Abstract
Anterior mid-tibial cortex stress fractures (ATCSF) are uncommon and notoriously challenging to treat. They are termed high risk due to their predilection to prolonged recovery, nonunion and complete fracture. Early diagnosis is essential to avoid progression and reduce fracture complications. Imaging plays a key role in confirming the diagnosis. Magnetic resonance imaging (MRI) is accepted as the gold standard modality due to its high accuracy and nonionizing properties. This report describes three cases of ATCSFs in recreational athletes who had positive radiographic findings with no significant MRI changes. Two athletes had multiple striations within their tibias. Despite the radiographic findings, their severity of symptoms were low with mild or no tenderness on examination. Clinicians should be mindful that the ATCSFs may not present with typical acute stress fracture symptoms. We recommend that plain radiographs should be used as the first line investigation when suspecting ATCSFs. Clinicians should be aware that despite MRI being considered the gold standard imaging modality, we report three cases where the MRI was unremarkable, whilst radiographs and computed tomography confirmed the diagnosis. We urge clinicians to continue to use radiographs as the first line imaging modality for ATCSFs and not to directly rely on MRI. Those who opt directly for MRI may be falsely reassured causing a delay in diagnosis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Stress fractures represent up to 20% of all injuries seen at a sport and exercise medicine (SEM) clinic [1, 2]. Common sites include the tibia, fibula and metatarsals. There is a subset of stress fracture locations, which are termed high risk due to their predilection to prolonged recovery, nonunion, complete fracture and chronic pain. These include the anterior tibia, femoral neck (lateral), patella, medial malleolus, talus, navicular, fifth metatarsal diametaphysis and great toe seasmoids [3].
Tibial stress fractures often occur along the medial aspect [4]. However, less commonly the anterior cortex of the mid diaphysis is affected, reported to represent 5–10% of all tibial stress fractures [5, 6]. In our experience, they are an infrequent presentation. These fractures are vulnerable to nonunion or delayed union and even complete fracture. The combination of tibial morphology, constant tension from the posterior muscles and reduced vascular supply to the anterior cortex are influencing factors. They are notoriously challenging to treat with long periods of relative rest and can result in surgery with lengthy return to play times [7].
Imaging plays a key role in diagnosis confirmation and guiding management to avoid fracture progression and nonunion. In typical stress fractures, radiographs have a low yield for the diagnosis of early stress fractures with up to 85% not identified on initial imaging [8, 9]; however, if positive, radiographs are clearly helpful. In the case of mid-tibial cortex stress fracture (ATCSF) the classic radiographic findings of an ATCSF include a transverse linear lucency within the anterior mid tibial cortex that is known as ‘the dreaded black line’ with adjacent cortical thickening.
Magnetic resonance imaging (MRI) has been shown to be the imaging modality of choice for diagnosing patients with suspected lower extremity stress injuries [8]. Classically, as described by Fredericson’s classification, early tibial bone stress injuries often present on MRI with periosteal oedema and progress to show marrow edema with worsening of the injury [10]. This has been shown to grade injury severity and predict return to play [11, 12]. However, much of this literature is based on medial tibial stress syndrome (MTSS) and posteromedial tibial stress fractures, rather than the less common fractures of the anterior cortex [10, 11, 13]. ATCSFs may require a different imaging approach.
This report describes three cases of ATCSFs in recreational athletes who presented with mild symptoms with minimal examination findings who all had positive radiographic findings without significant MRI changes. Their injuries would have been undetected without the use of plain radiographs. It discusses the athletes’ presenting features and suggests radiographs still have a crucial role in the ATCSFs imaging algorithm to prevent delays in diagnosis.
Method
All cases were managed in the multi-disciplinary SEM clinic at the Nuffield Orthopedic Centre, Oxford, United Kindgom during January 2015 to June 2016. Written consent was gained from all subjects.
Case 1
A 49-year-old female recreational runner presented with a 3-month history of right mid tibial pain. She had been training for her first marathon. Since completing a half marathon (13.1 miles/21 km) 5 months previously, she had increased her running distance and frequency gradually over the next 2 months. She noticed a ‘bruised like sensation’ over the mid anterior aspect of her right shin whilst having a sports massage. She denied having any pain on running, walking or nocturnal pain and continued to train over the next 2 months. Due to the ongoing tenderness on palpation, she was advised to see her general practitioner (GP) who requested a radiograph.
