Abstract
The liver is an important digestive gland, and acute liver failure results in high mortality. Probiotics are considered potential adjuvant therapies for liver disease. This study aimed to investigate the beneficial effects of Lactobacillus helveticus R0052 on acute liver injury and the underlying mechanisms. Sprague-Dawley rats were gavaged with L. helveticus R0052 suspensions (3 × 109 CFU) for 1 week. Subsequently, acute liver injury was induced by intraperitoneal d-galactosamine injection on the eighth day. After 24 h, samples (blood, liver, ileum, faeces) were collected and assessed for histological injury, inflammation, intestinal barrier, gut microbiome and metabolome. L. helveticus R0052 alleviated aminotransferase, bilirubin and total bile acid elevation and histological hepatic injuries. Additionally, L. helveticus R0052 exhibited anti-inflammatory properties by downregulating Toll-like receptors, tumour necrosis factor-α and nuclear factor-κb transcription in liver samples and decreasing proinflammatory cytokine plasma concentrations. Additionally, L. helveticus R0052 ameliorated intestinal abnormalities and regulated Toll-like receptors, claudin2 and mucin3 gene transcription in the intestine. These effects were associated with gut microbiome and metabolome modulation by L. helveticus R0052. Probiotic pretreatment enriched Lactobacillus and Bacteroides and depleted Flavonifractor and Acetatifactor in the gut microbiome. Meanwhile, L. helveticus R0052 improved carbohydrate and fatty acid metabolism and reduced lithocholic acid levels. These results indicate that L. helveticus R0052 is promising for alleviating acute liver injury and provide new insights regarding the correlations among the microbiome, the metabolome, the intestinal barrier and liver disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The liver is a vital organ for health, with important functions, such as metabolism and detoxification. It has a strong capability of regeneration, but persistent liver injury can be induced by several pathogeneses, such as those induced by medicines, viruses, alcohol or autoimmune hepatitis (Bernal et al. 2010). Without appropriate treatment, serious liver injuries may manifest as liver failure in clinical practice, especially acute liver failure (ALF), which can even result in high mortality exceeding 90% (Lee 2012).
Recently, the human microbiome has been closely linked with liver disease. The liver is perpetually exposed to gut microbes and their metabolites due to the close anatomical and functional relationships between the liver and intestine (Tripathi et al. 2018). However, the intestinal barrier prevents the translocation of most microbes and their metabolites, while hepatic Kupffer cells act as the first line to eliminate invading pathogens (Tilg et al. 2016). Most liver injuries will damage the gut barrier and disrupt the homeostasis of the gut-liver axis. Under this situation, some microbes and their metabolites, such as endotoxin, can permeate into the portal vein, induce immune response and aggravate liver injury.
Probiotic is defined as ‘live microorganisms that, when administered in adequate amounts, confer a health benefit on the host’ by expert consensus (Hill et al. 2014). Predictive, preventive and personalized application of probiotics can be a safe therapy to reduce hepatic inflammation and alleviate liver injury (Bubnov et al. 2015). Based on available studies, probiotic strains can promote health by different mechanisms and most probiotics are capable of supporting a healthy digestive tract and a balanced immune system by inhibiting potential pathogens and producing useful metabolites or enzymes (Hill et al. 2014). Although probiotic strains mainly colonize in intestine, they can interact with liver at distant site by gut-liver axis and metabolism (Reid et al. 2017). For example, Lactobacillus GG can ameliorate acute alcohol-induced liver injury by protecting the intestinal barrier and suppressing endotoxaemia (Wang et al. 2012). Our previous studies indicated that L. salivarius LI01, Pediococcus pentosaceus LI05 and Bifidobacterium adolescentis CGMCC 15058 can alleviate ALF by modulating gut microbiome (Li et al. 2019; Lv et al. 2014).
Among probiotic strains, the genus Lactobacillus is an important member of the human and animal microbiomes and plays a role on host’s health (Zhang et al. 2018). Lactobacillus helveticus is a food-associated species and products bioactive peptides with potential therapeutic values (Slattery et al. 2010). The strain L. helveticus R0052 was originally isolated from a North American dairy starter culture. L. helveticus R0052 can utilize a broader spectrum of carbohydrates, produce lactic acid and is widely used as a probiotic (Naser et al. 2006). Its safety and tolerability have been proven in multi-centre randomized, double-blind, placebo-controlled trials (Manzano et al. 2017). In addition, it was reported that L. helveticus R0052 might protect hepatocytes during liver disease. For example, the administration of L. helveticus R0052 can alleviate alcoholic liver disease by downregulating the expression of innate immunity-associated Toll-like receptors (TLRs) and improving immunologic status as well as regulating the gut-liver axis (Bang et al. 2014; Hong et al. 2015).
In this work, the effect of L. helveticus R0052 on acute liver injury was investigated using a d-galactosamine (d-GalN)-induced acute liver injury rat model, which is a classic model to study inflammatory liver injury and test the hepatoprotective effects of probiotics (Maes et al. 2016). d-GalN treatment can cause significant release of proinflammatory cytokines and induce systemic inflammatory response syndrome which is associated with the rapid progressing of disease and poor outcome in ALF patients (Antoniades et al. 2008). Additionally, comprehensive analyses of inflammation, the intestinal barrier, and the gut microbiome and metabolome were performed to explore the mechanism underlying the beneficial effects of L. helveticus R0052.
Materials and methods
Strain and culture conditions
The probiotic strain L. helveticus R0052 (CNCM I-1722) was supplied by Lallemand Heath Solutions Inc. (Blagnac, France). After anaerobically culturing in Man–Rogosa–Sharpe broth (Thermo Fisher, Shanghai, China) at 37 °C for 24 h, the broth was centrifuged at 8000×g at 4 °C for 10 min. Then, the precipitation was washed twice with sterile normal saline and resuspended in sterile normal saline at a final concentration of 3 × 109 colony-forming units (CFU)/mL for gavage.
Animals and experimental design
Twenty-four male specific pathogen-free (SPF) Sprague-Dawley rats (Shanghai SLAC Laboratory Animal Co. Ltd., China) weighing 250–300 g were included in this randomized controlled trial to avoid the effects of menstruation in female rats. All animals were fed standard rat chow and kept at an SPF facility. The rats were randomly divided into three experimental groups named the normal control (NC) group, positive control (PC) group and L. helveticus R0052-pretreated (R0052) group. Each group contained eight rats. In the first 7 days, the R0052 group was gavaged daily with 1 mL of freshly prepared L. helveticus R0052 suspensions. Meanwhile, the NC group and the PC group were gavaged with 1 mL of normal saline as a placebo. On the eighth day, d-GalN (Sigma, Saint Louis, MO, USA) was intraperitoneally injected into rats in the PC group and the R0052 group at a dose of 1.1 g/kg body weight to establish the acute liver injury model, while the rats in the NC group received the same dose of normal saline by intraperitoneal injection.
