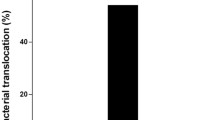Abstract
This work investigated the effect of the intragastric administration of five lactic acid bacteria from healthy people on acute liver failure in rats. Sprague–Dawley rats were given intragastric supplements of Lactobacillus salivarius LI01, Lactobacillus salivarius LI02, Lactobacillus paracasei LI03, Lactobacillus plantarum LI04, or Pediococcus pentosaceus LI05 for 8 days. Acute liver injury was induced on the eighth day by intraperitoneal injection of 1.1 g/kg body weight d-galactosamine (d-GalN). After 24 h, samples were collected to determine the level of liver enzymes, liver function, histology of the terminal ileum and liver, serum levels of inflammatory cytokines, bacterial translocation, and composition of the gut microbiome. The results indicated that pretreatment with L. salivarius LI01 or P. pentosaceus LI05 significantly reduced elevated alanine aminotransferase and aspartate aminotransferase levels, prevented the increase in total bilirubin, reduced the histological abnormalities of both the liver and the terminal ileum, decreased bacterial translocation, increased the serum level of interleukin 10 and/or interferon-γ, and resulted in a cecal microbiome that differed from that of the liver injury control. Pretreatment with L. plantarum LI04 or L. salivarius LI02 demonstrated no significant effects during this process, and pretreatment with L. paracasei LI03 aggravated liver injury. To the best of our knowledge, the effects of the three species—L. paracasei, L. salivarius, and P. pentosaceus—on d-GalN-induced liver injury have not been previously studied. The excellent characteristics of L. salivarius LI01 and P. pentosaceus LI05 enable them to serve as potential probiotics in the prevention or treatment of acute liver failure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The liver is the largest digestive gland and one of the five vital organs in humans; it has been described as the body’s chemical factory because it performs many critical functions, such as the secretion of bile and bile salts; the phagocytosis of bacteria and dead or foreign materials; the metabolism of carbohydrates, proteins, and fats; detoxification; and immune defense. Acute liver failure or fulminant hepatic failure is a drastic, unpredictable clinical syndrome in which the severe deterioration of liver function rapidly occurs and is typically followed by hepatic encephalopathy, coagulopathy and, in many cases, progressive multiorgan failure in patients with no reported underlying chronic liver disease (Bernal et al. 2010; Wlodzimirow et al. 2012). Acute liver failure is a life-threatening event with a high mortality ranging between 60 and 80 % (Wlodzimirow et al. 2012). The incidence of acute liver failure is between one and six cases per million people every year in the developed world but is higher in locations where infective hepatitis is prevalent (Bernal et al. 2010).
Many factors, such as viral hepatitis, excessive alcohol intake, idiosyncratic reactions to medication, and acute fatty liver during pregnancy, can cause acute liver failure (Bernal et al. 2010). Typically, liver transplantation is the most efficient salvage therapy, which has improved survival from less than 20 % to approximately 60 %; however, this option is strongly limited by the availability of donor livers (O’Grady 2012). Consequently, an artificial liver support system is useful to bridge the gap between the onset of acute liver failure and liver transplantation in addition to the regeneration of patient livers (Du et al. 2005). In addition, prevention or adjuvant treatment through the modulation of the gut microbiome composition has drawn increasing attention because several signs and symptoms such as infection, inflammation, sepsis, and hepatic encephalopathy demonstrate strong correlations between acute liver failure and the gut microbiome (Festi et al. 2006; Tranah et al. 2013). Patients are vulnerable to enteric infections by both gut microbes (particularly Gram-negative bacteria such as Klebsiella pneumonia, Escherichia coli, Pseudomonas aeruginosa, and other Enterobacteriaceae) and their products (Schnabl 2013) and thereby develop immune dysfunction. Pretreatment with Lactobacillus plantarum DSM 9843 (Adawi et al. 1997, 1998; Kasravi et al. 1997), Lactobacillus rhamnosus ATCC 53103 (Adawi et al. 2001; Osman et al. 2007), or Lactobacillus casei Zhang (Wang et al. 2013) was found to significantly improve d-galactosamine (d-GalN)-induced liver injury in rats. A recent review indicates that some probiotics appear to reduce the plasma ammonia concentration in comparison to placebo or no intervention, thus relieving hepatic encephalopathy in patients (McGee et al. 2011). However, because the gut hosts up to 100 trillion (1014) microbes from 500 to 1,000 different species, the effects of most gut microbes on acute liver failure are unclear.
In this study, the effects of five lactic acid bacteria from healthy people on liver enzymes, liver injury, and liver function were evaluated using a d-galactosamine-induced liver injury model in rats. Additionally, the effects of these bacteria on the serum levels of inflammatory cytokines, intestinal barriers, bacterial translocation, and the composition of the cecal microbiome were investigated to determine the possible mechanism of their effects on acute liver failure.