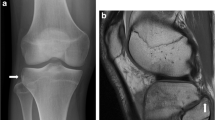
She had no previous bone stress injuries and no specific metabolic bone risk factors were identified. On examination, there was palpable bony irregularity in the middle third of the right tibia anteriorly with mild tenderness over this area. A right tibial radiograph revealed focal cortical thickening in the mid-tibial diaphysis anteriorly with a lucent line extending through the anterior cortex in keeping with a stress fracture (Fig. 1a).
(a) Lateral radiograph of the right tibia showing focal cortical thickening with a horizontal lucency in the anterior cortex of the mid tibia indicating an incomplete stress fracture (arrowhead). MRI Sagittal STIR (b) and T1 weighted images (c) showing focal cortical thickening but no periostitis, fracture line or evidence of marrow oedema (arrows). (d) Sagittal CT reformat of right tibia demonstrating presence of horizontal fracture line (arrow) seen on the radiograph
A subsequent MRI showed no evidence of bone oedema or periosteal reaction (Fig. 1b, c). Computer tomography (CT) confirmed the presence of the fracture (Fig. 1d). Dual energy x-ray absorptiometry (DEXA) showed a T-score within the normal range. Following 6 months of conservative management with complete resolution of tenderness and confirmation of bony union on CT. She successfully gradually returned to running.
Case 2
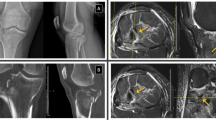
A 43-year-old male elite level ironman triathlete and ultra-marathon runner was referred with an insidious onset of exertional left mid-tibial pain. Prior to the onset of pain, he was conducting his normal weekly training load of 20 h of triathlon training (including swimming, cycling and running a total of 30–40 miles/week). He noticed a mild swelling in the mid part of his shin and some minor discomfort when running. He was reviewed by his GP who performed a radiograph (Fig. 2a). This showed several short linear lucencies within the anterior cortex of the mid-shaft of the tibia, representing incomplete stress fractures. He was subsequently referred to the clinic and was advised to cease running. There were no previous bone stress injuries and no specific metabolic bone risk factors were identified.
(a)HSR72773 Lateral radiograph of the left tibia demonstrating several short linear lucencies within the anterior cortex of the mid-shaft of the tibia, representing incomplete stress fractures (arrows). Sagittal T1 (b) and STIR (c) images of left tibia showing no evidence of marrow oedema or periosteal change. (d) Sagittal CT of left tibia confirming the fracture sites demonstrated on radiographs (arrows)
On examination in clinic, 2 months following the onset of his symptoms, there was no significant tenderness on either border of the left tibia. He was able to fully load the left leg and hopped repetitively without any pain. Subsequent MRI revealed the absence of any bone marrow or muscle abnormalities and no stress fractures were identified (Fig. 2b, c). Computer tomography (CT) confirmed the radiographic findings (Fig. 2d). Following a multi-disciplinary case discussion, a conservative approach was undertaken. After 9 months of conservative management (Table 1), CT confirmed cortical bridging at all fracture sites and he gradually returned to running.
Case 3
A 33-year-old male heating engineer was referred with a 4-year history of intermittent bilateral leg pain, which had initially started whilst training for a military entry fitness test. He had reported a dull ache over the anterior aspect of both shins exacerbated by running short distances and prolonged walking. The dull ache would gradually subside over several days following the activity; therefore, he chose to avoid exacerbating activities. However, his symptoms had been particularly worse over the last 8 months influencing his job and he had sought review; additionally, he denied having any nocturnal pain.
On examination, there were multiple nontender bony ridges in the mid anterior of both tibias. Single leg hop on both legs was not painful. Radiographs showed several short linear lucencies within the anterior cortex of the mid-shaft of both tibias, representing incomplete stress fractures (Fig. 3a). MRI showed no evidence of periosteal reaction or bony oedema (Fig. 3b, c). Subsequent CT confirmed the presence of the fractures (Fig. 3d–f).