Sample collection
Twenty-four hours after the injection of d-GalN, faecal samples were collected upon defecation. Rats were anaesthetized with 30 mg/kg pentobarbital sodium (Sigma, Saint Louis, MO, USA) and 10 mg/kg pethidine (Sigma, Saint Louis, MO, USA). The blood was collected from the inferior vena cava and centrifuged at 3000×g for 10 min to collect plasma and serum samples. Samples of liver and terminal ileum for histological evaluation were immediately fixed in 10% paraformaldehyde once isolated from rats. Terminal ileum samples for scanning electron microscopy observation were fixed in 2.5% glutaraldehyde in phosphate buffer. Other liver and terminal ileum samples were stored in liquid nitrogen for further study.
Histological evaluation
After 24 h of fixation, the samples were embedded in paraffin, cut into 2-μm sections and stained with haematoxylin and eosin (H&E). At least five fields of each section were assessed under a microscope. The liver biopsies were evaluated according to the histological activity index (HAI) (Knodell et al. 1981). In brief, specimens were graded in four categories: periportal with or without bridging hepatocellular necrosis, intralobular degeneration and focal hepatocellular necrosis, portal inflammation, and fibrosis.
Intestinal epithelium abnormalities were graded as suggested by Chiu (1970). Subepithelial Gruen Hagen’s space, epithelial lifting and denudation of villi were taken into consideration in this scoring system.
Scanning electron microscopy
The terminal ileum samples were secondarily fixed with 1% OsO4 in phosphate buffer for 1 h and washed three times in phosphate buffer for 15 min. The fixed samples were dehydrated by a graded series of ethanol (30%, 50%, 70%, 80%, 90% and 95%) for 15 min at each step and pure ethanol for 20 min. Finally, the samples were dehydrated in a Hitachi Model HCP-2 critical point dryer (Hitachi, Tokyo, Japan) and coated with gold-palladium in a Hitachi Model E-1010 ion sputter (Hitachi, Tokyo, Japan) for 5 min. A Nova Nano 450 scanning electron microscope (FEI, Hillsboro, USA) was used for observation.
RT-qPCR analysis
The transcription of inflammation-associated genes was evaluated by a two-step reverse transcription quantitative PCR (RT-qPCR) method. Total RNA was extracted from the liver and terminal ileum samples with an RNeasy Plus Mini kit (Qiagen, Valencia, CA, USA) and immediately transformed into cDNA using a PrimeScript™ RT reagent Kit (Takara Biomedicals, Kusatsu, Japan). Subsequently, the transcription of genes was measured by qPCR in triplicate with Premix Ex Taq (Takara Biomedicals, Kusatsu, Japan) on a ViiA7 Real-time PCR system (Applied Biosystems, Waltham, Massachusetts, USA). The housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was defined as the internal control. The transcription of genes was transformed into relative expression by the internal control for further analysis. The primer sequences of the indicated genes for RT-qPCR analysis are provided in Table 1.
Serum liver function test
The serum samples were analysed by a biochemical analyser (Hitachi 7600–210; Tokyo, Japan). This analyser is able to detect the concentration of liver function biochemical indicators, including total bile acid (TBA), total bilirubin (TB), direct bilirubin (DB), indirect bilirubin (IB), alanine aminotransferase (ALT), and aspartate aminotransferase (AST).
Plasma cytokine analysis
The Bio-Plex Pro Rat Cytokine Panel Assay (Bio-Rad, Hercules, California, USA) was conducted to determine the levels of plasma cytokines or chemokines, including interleukin-2 (IL-2), IL-6, IL-12, IL-17, tumour necrosis factor-α (TNF-α), regulated upon activation normal T-cell expressed and secreted (RANTES) protein, macrophage inflammatory protein-3α (MIP-3α).
16S rRNA gene sequencing and LEfSe analysis
Total genomic DNA was extracted from faecal samples using a QIAamp Fast DNA Stool Mini Kit (Qiagen, Valencia, USA). The quality of the DNA was verified by electrophoresis. The 16S rRNA genes were amplified by the universal primers 338F (5′-ACTCCTACGGGAGGCAGCAG-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′). The PCR products were purified with AMPure XP beads (Agencourt, Beckman Coulter, Brea, California, USA) to establish qualified libraries. The qualified libraries were sequenced on an Illumina MiSeq platform (Illumina, San Diego, CA) with a MiSeq Reagent Kit V3 (Illumina, San Diego, CA). To restore the sequence of V3-V4 regions, overlapping paired-end reads were merged to form long tags with FLASH software (version 1.2.8) (Magoč and Salzberg 2011). In addition, low-quality reads and chimaeras were excluded, and the clean data were clustered into operational taxonomic units (OTUs) with a 97% threshold by Vsearch software (v2.3.4, Vsearch) (Rognes et al. 2016). A representative sequence of each OTU was selected and annotated with RDP classifier according to the Ribosomal Database Project (RDP, database v.11.3) (Cole et al. 2014). The Chao1 index, Shannon index and principal coordinates analysis (PCoA) of unweighted UniFrac beta diversity were calculated by QIIME software (version 1.8.0) (Caporaso et al. 2010) to investigate the richness and diversity of each gut microbiome. Linear discriminant analysis effect size (LEfSe) analysis was performed on Galaxy web platform (Afgan et al. 2018). The effect size of each differential taxon was estimated by linear discriminant analysis (LDA) score, and the results with LDA scores greater than 3.5 were defined as discriminative taxa.
Metabolomic analysis
Analysis of the faecal metabolome was based on the gas chromatography-mass spectrometer (GC-MS) method, which was proposed in our previous study (Ye et al. 2018). In brief, 15 mg of faeces was homogenized in 800 mL of ice-cold methanol (Sigma-Aldrich, St. Louis, MO, USA) and centrifuged at 14,000 rpm for 15 min. The supernatant was mixed with 20 mL of heptadecanoic acid (1 mg/mL, Sigma-Aldrich, St. Louis, MO, USA) and dried by nitrogen stream. The residue was dissolved in 50 mL of anhydrous pyridine with 15 mg/mL methoxylamine hydrochloride (Sigma-Aldrich, St. Louis, MO, USA) and incubated at 37 °C for 24 h. Then, the samples were mixed with 50 mL of 1% trimethylsilyl chloride in N,O-bistrifluoroacetamide (Sigma-Aldrich, St. Louis, MO, USA) and incubated at 70 °C for 120 min. After these preparations, the mixtures were analysed on a GC-MS Agilent 7890A GC system and an Agilent 5975C inert mass-selective detector system (Agilent Technologies, Santa Clara, CA, USA).
