on radiographs were treated for their injuries without being further evaluated with MRI. Furthermore, some patients with fibular stress injuries and normal radiographs may have been further evaluated with computed tomography or nuclear medicine scintigraphy. A third limitation of our study was that oblique radiographs were not used to evaluate our patients with fibular stress injuries. Oblique radiographs may have provided better detection of subtle periosteal reaction on the fibular cortex in patients with stress injuries.
In conclusion, our study summarizes the radiographic and MRI findings in a relatively large number of patients with fibular stress injuries. Initial and follow-up radiographs have a low sensitivity for detecting fibular stress injuries. However, identifying periosteal reaction on radiographs has prognostic value and indicates that a high-grade fibular stress injury is likely present. The MRI findings of fibular stress injuries include periosteal edema, bone marrow edema, and intracortical signal abnormality. Fibular stress injuries are most commonly located in the distal third of the fibula with the proximal third of the fibula being the second most common location. Distal fibular stress injuries most commonly involve the lateral fibular cortex, while most proximal fibular stress injuries involve the posterior fibular cortex.
References
Matheson GO, Clement DB, McKenzie DC, Taunton JE, Lloyd-Smith DR, MacIntyre JG. Stress fractures in athletes: a study of 320 cases. Am J Sports Med 1987; 15: 45–58.
Orava S, Hulkko A. Stress fractures in athletes. J Sports Med 1987; 8: 224–226.
Bennell KL, Bruckner PD. Epidemiology and site specificity of stress fractures. Clin Sports Med 1997; 16: 179–196.
Jones BH, Harris JM, Vinh TN, Rubin C. Exercise-induced stress fractures and stress reactions of bone: epidemiology, etiology, and classification. Exerc Sport Sci Rev 1989; 17: 379–422.
Johnson LC, Stradford HT, Geis RW, Dineen JR, Kerley E. Histiogenesis of stress fractures [abstract]. J Bone Joint Surg Am 1963; 45: 1542.
Li GP, Zhang SD, Chen G, Chen H, Wang AM. Radiographic and histologic analyses of stress fracture in rabbit tibias. Am J Sports Med 1985; 13: 285–294.
Bentolila V, Boyce TM, Fyhrie DP, Drumb R, Skerry TM, Schaffler MB. Intracortical remodeling in adult rat long bones after fatigue loading. Bone 1998; 23: 275–281.
Colopy SA, Benz-Dean J, Barrett JG, Sample SJ, Lu Y, Danova NA, et al. Response of the osteocyte syncytium adjacent to and distant from linear microcracks during adaptation to cyclic loading. Bone 2004; 35: 881–891.
Hopfenfärtner. Zur Entstehung von Wadenbeinbrüchen. Dtsch Mil Arztl Z 1907; 36: 100–106.
Devas MB, Sweetnam R. Stress fractures of the fibula. J Bone Joint Surg Br 1956; 38B: 818–829.
Symeonides PP. High stress fractures of the fibula. J Bone Joint Surg Br 1980; 2: 192–193.
Slawson SH, Arendt E, Engebretsen L, Everson LI, Griffiths HJ. Fibular stress fracture in a 20-year old woman. Orthopedics 1994; 17: 378–379.
DiFiori JP. Stress fracture of the proximal fibula in a young soccer player: a case report and a review of the literature. Med Sci Sports Exerc 1999; 31: 925–928.
Al-Kashmiri A, Delaney JS. Fatigue fracture of the proximal fibula with secondary common peroneal nerve injury. Clin Orthop Relat Res 2007; 463: 225–228.
Ballinger PW, Frank ED. Merrill’s atlas of radiographic positions and radiologic procedures. 9th ed. Philadelphia: Mosby; 1999: 285–287.
Fredericson M, Bergman AG, Hoffman KL, Dillingham MS. Tibial stress reaction in runners. Correlation of clinical symptoms and scintigraphy with a new magnetic resonance imaging grading system. Am J Sports Med 1995; 23: 472–481.
Lambert KL. The weight-bearing function of the fibula. A strain gauge study. J Bone Joint Surg 1971; 53: 507–513.
Muir P, Sample SJ, Barrett JG, et al. Effect of fatigue loading and associated matrix microdamage on bone blood flow and interstitial fluid flow. Bone 2007; 40: 948–956.
Ogden JA. The anatomy and function of the proximal tibiofibular joint. Clin Orthop 1974; 101: 186–191.
Takebe K, Nakagawa A, Minami H, Kanazawa H, Hirohata K. Role of the fibula in weight-bearing. Clin Orthop 1984; 187: 188–192.
Giladi M, Nili E, Ziv Y, Danon YL, Aharonson Z. Comparison between radiography, bone scan, and ultrasound in the diagnosis of stress fractures. Mil Med 1984; 149: 459–461.
Greaney RB, Gerber FH, Laughlin RL, et al. Distribution and natural history of stress fractures in U.S. Marine recruits. Radiology 1983; 146: 339–346.
Prather JL, Nusynowitz ML, Snowdy HA, Hughes AD, McCartney WH, Bagg RJ. Scintigraphic findings in stress fractures. J Bone Joint Surg 1977; 59: 869–874.
Zwas ST, Elkanovitch R, Frank G. Interpretation and classification of bone scintigraphic findings in stress fractures. J Nucl Med 1987; 28: 452–457.
Kijowski R, Choi J, Mukharjee R, De Smet A. Significance of radiographic abnormalities in patients with tibial stress injuries. Skeletal Radiol 2007; 7: 633–640.
Anderson MW, Greenspan A. Stress fractures. Radiology 1996; 199: 1–12.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Woods, M., Kijowski, R., Sanford, M. et al. Magnetic resonance imaging findings in patients with fibular stress injuries. Skeletal Radiol 37, 835–841 (2008). https://doi.org/10.1007/s00256-008-0488-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-008-0488-6