His serum 25(OH)D level was was deficient at 17 nmol/L (Table 1) and was treated with oral supplementation. The patient is currently undergoing a period of conservative rehabilitation prior to review. No other specific metabolic bone risk factors were identified.
MRI protocol used
MRI was performed using a 3-T Philips scanner using a phased-array extremity coil. Both examinations included axial T1-weighted spin-echo sequence (TR range/TE range, 400–600/15–30) and fat-suppressed T2-weighted fast spin-echo sequence, and sagittal and coronal T1-weighted spin-echo sequence and either a fat-sequence-suppressed T2-weighted fast spin sequence or a STIR sequence. All examinations were performed with an FOV between 16 and 24 cm, a slice thickness of 3-4 mm with an inter slice gap between 0.4 and 0.8 mm.
Discussion
The tibia is a common site for stress fractures; however, the anterior mid-tibial diaphysis is infrequently involved [5, 6]. Originally described in ballet dancers in 1956 [14], it has since been described in a variety of sports including netball, volleyball, soccer and distance running [7].
Stanitsky et al. [15] proposes that ATCSFs unique etiology centers on the repetitive forceful contraction of the posterior leg musculature creating significant tensile force across the anterior tibial cortex. In conjunction with the hypovascularity of the anterior cortex, these fractures are at a high risk of nonunion [16].
It is often a combination of intrinsic and extrinsic factors such as errors in training load, predisposing biomechanics, muscle weakness and bone health risk factors which all contribute to an imbalance in bone remodeling and consequent bone stress [2]. Specific to the tibia: a reduced calf circumference [1], smaller bone geometry [17], greater bending moments about the medial-lateral axis of the tibia [18] and greater vertical loading rate [19] may all play a significant role.
Some reports suggest that symptoms of ATCSFs may vary in severity and are often vague in nature [20, 21]. Classically, one would expect patients to complain of progressively worsening exertional shin pain with significant bony tenderness. However, despite the radiographic findings, the presented cases had a surprisingly low level of symptoms. Brukner et al. [20] reports similar findings in their case of a patient with bilateral anterior ATCSFs, they highlight the remarkable absence of the typical symptoms of an acute stress fracture and the participation in competitive sport for 5 years without extensive limitations. There are other reports of athletes continuing athletic activity for significant periods with either mild or no symptoms [21, 22]. The combination of the potential absence of acute stress fracture symptoms, limited examination findings and the diverse differential diagnosis for exertional leg pain may explain why mean duration of preceding symptoms prior to diagnosis can be up to 26 months [7]. Clinicians must be mindful that the ATCSF may not present with typical stress fracture symptoms.
Diagnostic imaging
Imaging plays a critical role in the evaluation of potential bone stress injuries. Radiographs are still recommended as the initial diagnostic modality in suspected stress fractures [23, 24] and if positive are clearly helpful. However, the requesting clinician must be aware of their poor sensitivity (15%) and are often unremarkable despite significant time intervals [8]. Their role appears still to be valid in ATCSF. Classic radiographic findings of ATCSF include a transverse linear lucency within the anterior cortex (‘the dreaded black line’) with adjacent cortical thickening alone or associated with subacute or chronic periosteal bone formation.