Metabolites were identified by the NIST databases (score > 80). Principal component analysis (PCA) and orthogonal partial least squares-discriminant analysis (OPLS-DA) were both performed by SIMCA software (version 14.1) (Sartorius Stedim Biotech, Umeå, Sweden). Variables within each model were scaled by the Pareto scaling method to reduce the impact of noise and artefacts in the models. To identify the differential metabolites between the NC group and the PC group or between the R0052 group and the PC group, metabolites with a variable importance in the projection (VIP) value more than 1 and absolute p(corr) values larger than 0.3 were retained in each model (Zeng et al. 2014). The metabolites observed in both models were identified as the most differential metabolites for L. helveticus R0052 pretreatment. In addition, the Mann-Whitney U test was applied to the peak area of these differential metabolites to examine whether their levels were significantly different among experimental groups.
Statistical analysis and illustrations
Statistical analyses were performed using SPSS 20.0 (SPSS Inc., Chicago, IL, USA). The data for liver function, relative expression of genes, cytokine concentrations, Chao1 index and Shannon index were analysed using one-way ANOVA followed by LSD multi-comparison test. The data for HAI score, ileum histology score and metabolites were analysed by the pairwise Mann-Whitney U test. Correlation analysis was based on Spearman’s rank correlation analysis.
Statistical significance was defined as two-tailed P values < 0.05.
Nucleotide sequence accession number
The 16S sequencing data of the rat gut microbiome was deposited in the Sequence Read Archive (SRA) database and can be accessed by the SRA accession number PRJNA552945.
Result
Pretreatment with L. helveticus R0052 alleviated liver injury in rat model
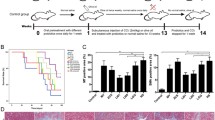
None of the rats died in this study after the induction of liver injury. As shown in the photomicrographs of H&E staining (Fig. 1a), the rat livers of the NC group exhibited normal lobular structure, whereas the portal tract in those of the PC group was eroded by inflammatory cells. Meanwhile, foci of lobular necroinflammation, degeneration and apoptosis were observed in the livers of the PC group. In the group pretreated with L. helveticus R0052, these histological injuries as well as the elevation of HAI score when d-GalN was administered were alleviated.
L. helveticus R0052 alleviated liver injury and inflammation. a Representative liver samples stained by H&E. Grading of the HAI score was based on these images. b Concentration of ALT and AST in serum samples. c Concentration of TBA, TB, DB and IB in serum samples. d The transcription of TLR2, TLR4, TLR5, TNF-α and NF-κb in liver samples was detected by RT-qPCR and is presented as relative expression. e Concentration of proinflammatory cytokines in plasma samples. Data are exhibited as the mean ± SEM. *P < 0.05, **P < 0.01 and ***P < 0.001 comparing to the PC group. ‘ns’ means the difference is not significant
Serum ALT is highly sensitive and specific to liver injury and the gold standard clinical chemistry marker of hepatotoxicity (Ozer et al. 2008). We found that the concentrations of serum ALT and AST were significantly elevated during d-GalN-induced liver injury (Fig. 1b). Bilirubin is the product of haemoglobin degradation and is a better indicator of disease severity than ALT in the acute phase of liver injury, while TBA is usually elevated with liver injury or functional change (Dufour et al. 2000). Pretreatment with L. helveticus R0052 reduced the increase in ALT, AST, TB and TBA induced by d-GalN, indicating a reduction in hepatocellular damage in the R0052 group during this process (Fig. 1c).
TLR2, TLR4 and TLR5 can recognize pathogen-associated molecular patterns (PAMPs) and activate the host innate immune reaction (Kaisho and Akira 2006). Nuclear factor-κb (NF-κb) and TNF-α are both important inflammation factors, and their transcription can be induced by TLR signalling (Lim and Staudt 2013). Pretreatment with L. helveticus R0052 downregulated the transcription of TLR2, TLR4, TLR5, NF-κb and TNF-α in the liver induced by d-GalN (Fig. 1d). In addition, the increase in several proinflammatory cytokines, including IL-2, IL-6, IL-12, IL-17, TNF-α, RANTES and MIP-3α, in plasma induced by d-GalN was attenuated by pretreatment with L. helveticus R0052 (Fig. 1e). These results indicated the anti-inflammatory effects of L. helveticus R0052 in the d-GalN-induced liver injury model.
Pretreatment with L. helveticus R0052 enhanced the intestinal barrier in rat model
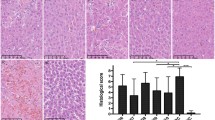
The terminal ileum exhibited histological abnormalities during d-GalN-induced liver injury. Subepithelial Gruen Hagen’s space, epithelial lifting and even denuded villi were observed in the PC group, while L. helveticus R0052 pretreatment ameliorated these histological abnormalities (Fig. 2c). During the evaluation of the ileum histology score, the R0052 group was graded with a lower histology score than the PC group (Fig. 2d). To further investigate the ultrastructure of intestinal villi, the ileum samples were observed by scanning electron microscopy. The normal intestinal villi in the NC group were small, finger-like projections, while the abnormal intestinal villi in the PC group were atrophied (Fig. 2a). In addition, the microvilli on the brush border of villi were disorganized in the PC group (Fig. 2b). These abnormalities of ultrastructures were also alleviated by L. helveticus R0052 pretreatment.
L. helveticus R0052 enhanced the intestinal barrier. a, b Representative images of intestinal villus ultrastructure under scanning electron microscopy (× 200, × 30,000, respectively). c Representative images of H&E staining of terminal ileum samples. d Ileum histological score was graded by H&E staining of terminal ileum samples. e Relative expression of TLR2, TLR4, TLR5, MUC3 and claudin2 in ileum samples. Data are exhibited as the mean ± SEM. *P < 0.05, **P < 0.01 and ***P < 0.001 comparing to the PC group. ‘ns’ means the difference is not significant
The intestinal epithelium, mucosal layer and tight junction together form the mechanical intestinal barrier, which is the forefront of defence against gut-derived pathogens. Compared with the rats of PC group, those pretreated with L. helveticus R0052 showed enhanced transcription of the mucosa-associated gene mucin3 (MUC3) (Fig. 2e). The transcription of claudin2, which forms paracellular cation pores in tight junction, was decreased after L. helveticus R0052 pretreatment (Amasheh et al. 2002). These results indicated that L. helveticus R0052 might enhance the intestinal barrier during liver injury. No significant difference in TLR relative expression was found between the NC group and the PC group in the ileum. However, it is interesting that the R0052 group had lower relative expression levels of TLR2, TLR4 and TLR5 than the PC group.
Pretreatment with L. helveticus R0052 reduced potential gut pathogens and restored normal gut flora in rat model
16S rRNA gene sequencing demonstrated the alteration of gut microbiome structure by L. helveticus R0052 during d-GalN-induced liver injury, which can be potential predictor for liver protective effect. The gut microbiome of all 24 rats was sequenced, and a total of 2242079 valid tags were clustered into 5818 qualified OTUs. As shown by the Chao1 index, the microbiome of the R0052 group exhibited lower richness than that of the PC group (Fig. 3a). As shown in the Shannon index, there was no significant difference in microbiome diversity between the R0052 group and the PC group (Fig. 3b). The PCoA results showed that the microbiome profile of the L. helveticus R0052 group was closer to that of the NC group than to that of the PC group (Fig. 3c).
L. helveticus R0052 reduced potential gut pathogens but increased normal gut flora. a Richness of the three groups was evaluated by the Chao1 index. b Alpha diversity was evaluated by the Shannon index. c Beta diversity was determined by the UniFrac unweighted distance method and PCoA analysis. d Sizes of circles indicate the relative abundance of the taxa, and the discriminative taxa were annotated in a cladogram (LDA score > 3.5). The taxa enriched in the PC group are green, and the taxa enriched in the NC group are red. e Taxa enriched in the PC group are green, while taxa enriched in the R0052 group are red. Data are exhibited as the mean ± SEM. *P < 0.05 comparing to the PC group
Discriminative taxa were identified by LEfSe analysis at multiple levels, and the most differential results (LDA score > 3.5) were retained to investigate the alteration of the microbiome structure. Compared with the NC group, the PC group exhibited depletion of the phylum Bacteroidetes as well as of the affiliated class Bacteroidia, order Bacteroidales, and families Bacteroidaceae and Prevotellaceae (Fig. 3d). At the genus level, Bacteroides, Prevotella, Alloprevotella and Barnesiella were depleted in the PC group compared with in the NC group. Meanwhile, enrichment of the phylum Firmicutes, class Clostridia, order Clostridiales, family Ruminococcaceae, and genera Ruminococcus, Flavonifractor and Acetatifactor contributed to gut microbiome dysbiosis in the PC group compared with that in the NC group.
Compared with the PC group, the R0052 group exhibited depletion of Flavonifractor and Acetatifactor, which indicated that L. helveticus R0052 could compete with some potential pathogens (Fig. 3e). In addition, the genera Lactobacillus and Bacteroides as well as the class Bacilli, order Lactobacillales, and family Lactobacillaceae were enriched in the R0052 group compared with those in the PC group, indicating the potential role of L. helveticus R0052 in promoting normal gut flora.
L. helveticus R0052 improved the faecal metabolomic profile in rat model
The faecal metabolome was analysed by the GC-MS method, and a total of 74 metabolites were identified from the faecal samples. To provide an overview of the metabolomic profile, unsupervised PCA analysis showed a trend in which the NC group, the PC group and the R0052 group clustered separately, which suggested differences in the metabolome profile among the experimental groups (Fig. 4a). The results of supervised OPLS-DA analysis revealed that the NC group and the R0052 group were significantly separated from the PC group (Fig. 4b). To identify the differential metabolites that contributed to the alterations of the metabolome, 26 important metabolites between the NC group and the PC group and 16 important metabolites between the R0052 group and the PC group were selected based on VIP values (VIP value > 1) because they were most relevant for explaining the OPLS-DA models (Fig. 4c). Subsequently, the p(corr) value, which reflects the reliability of variables, was taken into consideration, and metabolites with absolute p(corr) values greater than 0.3 were retained in a V plot (Zeng et al. 2014). Based on the results of the V plot, seven differential metabolites, namely lactose, galactose, maltose, talose, myo-inositol, oleic acid and lithocholic acid (LCA), were observed not only in the V plot between the NC and the PC groups but also in the V plot between the R0052 and the PC groups, indicating their important role in discriminating these groups (Fig. 4d).
L. helveticus R0052 modulated the faecal metabolome. a PCA score plots of three groups. b OPLS-DA score plots between the NC group vs. the PC group and the R0052 group vs. the PC group. c VIP values with jack-knifed confidence intervals. Only metabolites with a VIP > 1 are shown above. d Differential metabolites (VIP > 1, absolute p(corr) > 0.3) were selected by V plot. Differential metabolites between the NC group and the PC group are in red, while differential metabolites between the R0052 group and the PC group are in green. The triangles with annotated names indicate that metabolites were differentially expressed in both models. e Peak areas of metabolites were compared by the Mann-Whitney U test. Data are exhibited as the mean ± SEM. *P < 0.05 and **P < 0.01 comparing to the PC group. ‘ns’ means the difference is not significant
The results of the Mann-Whitney U test showed that compared with that of the NC group, the level of LCA, a toxic bile acid, was significantly increased in the PC group and that the levels of galactose, myo-inositol and maltose were decreased in the PC group (Fig. 4e). The levels of lactose, talose and oleic acid were not significantly different between the NC group and the PC group. However, L. helveticus R0052 pretreatment improved the faecal metabolomic profile by reducing the levels of LCA, lactose and talose and increasing the levels of galactose, myo-inositol, oleic acid and maltose comparing to those of the PC group.
The correlation between gut microbiome, faecal metabolome and liver injury in rat model
The correlations of the discriminative bacterial genera and differential metabolites from all three groups with liver function biochemical indicators, plasma proinflammatory cytokines and relative expression of inflammation-associated genes in the liver were determined using Spearman’s rank correlation analysis (Fig. 5). First, the relative abundances of Bacteroides, Prevotella, Alloprevotella and Barnesiella were negatively correlated with serum levels of ALT and TB and the relative expression of TNF-α in liver, as well as Bacteroides with plasma TNF-α and Prevotella with the relative expression of TLR4 in the liver. In contrast, the relative abundance of Ruminococcus was positively correlated with the circulating levels of ALT, AST, TB, TBA, IL-12, IL-17, RANTES, and MIP-3α and the relative expression of TLR5 in the liver. Second, the peak area of myo-inositol was positively correlated with the relative abundances of Bacteroides, Prevotella and Alloprevotella and negatively correlated with the circulating levels of ALT, TB, TBA, IL-2, IL-6, IL-12, RANTES, and MIP-3α and the relative expression of TLR2, TLR4, TLR5, NF-κb and TNF-α in the liver. In contrast, harmful bacterial metabolites, such as LCA, exhibited positive correlations with circulating levels of ALT, AST, TB, TBA, IL-2, IL-6, IL-12, IL-17, RANTES and MIP-3α, and the relative expression of TLR2, TLR4, TLR5 and TNF-α in the liver.