Bone scintigraphy was once considered gold standard for advanced imaging due to its superior sensitivity to plain radiographs. However, MRI now supersedes it due to its nonionizing radiation properties and higher diagnostic accuracy [8, 25]. A recent review by Wright et al. [8] suggests that MRI is the modality of choice to detect stress fractures in the lower extremity. Clinicians should always consider MRI despite normal radiographs where their index of suspicion remains high. For the tibia, sensitivities have been recorded as high as 80–100% and specificity of 96%. Classification systems aid severity grading [10, 12, 26] and have been correlated to return to play times [26, 27]. However, the focus is primarily on the commoner lower risk presentation of medial/posterior tibial bone stress [10,11,12,13]. Whilst MRI has shown to be successful at highlighting soft tissue changes, it can struggle to delineate a fracture line. Brukner et al. [20] noted MRI findings comparable to our cases with no evidence of a transverse line through the tibia nor evidence of bone marrow oedema with only a slight increase in the signal intensity in the posterior aspect of the periosteum. They suggest that there may be a different underlying mechanism to a typical stress fracture. Seki et al. [28] reports a rare case of a 16-year-old distance runner with a femoral neck stress fracture (FNSF) which was not detected on MRI with follow up radiographs confirming the diagnosis. This was similar to a case by Sato et al., in which a FNSF was undetected on MRI in a pilgrimage walker, it was suggests that the bone marrow oedema and fracture site were too small to be detected on the slice thickness settings of 6 mm that were used [29]. Sato et al. [29] reports as the fracture became larger with daily distance walking, it was detected on MRI a month later. However, in the presented cases the slice thickness used was 3-4 mm and the athletes had symptoms for more than 1 month. The presence of fractures on the radiographs coupled with the lack of bone oedema on MRI suggest chronicity. Additionally, the hypovascular characteristics of the anterior tibia may explain the lack of MRI findings; however, an alternative explanation for the lack of periosteal oedema seen on MRI is the time delay in the presented cases between obtaining the MRI study and initial radiographs. In light of the radiographic findings, all patients were advised to stop running and reduce loading activities. It is conceivable that periosteal oedema resolved during this short period.
In the absence of radiographs, the clinician who had opted initially for the gold standard modality, MRI, would have been falsely reassured. This finding may be unique to the ATCSFs and should prompt awareness amongst clinicians. In this series, MRI was requested in light of the positive radiographic in an attempt to gain further evaluation of the bony lesion, periosteal tissues, endosteal tissue and cortical bone assessing particularly for the presence of bone oedema. In typical stress fractures, the presence of bone oedema (high signal) around the fracture site indicates a stress response and metabolic activity with healing potential. Conversely, the lack of oedema may suggest nonunion; nevertheless, at least two of the presented cases had subsequently confirmed union on CT following conservative management.
Bone scintigraphy with single-photon emission computed tomography (SPECT) may be a useful tool in such scenarios and has an increasing use in musculoskeletal medicine. It has shown to be superior to MRI and CT in the detection of spondylolysis and has been used for young athletes with lower back pain [30]. It allows visualization of bone morphology and metabolism in a single image session correlating the relationship between metabolic activity and anatomical site. Areas of high uptake ‘hot spots’ may indicate healing potential where as those with no or little uptake ‘cold spots’ over the fracture site suggest nonunion, a helpful adjunct when considering surgical management.
On biopsy, the chronic anterior mid-tibial stress fracture appears to have limited or no evidence of bone remodeling. Rolf et al. [31] described it as an “atrophic pseudoarthrosis”. Compact cortical bone with empty lacunae surrounds the fracture defect, which is filled with fibrous material or granulation tissue without notable callous [32].
CT was used to define the fracture architecture and cortical change. As it is more sensitive than radiographs at delineating the bony bridging across the fracture site in multiple planes [33], it was therefore used in conjunction with clinical findings to confirm osseous union. Using local osteopenia as the earliest finding of fatigue, Gaetae et al. found CT to be superior to MRI for specifically detecting early cortical abnormalities. In their study of 50 tibias, of 42 patients who had early tibial stress injuries with normal radiographs, six patients had negative MRI findings; however, cortical abnormalities were noted on CT in these cases [25].
Multiple anterior tibial cortex stress fractures
Multiple tibial stress fractures in the same bone are rare. The first reports are in the 1970s amongst ballet dancers [34, 35]. Since then they have been included within several case series [20, 36] with some progressing to complete fracture [37]. Stress fractures are seen at multiple sites in the cortex with cortical thickening and narrowing of the medullary canal. This suggests a different etiology compared to the typical stress fracture where there is a single loss of focal discontinuity in a perceived normal bone. Gaeta et al. [25] noted tibial striations on CT (subtle intracortical linear hypoattenuation) in 11/50 cases of subjects with early tibial stress injuries. They believe that these changes were accompanied by osteopenia and resorption cavities represent prefracture lesions.