The correlations of the discriminative bacterial genera and differential metabolites from all three groups with liver function biochemical indicators, proinflammatory cytokines and relative expression of inflammation-associated genes were determined using Spearman’s rank correlation analysis. The hierarchical clustering was based on the Euclidean distance method. These results with P values < 0.05 are shown in the heatmap. The colour key and circle size indicate the strength of the correlation (r value). Red indicates positive correlations; blue indicates negative correlations
Discussion
The crosstalk between the gut and liver plays an important role in the onset and progression of liver diseases. Gut microbiome dysbiosis and intestinal barrier impairment will increase the translocation of microbes and their metabolites, which induce inflammation and liver damage (Tripathi et al. 2018). L. helveticus R0052 is considered a potential probiotic for treating liver disease (Bang et al. 2014; Hong et al. 2015). However, to the best of our knowledge, few studies have focused on its effect on acute liver injury. In our research, pretreatment with L. helveticus R0052 alleviated acute liver injury and systemic inflammation in d-GalN-treated rats. The beneficial effects of L. helveticus R0052 might be associated with the enhancement of the intestinal barrier as well as the modulation of gut microbiome and metabolome.
Inflammatory-mediated liver injury was the crucial mechanism for d-GalN-induced rat model. The pathophysiology of d-GalN-induced liver injury is first due to the binding of gut-derived PAMPs to protective immune receptors, such as TLRs on hepatic immune cells (Maes et al. 2016). TLR signalling culminates in NF-κB activation and provokes inflammatory cytokine production, particularly that of TNF-α (Abreu 2010). These inflammatory cytokines are responsible for neutrophil recruitment and cause liver injury (Zou et al. 2011). The results in our study were consistent with a previous study showing that L. helveticus R0052 could reduce the relative expression of TLRs and subsequently helped suppress the transcription of NF-κB and TNF-α in liver samples (Bang et al. 2014; Hong et al. 2015). In addition, the decreased plasma level of TNF-α and other cytokines indicated the overall anti-inflammatory effect of L. helveticus R0052 in rat model.
Impairment of the intestinal barrier aggravates liver injury due to the translocation of gut-derived pathogens and their products (Tilg et al. 2016). Increased permeability of tight junctions can be induced by overexpression of claudin-2, a member of the tight junction proteins forming paracellular cation-selective pores (Amasheh et al. 2002). The decrease in TNF-α production might contribute to the reduced claudin-2 relative expression after L. helveticus R0052 pretreatment in our study (Mankertz et al. 2009). MUC3 is a member of the transmembrane mucins that forms the enterocyte glycocalyx that is impermeable to commensal microorganisms (Johansson et al. 2008). In a previous study, Lactobacillus strains enhanced the transcription and production of MUC3, which led to reduced adherence ability of the enteropathogen Escherichia coli E2348/69 (Mack et al. 2003). Our research consistently found that the transcription of MUC3 was increased in the R0052 group compared with that in the PC group. Additionally, the abnormalities of intestinal epithelial cells were ameliorated after L. helveticus R0052 pretreatment. In addition, we found that the R0052 group had lower relative expression levels of TLR2, TLR4 and TLR5 than the PC group. However, intestinal TLR signalling not only triggers inflammatory responses but also facilitates intestinal barrier functions, such as epithelial cell proliferation, tight junction maintenance and immunoglobulin production (Abreu 2010; Lebeis et al. 2007). Whether the reduced transcription of TLRs contributed to the beneficial effects of L. helveticus R0052 on liver injury needs further investigation. Overall, L. helveticus R0052 might alleviate liver injury by enhancing the intestinal barrier in rat model.
Accumulating evidence has indicated that the gut microbiome is involved in the pathogenesis of liver diseases by influencing the host’s immunity and metabolism (Cani 2018; Tripathi et al. 2018). We found that Bacteroides, Prevotella, Alloprevotella and Barnesiella were depleted during d-GalN-induced liver injury, and the negative correlation between these normal gut flora and liver function biochemical indicators, especially ALT, suggested their beneficial role in liver health. Based on available research, Bacteroides is the most predominant anaerobe in the gut and can ferment undigested polysaccharides (Wexler 2007). The genera Alloprevotella and Prevotella both belong to the family Prevotellaceae, whose depletion contributes to nonalcoholic fatty liver disease and microbiome dysbiosis (Shen et al. 2017). In addition, genus Prevotella is considered beneficial for promoting hepatic glycogen storage and improving glucose metabolism (Kovatcheva-Datchary et al. 2015). Meanwhile, the overgrowth of Ruminococcus, Flavonifractor and Acetatifactor may lead to metabolic dysfunction and aggravation of liver injury in the PC group. The genus Ruminococcus is considered a gut microbiota signature of nonalcoholic fatty liver disease and was positively correlated with the levels of ALT, AST, TB and TBA in our study (Del Chierico et al. 2017). The genus Acetatifactor has only one species, named Acetatifactor muris, which has highly active 7α-dehydroxylases to convert bile acid into LCA, a hepatotoxic microbial metabolite (Pathak et al. 2018). L. helveticus R0052 pretreatment improved the gut microbiome structure by antagonistic interactions with potential pathogens such as Flavonifractor and Acetatifactor in our study (García-Bayona and Comstock 2018). Moreover, the colonization of L. helveticus R0052 enriched the normal gut flora, especially Lactobacillus, which can ferment nutrients into lactic acid and benefit health (Klaenhammer et al. 2005). The decrease in potential pathogens and restoration of the normal gut flora by L. helveticus R0052 might improve host metabolism and alleviate liver injury.