Conclusion
Clinicians should be mindful that the ATCSFs may not present with typical stress fracture symptoms. Severity of symptoms may be low with mild or no tenderness on examination. We recommend plain radiographs to be used as the first line investigation when suspecting tibial stress fractures. Clinicians should be aware that despite being considered the gold standard imaging modality, MRI can be unremarkable. We urge clinicians to continue using radiographs as the first line imaging modality for ATCSFs and not to directly rely on MRI. Those who opt directly for MRI may be falsely reassured causing a delay in diagnosis.
References
Bennell KL, Malcolm SA, Thomas SA, Wark JD, Brukner PD. The incidence and distribution of stress fractures in competitive track and field athletes: a twelve-month prospective study. Am J Sports Med. 1996;24(2):211–7.
Pegrum J, Crisp T, Padhiar N. Diagnosis and management of bone stress injuries of the lower limb in athletes. BMJ. 2012;344:e2511.
McInnis KC, Ramey LN. High-risk stress fractures: diagnosis and management. PM R. 2016;8(3 Suppl):S113–24.
Matheson GO, Clement DB, McKenzie DC, Taunton JE, Lloyd-Smith DR, MacIntyre JG. Stress fractures in athletes: a study of 320 cases. Am J Sports Med. 1987;15(1):46–58.
Liimatainen E, Sarimo J, Hulkko A, Ranne J, Heikkilä J, Orava S. Anterior mid-tibial stress fractures: results of surgical treatment. Scand J Surg. 2009;98(4):244–9.
Orava S, Hulkko A. Stress fracture of the mid-tibial shaft. Acta Orthop Scand. 1984;55(1):35–7.
Robertson GA, Wood AM. Return to sports after stress fractures of the tibial diaphysis: a systematic review. Br Med Bull. 2015;114(1):95–111.
Wright AA, Hegedus EJ, Lenchik L, Kuhn KJ, Santiago L, Smoliga JM. Diagnostic accuracy of various imaging modalities for suspected lower extremity stress fractures: a systematic review with evidence-based recommendations for clinical practice. Am J Sports Med. 2016;44(1):255–63.
Moran DS, Evans RK, Hadad E. Imaging of lower extremity stress fracture injuries. Sports Med. 2008;38(4):345–56.
Fredericson M, Bergman AG, Hoffman KL, Dillingham MS. Tibial stress reaction in runners: correlation of clinical symptoms and scintigraphy with a new magnetic resonance imaging grading system. Am J Sports Med. 1995;23(4):472–81.
Beck BR, Bergman AG, Miner M, Arendt EA, Klevansky AB, Matheson GO, et al. Tibial stress injury: relationship of radiographic, nuclear medicine bone scanning, MR imaging, and CT severity grades to clinical severity and time to healing. Radiology. 2012;263(3):811–8.
Kijowski R, Choi J, Shinki K, Del Rio AM, De Smet A. Validation of MRI classification system for tibial stress injuries. AJR Am J Roentgenol. 2012;198(4):878–84.
Mammoto T, Hirano A, Tomaru Y, Kono M, Tsukagoshi Y, Onishi S, et al. High-resolution axial MR imaging of tibial stress injuries. Sports Med Arthrosc Rehabil Ther Technol. 2012;4(1):16.
BURROWS HJ. Fatigue infraction of the middle of the tibia in ballet dancers. J Bone Joint Surg Br. 1956;38-B(1):83–94.
Stanitski CL, McMaster JH, Scranton PE. On the nature of stress fractures. Am J Sports Med. 1978;6(6):391–6.
Boden BP, Osbahr DC. High-risk stress fractures: evaluation and treatment. J Am Acad Orthop Surg. 2000;8(6):344–53.
Bennell K, Crossley K, Jayarajan J, Walton E, Warden S, Kiss ZS, et al. Ground reaction forces and bone parameters in females with tibial stress fracture. Med Sci Sports Exerc. 2004;36(3):397–404.
Meardon SA, Willson JD, Gries SR, Kernozek TW, Derrick TR. Bone stress in runners with tibial stress fracture. Clin Biomech (Bristol, Avon). 2015;30(9):895–902.
Milner CE, Ferber R, Pollard CD, Hamill J, Davis IS. Biomechanical factors associated with tibial stress fracture in female runners. Med Sci Sports Exerc. 2006;38(2):323–8.