The intestinal microbiome is considered an additional metabolic organ for the host, and its metabolites can influence the host’s health by binding to specific host membranes or nuclear receptors (Backhed et al. 2005; Husted et al. 2017). In our study, pretreatment with L. helveticus R0052 improved carbohydrate metabolism by increasing faecal levels of galactose and maltose and decreasing faecal levels of lactose and talose. Lactose is an important nutrition from diet, and the elevated lactose level in our study may be due to the inefficiency of lactose digestion (Azcarate-Peril et al. 2017). Intestinal lactase is decreased after the weaning period; therefore, lactose-fermenting bacteria are vital for lactose absorption (Brüssow 2013). Notably, L. helveticus R0052 is capable of fermenting lactose into the easily absorbed lactic acid and providing additional nutrition for hosts (Naser et al. 2006). Galactose is released from lactose and plays an important role in hepatic metabolism because a major part of the ingested galactose is retained in the liver (Leturque et al. 2009). In ALF, galactose is able to protect hepatocytes against TNF-α-induced injury (Liu et al. 2015). Additionally, L. helveticus R0052 improved the metabolism of fatty acids, such as increasing oleic acid levels, compared with that of the PC group. Oleic acid is a monounsaturated fatty acid beneficial for reducing cholesterol levels and ameliorating inflammation (Sales-Campos et al. 2013). In addition, L. helveticus R0052 rescued the decrease in myo-inositol, which has promising lipotropic function and was negatively associated with ALT and TNF-α transcription in our study (Holub 1986). L. helveticus R0052 pretreatment also decreased the level of harmful bacterial metabolites, such as LCA. Potential pathogens such as Acetatifactor can transform non-toxic bile acid into LCA, which can cause liver damage (Hofmann 2004). It was reported that some Lactobacillus strains can degrade and transform bile acids and subsequently benefit host’s health by regulating the nuclear farnesoid X receptor and cholesterol metabolism (Bubnov et al. 2017, 2018). In our research, L. helveticus R0052 pretreatment decreased both the LCA level and Acetatifactor abundance, which might have helped alleviate liver injury. Future studies are needed to assess cholesterol lowering efficacy and hepatoprotective effect of L. helveticus R0052 in animal model and even human.
In conclusion, our study demonstrated that L. helveticus R0052 alleviated d-GalN-induced liver injury in rats by suppressing hepatic inflammation, enhancing the intestinal barrier, modulating the gut microbiome and regulating metabolome (Fig. 6). These results indicate that L. helveticus R0052 is promising for alleviating ALF and provide new insights regarding the correlations among the microbiome, the metabolome, the intestinal barrier and liver disease. L. helveticus R0052 is a potential strain for liver disease, and its effect on human patients needs to be proved in future clinical trial.
Mechanisms of L. helveticus R0052’s beneficial effects on acute liver injury. d-GalN can cause significant inflammation in liver. At the same time, the gut barrier is disrupted due to the close relationships between the liver and intestine. Subsequently, the intestinal barrier impairment, gut microbiome dysbiosis and metabolic dysfunction lead to immune response in liver and aggravate acute liver injury. L. helveticus R0052 might enhance the intestinal barrier by increasing the transcription of MUC3 and decreasing the transcription of claudin2. Secondly, L. helveticus R0052 modulates the gut microbiome by enriching Lactobacillus and Bacteroides and depleting Flavonifractor and Acetatifactor. Furthermore, L. helveticus R0052 regulates gut metabolome by increasing the levels of galactose, myo-inositol, oleic acid and maltose and reducing the levels of LCA, lactose and talose. Besides, L. helveticus R0052 alleviates the hepatic inflammation by downregulating the transcription of TLR2, TLR4, TLR5, TNF-α and NF-κb as well as decreasing the level of proinflammatory cytokines
References
Abreu MT (2010) Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat Rev Immunol 10:131–144. https://doi.org/10.1038/nri2707
Afgan E, Baker D, Batut B, van den Beek M, Bouvier D, Cech M, Chilton J, Clements D, Coraor N, Gruning BA, Guerler A, Hillman-Jackson J, Hiltemann S, Jalili V, Rasche H, Soranzo N, Goecks J, Taylor J, Nekrutenko A, Blankenberg D (2018) The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2018 update. Nucleic Acids Research 46(W1):W537–W544. https://doi.org/10.1093/nar/gky379
Amasheh S, Meiri N, Gitter AH, Schöneberg T, Mankertz J, Schulzke JD, Fromm M (2002) claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J Cell Sci 115:4969–4976. https://doi.org/10.1242/jcs.00165
Antoniades CG, Berry PA, Wendon JA, Vergani D (2008) The importance of immune dysfunction in determining outcome in acute liver failure. J Hepatol 49(5):845–861. https://doi.org/10.1016/j.jhep.2008.08.009
Azcarate-Peril MA, Ritter AJ, Savaiano D, Monteagudo-Mera A, Anderson C, Magness ST, Klaenhammer TR (2017) Impact of short-chain galactooligosaccharides on the gut microbiome of lactose-intolerant individuals. Proc Natl Acad Sci USA 114(3):E367–e375. https://doi.org/10.1073/pnas.1606722113
Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI (2005) Host-bacterial Mutualism in the human intestine. Science 307:1915–1920. https://doi.org/10.1126/science.1104816
Bang CS, Hong SH, Suk KT, Kim JB, Han SH, Sung H, Kim EJ, Kim MJ, Kim MY, Baik SK, Kim DJ (2014) Effects of Korean red ginseng (Panax ginseng), urushiol (Rhus vernicifera Stokes), and probiotics (Lactobacillus rhamnosus R0011 and Lactobacillus acidophilus R0052) on the gut–liver axis of alcoholic liver disease. J Ginseng Res 38:167–172. https://doi.org/10.1016/j.jgr.2014.04.002
Bernal W, Auzinger G, Dhawan A, Wendon J (2010) Acute liver failure. Lancet 376:190–201. https://doi.org/10.1016/s0140-6736(10)60274-7
Brüssow H (2013) Nutrition, population growth and disease: a short history of lactose. Environ Microbiol 15:2154–2161. https://doi.org/10.1111/1462-2920.12117
Bubnov RV, Babenko LP, Lazarenko LM, Mokrozub VV, Demchenko OA, Nechypurenko OV, Spivak MY (2017) Comparative study of probiotic effects of and strains on cholesterol levels, liver morphology and the gut microbiota in obese mice. EPMA j 8(4):357–376. https://doi.org/10.1007/s13167-017-0117-3
Bubnov RV, Babenko LP, Lazarenko LM, Mokrozub VV, Spivak MY (2018) Specific properties of probiotic strains: relevance and benefits for the host. EPMA j 9(2):205–223. https://doi.org/10.1007/s13167-018-0132-z
Bubnov RV, Spivak MY, Lazarenko LM, Bomba A, Boyko NV (2015) Probiotics and immunity: provisional role for personalized diets and disease prevention. EPMA j 6(1):14. https://doi.org/10.1186/s13167-015-0036-0
Cani PD (2018) Human gut microbiome: hopes, threats and promises. Gut 67:1716–1725. https://doi.org/10.1136/gutjnl-2018-316723
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R (2010) QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7(5):335–336. https://doi.org/10.1038/nmeth.f.303
Chiu C-J (1970) Intestinal mucosal lesion in low-flow states. Arch Surg 101:478–483. https://doi.org/10.1001/archsurg.1970.01340280030009
Cole JR, Wang Q, Fish JA, Chai B, McGarrell DM, Sun Y, Brown CT, Porras-Alfaro A, Kuske CR, Tiedje JM (2014) Ribosomal Database Project: data and tools for high throughput rRNA analysis. Nucleic Acids Res 42(Database issue):D633–D642. https://doi.org/10.1093/nar/gkt1244
Del Chierico F, Nobili V, Vernocchi P, Russo A, Stefanis CD, Gnani D, Furlanello C, Zandonà A, Paci P, Capuani G, Dallapiccola B, Miccheli A, Alisi A, Putignani L (2017) Gut microbiota profiling of pediatric nonalcoholic fatty liver disease and obese patients unveiled by an integrated meta-omics-based approach. Hepatology 65:451–464. https://doi.org/10.1002/hep.28572
Dufour DR, Lott JA, Nolte FS, Gretch DR, Koff RS, Seeff LB (2000) Diagnosis and monitoring of hepatic injury. II. Recommendations for use of laboratory tests in screening, diagnosis, and monitoring. Clin Chem 46:2050–2068
García-Bayona L, Comstock LE (2018) Bacterial antagonism in host-associated microbial communities. Science 361:eaat2456. https://doi.org/10.1126/science.aat2456
Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, Calder PC, Sanders ME (2014) The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 11:506–514. https://doi.org/10.1038/nrgastro.2014.66
Hofmann AF (2004) Detoxification of lithocholic acid, a toxic bile acid: relevance to drug hepatotoxicity. Drug Metabol Rev 36:703–722. https://doi.org/10.1081/dmr-200033475
Holub B (1986) Metabolism and function of myo-inositol and inositol phospholipids. Annu Rev Nutr 6:563–597. https://doi.org/10.1146/annurev.nutr.6.1.563
Hong M, Kim SW, Han SH, Kim DJ, Suk KT, Kim YS, Kim MJ, Kim MY, Baik SK, Ham YL (2015) Probiotics (Lactobacillus rhamnosus R0011 and Acidophilus R0052) reduce the expression of toll-like receptor 4 in mice with alcoholic liver disease. PLoS One 10:e0117451. https://doi.org/10.1371/journal.pone.0117451
Husted AS, Trauelsen M, Rudenko O, Hjorth SA, Schwartz TW (2017) GPCR-mediated signaling of metabolites. Cell Metabolism 25:777–796. https://doi.org/10.1016/j.cmet.2017.03.008
Johansson MEV, Phillipson M, Petersson J, Velcich A, Holm L, Hansson GC (2008) The inner of the two MUC2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci U S A 105:15064–15069. https://doi.org/10.1073/pnas.0803124105
Kaisho T, Akira S (2006) Toll-like receptor function and signaling. J Allergy Clin Immunol 117:979–987. https://doi.org/10.1016/j.jaci.2006.02.023
Klaenhammer T, Barrangou R, Buck B, Azcarateperil M, Altermann E (2005) Genomic features of lactic acid bacteria effecting bioprocessing and health. FEMS Microbiol Rev 29:393–409. https://doi.org/10.1016/j.femsre.2005.04.007
Knodell RG, Ishak KG, Black WC, Chen TS, Craig R, Kaplowitz N, Kiernan TW, Wollman J (1981) Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology 1:431–435. https://doi.org/10.1016/s0168-8278(03)00005-9
Kovatcheva-Datchary P, Nilsson A, Akrami R, Ying SL, De Vadder F, Arora T, Hallen A, Martens E, Björck I, Bäckhed F (2015) Dietary fiber-induced improvement in glucose metabolism is associated with increased abundance of Prevotella. Cell Metab 22:971–982. https://doi.org/10.1016/j.cmet.2015.10.001
Lebeis SL, Bommarius B, Parkos CA, Sherman MA, Kalman D (2007) TLR signaling mediated by MyD88 is required for a protective innate immune response by neutrophils to Citrobacter rodentium. J Immunol 179:566–577. https://doi.org/10.4049/jimmunol.179.1.566
Lee W (2012) Acute liver failure. Semin Respir Crit Care Med 33:36–45. https://doi.org/10.1055/s-0032-1301733
Leturque A, Brot-Laroche E, Le Gall M (2009) GLUT2 mutations, translocation, and receptor function in diet sugar managing. Am J Physiol Endocrinol Metabol 296:E985–E992. https://doi.org/10.1152/ajpendo.00004.2009
Li Y, Lv L, Ye J, Fang D, Shi D, Wu W, Wang Q, Wu J, Yang L, Bian X, Jiang X, Jiang H, Yan R, Peng C, Li L (2019) Bifidobacterium adolescentis CGMCC 15058 alleviates liver injury, enhances the intestinal barrier and modifies the gut microbiota in D-galactosamine-treated rats. Appl Microbiol Biotechnol 103:375–393. https://doi.org/10.1007/s00253-018-9454-y
Lim KH, Staudt LM (2013) Toll-like receptor signaling. Cold Spring Harb Perspect Biol 5:a011247. https://doi.org/10.1101/cshperspect.a011247
Liu Y, Zhu L, Liang S, Yao S, Li R, Liu S, Ma Y, Zhou X, Zhang J, Zeng H, Wang X (2015) Galactose protects hepatocytes against TNF-α-induced apoptosis by promoting activation of the NF-κB signaling pathway in acute liver failure. Lab Investig 95:504–514. https://doi.org/10.1038/labinvest.2015.34