Brukner P, Fanton G, Bergman AG, Beaulieu C, Matheson GO. Bilateral stress fractures of the anterior part of the tibial cortex: a case report. J Bone Joint Surg Am. 2000;82(2):213–8.
Gigis I, Rallis I, Gigis P, Goulios V. Anterior tibial cortex stress fracture in a high demand professional soccer player. J Med Cases. 2011;2(5):210–5.
Brahms MA, Fumich RM, Ippolito VD. Atypical stress fracture of the tibia in a professional athlete. Am J Sports Med. 1980;8(2):131–2.
Manaster BJ, Dalinka MK, Alazraki N, Berquist TH, Daffner RH, DeSmet AA, et al. Stress/insufficiency fractures (excluding vertebral): ACR appropriateness criteria. Radiology. 2000;215(Suppl):265–72.
Dixon S, Newton J, Teh J. Stress fractures in the young athlete: a pictorial review. Curr Probl Diagn Radiol. 2011;40(1):29–44.
Gaeta M, Minutoli F, Scribano E, Ascenti G, Vinci S, Bruschetta D, et al. CT and MR imaging findings in athletes with early tibial stress injuries: comparison with bone scintigraphy findings and emphasis on cortical abnormalities. Radiology. 2005;235(2):553–61.
Kijowski R, Choi J, Mukharjee R, de Smet A. Significance of radiographic abnormalities in patients with tibial stress injuries: correlation with magnetic resonance imaging. Skelet Radiol. 2007;36(7):633–40.
Nattiv A, Kennedy G, Barrack MT, Abdelkerim A, Goolsby MA, Arends JC, et al. Correlation of MRI grading of bone stress injuries with clinical risk factors and return to play: a 5-year prospective study in collegiate track and field athletes. Am J Sports Med. 2013;41(8):1930–41.
Seki N, Okuyama K, Kamo K, Chiba M, Shimada Y. Negative magnetic resonance imaging in femoral neck stress fracture with joint effusion: a case report. Skelet Radiol. 2016;45(6):843–6.
Sato RYS, Mihashi M, et al. A case of femoral neck stress fracture without early diagnosis by magnetic resonance imaging. Chubuseisaishi. 2007;50:1095–6. (in Japanese)
Matesan M, Behnia F, Bermo M, Vesselle H. SPECT/CT bone scintigraphy to evaluate low back pain in young athletes: common and uncommon etiologies. J Orthop Surg Res. 2016;11(1):76.
Rolf C, Ekenman I, Törnqvist H, Gad A. The anterior stress fracture of the tibia: an atrophic pseudoarthosis? Scand J Med Sci Sports. 1997;7(4):249–52.
Orava S, Karpakka J, Hulkko A, Väänänen K, Takala T, Kallinen M, et al. Diagnosis and treatment of stress fractures located at the mid-tibial shaft in athletes. Int J Sports Med. 1991;12(4):419–22.
Kuhlman JE, Fishman EK, Magid D, Scott WW, Brooker AF, Siegelman SS. Fracture nonunion: CT assessment with multiplanar reconstruction. Radiology. 1988;167(2):483–8.
Miller EH, Schneider HJ, Bronson JL, McLain D. A new consideration in athletic injuries: the classical ballet dancer. Clin Orthop Relat Res. 1975;111:181–91.
Schneider HJ, King AY, Bronson JL, Miller EH. Stress injuries and developmental change of lower extremities in ballet dancers. Radiology. 1974;113(3):627–32.
Daffner RH. Anterior tibial striations. AJR Am J Roentgenol. 1984;143(3):651–3.
Burke R, Chiang AL, Lomasney LM, Demos TC, Wu K. Multiple anterior tibial stress fractures complicated by acute complete fracture of the distal tibia. Orthopedics. 2014;37(4):217, 274–218.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Presentation
The authors directly declare that the results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Smith, R., Moghal, M., Newton, J.L. et al. Negative magnetic resonance imaging in three cases of anterior tibial cortex stress fractures. Skeletal Radiol 46, 1775–1782 (2017). https://doi.org/10.1007/s00256-017-2773-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-017-2773-8