Lv L-X, Hu X-J, Qian G-R, Zhang H, Lu H-F, Zheng B-W, Jiang L, Li L-J (2014) Administration of Lactobacillus salivarius LI01 or Pediococcus pentosaceus LI05 improves acute liver injury induced by D-galactosamine in rats. Appl Microbiol Biotechnol 98:5619–5632. https://doi.org/10.1007/s00253-014-5638-2
Mack DR, Ahrne S, Hyde L, Wei S, Hollingsworth MA (2003) Extracellular MUC3 mucin secretion follows adherence of Lactobacillus strains to intestinal epithelial cells in vitro. Gut 52:827–833. https://doi.org/10.1136/gut.52.6.827
Maes M, Vinken M, Jaeschke H (2016) Experimental models of hepatotoxicity related to acute liver failure. Toxicol Appl Pharmacol 290:86–97. https://doi.org/10.1016/j.taap.2015.11.016
Magoč T, Salzberg SL (2011) FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics 27(21):2957–2963. https://doi.org/10.1093/bioinformatics/btr507
Mankertz J, Amasheh M, Krug SM, Fromm A, Amasheh S, Hillenbrand B, Tavalali S, Fromm M, Schulzke JD (2009) TNFα up-regulates claudin-2 expression in epithelial HT-29/B6 cells via phosphatidylinositol-3-kinase signaling. Cell Tissue Res 336:67–77. https://doi.org/10.1007/s00441-009-0751-8
Manzano S, De Andrés J, Castro I, Rodríguez JM, Jiménez E, Espinosa-Martos I (2017) Safety and tolerance of three probiotic strains in healthy infants: a multi-centre randomized, double-blind, placebo-controlled trial. Benef Microbes 8:569–578. https://doi.org/10.3920/bm2017.0009
Naser SM, Hagen KE, Vancanneyt M, Cleenwerck I, Swings J, Tompkins TA (2006) Lactobacillus suntoryeus cachat and priest 2005 is a later synonym of Lactobacillus helveticus (Orla-Jensen 1919) Bergey et al. 1925 (approved lists 1980). Int J Syst Evol Microbiol 56:355–360. https://doi.org/10.1099/ijs.0.64001-0
Ozer J, Ratner M, Shaw M, Bailey W, Schomaker S (2008) The current state of serum biomarkers of hepatotoxicity. Toxicology 245:194–205. https://doi.org/10.1016/j.tox.2007.11.021
Pathak P, Xie C, Nichols RG, Ferrell JM, Boehme S, Krausz KW, Patterson AD, Gonzalez FJ, Chiang JYL (2018) Intestine farnesoid X receptor agonist and the gut microbiota activate G-protein bile acid receptor-1 signaling to improve metabolism. Hepatology 68:1574–1588. https://doi.org/10.1002/hep.29857
Reid G, Abrahamsson T, Bailey M, Bindels LB, Bubnov R, Ganguli K, Martoni C, O'Neill C, Savignac HM, Stanton C, Ship N, Surette M, Tuohy K, van Hemert S (2017) How do probiotics and prebiotics function at distant sites? Benef Microbes 8(4):521–533. https://doi.org/10.3920/bm2016.0222
Rognes T, Flouri T, Nichols B, Quince C, Mahé F (2016) VSEARCH: a versatile open source tool for metagenomics. PeerJ 4:e2584
Sales-Campos H, de Souza PR, Peghini BC, da Silva JS, Cardoso CR (2013) An overview of the modulatory effects of oleic acid in health and disease. Mini Rev Med Chem 13:201–210. https://doi.org/10.2174/138955713804805193
Shen F, Zheng R-D, Sun X-Q, Ding W-J, Wang X-Y, Fan J-G (2017) Gut microbiota dysbiosis in patients with non-alcoholic fatty liver disease. Hepatobiliary Pancreat Dis Int 16:375–381. https://doi.org/10.1016/s1499-3872(17)60019-5
Slattery L, O'Callaghan J, Fitzgerald GF, Beresford T, Ross RP (2010) Invited review: Lactobacillus helveticus--a thermophilic dairy starter related to gut bacteria. J Dairy Sci 93(10):4435–4454. https://doi.org/10.3168/jds.2010-3327
Tilg H, Cani PD, Mayer EA (2016) Gut microbiome and liver diseases. Gut 65:2035–2044. https://doi.org/10.1136/gutjnl-2016-312729
Tripathi A, Debelius J, Brenner DA, Karin M, Loomba R, Schnabl B, Knight R (2018) The gut–liver axis and the intersection with the microbiome. Nat Rev Gastroenterol Hepatol 15:397–411. https://doi.org/10.1038/s41575-018-0011-z
Wang Y, Liu Y, Sidhu A, Ma Z, McClain C, Feng W (2012) Lactobacillus rhamnosus GG culture supernatant ameliorates acute alcohol-induced intestinal permeability and liver injury. Am J Physiol Gastrointest Liver Physiol 303:G32–G41. https://doi.org/10.1152/ajpgi.00024.2012
Wexler HM (2007) Bacteroides: the good, the bad, and the nitty-gritty. Clin Microbiol Rev 20:593–621. https://doi.org/10.1128/cmr.00008-07
Ye J, Lv L, Wu W, Li Y, Shi D, Fang D, Guo F, Jiang H, Yan R, Ye W, Li L (2018) Butyrate protects mice against methionine–choline-deficient diet-induced non-alcoholic steatohepatitis by improving gut barrier function, attenuating inflammation and reducing endotoxin levels. Front Microbiol 9:1967. https://doi.org/10.3389/fmicb.2018.01967
Zhang Z, Lv J, Pan L, Zhang Y (2018) Roles and applications of probiotic Lactobacillus strains. Appl Microbiol Biotechnol 102(19):8135–8143. https://doi.org/10.1007/s00253-018-9217-9
Zeng J, Yin P, Tan Y, Dong L, Hu C, Huang Q, Lu X, Wang H, Xu G (2014) Metabolomics study of hepatocellular carcinoma: discovery and validation of serum potential biomarkers by using capillary electrophoresis–mass spectrometry. J Proteome Res 13:3420–3431. https://doi.org/10.1021/pr500390y
Zou W, Roth RA, Younis HS, Malle E, Ganey PE (2011) Neutrophil–cytokine interactions in a rat model of sulindac-induced idiosyncratic liver injury. Toxicology 290:278–285. https://doi.org/10.1016/j.tox.2011.10.005
Funding
This study was supported by the National Natural Science Foundation of China (81790631, 81570512), the National Key Research and Development Program of China (2018YFC2000500), and Natural Science Foundation of Zhejiang Province, China (LQ19H030007).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was approved by the Animal Care and Use Committee of the First Affiliated Hospital, School of Medicine, Zhejiang University (permit no. 2019-1068).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, Q., Lv, L., Jiang, H. et al. Lactobacillus helveticus R0052 alleviates liver injury by modulating gut microbiome and metabolome in d-galactosamine-treated rats. Appl Microbiol Biotechnol 103, 9673–9686 (2019). https://doi.org/10.1007/s00253-019-10211-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-019-10211-8