Materials and methods
Strain and culture conditions
Five strains were used in this study: Lactobacillus salivarius LI01 (CGMCC 7045), Lactobacillus salivarius LI02 (CGMCC 7046), Lactobacillus paracasei LI03 (CGMCC 7047), Lactobacillus plantarum LI04 (CGMCC 7048), and Pediococcus pentosaceus LI05 (CGMCC 7049). All strains were isolated in our laboratory from healthy volunteers, stored at −80 °C in Man–Rogosa–Sharpe (MRS, Oxoid, Thermo Fisher Biochemicals Ltd., Beijing, China) broth with 20 % glycerol, and deposited in the China General Microbiological Culture Collection Center (CGMCC). These strains exhibited tolerance property to bile, acid, and strong antimicrobial activity against tested enteropathogens (data not shown). After revival using standard methods, the bacterial strains were anaerobically cultured in MRS broth for 18 h at 37 °C. Cells were obtained by centrifugation at 8,000g for 10 min at 4 °C. Subsequently, these cells were washed twice with physiological saline at 4 °C and resuspended to a concentration of 3 × 109 colony-forming units (CFU)/ml in physiological saline for further use.
Animals and experimental design
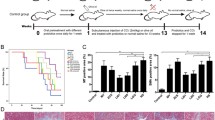
Forty-two male Sprague–Dawley rats (from the Experimental Animal Center of Zhejiang Province, Zhejiang, China), with a weight range of 200 to 300 g, were divided into seven groups of six rats: a normal group treated with physiological saline (saline + saline), a control group with acute liver injury (ALI) alone (GalN + saline), and five groups of liver injury treated with each of the following strains: L. salivarius LI01 (GalN + L. salivarius LI01), L. salivarius LI02 (GalN + L. salivarius LI02), L. paracasei LI03 (GalN + L. paracasei LI03), L. plantarum LI04 (GalN + L. plantarum LI04), and P. pentosaceus LI05 (GalN + P. pentosaceus LI05). The animals were fed normal food and were maintained at room temperature (22 °C) with a controlled 12-h:12-h light–dark cycle. The animals were treated with oral administration through an orogastric tube (once daily for 8 days) of 1 ml of normal saline in the liver injury control or 1 ml (3 × 109 CFU/ml) of bacterial cells that were freshly prepared as previously described. Acute liver injury was induced on the eighth day by an intraperitoneal injection of 1.1 g/kg body weight d-galactosamine (G0500, Sigma, Saint Louis, MO, USA). Twenty-four hours after the induction of liver injury, the rats were anesthetized by an intraperitoneal injection of 400 mg/kg body weight chloral hydrate (Sinopharm Chemical Reagent Co., Ltd., Shanghai, China) to collect the samples for analysis.
Liver enzymes and bilirubin
The serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), albumin, globulin, and bilirubin were assessed by standard methods using a 7600-210 automatic analyzer (Hitachi 7600-210; Hitachi, Tokyo, Japan).
Serum cytokines
The serum concentrations of tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), interleukin 6 (IL-6), interleukin 10 (IL-10), and interferon-γ (IFN-γ) were measured using commercially available enzyme-linked immunosorbent assay kits following the manufacturer’s recommendations (eBioscience Inc., San Diego, CA, USA).
Bacterial translocation
Samples from the caudate lobe of the liver and mesenteric lymph nodes (MLN) were collected, weighed, and milled under aseptic conditions in glass homogenizers that had been autoclaved at 121 °C for 15 min. After appropriate dilution with physiological saline, these milled samples and samples from arterial blood or portal blood were separately incubated under aerobic and anaerobic conditions on brain heart infusion agar (BHI, Oxoid, Thermo Fisher Biochemicals Ltd., Beijing, China) at 37 °C for 48 to 72 h. The number of colonies from each plate was counted. The bacterial translocation of tissue samples was expressed per gram of tissue, whereas that of blood samples was expressed per milliliter of blood.
DNA extraction, PCR amplification, and denaturing gradient gel electrophoresis (DGGE) analysis of the intestinal microbiome
DNA extraction
Cecal contents were collected from sacrificed rats. DNA was extracted from the cecal contents using a Qiagen Stool Kit (Qiagen, Hilden, Germany). The quality of the DNA was determined using agarose gel electrophoresis analysis and UV light visualization with ethidium bromide staining.
PCR amplification for DGGE
The V3 variable region of 16S ribosomal DNA (rDNA) was amplified using a hot start touchdown approach. The nucleotide sequences of the primers were as follows: primer 1, 5′-CCTACGGGAGGCAGCAG-3′; primer 2, 5′-ATTACCGCGGCTGCTGG-3′; and primer 3, 5′-CGCCCGCCGCGCGCGGCGGGCGGGGCGGGGGCACGGGGGGCCTACGGGAGGCAGCAG-3′ (Muyzer et al. 1993). A combination of primers 1 and 2 or primers 3 and 2 was used to amplify the V3 variable region of 16S rDNA. PCR was performed in a final volume of 50 μl containing 2 μl of template DNA, 25 pmol of each primer, 200 μM of each dNTP mixture, 5 μl of 10 × Ex Taq buffer, and 0.5 μl of TaKaRa Ex Taq polymerase (TaKaRa, Dalian, China). Initial denaturation was performed at 95 °C for 3 min, and amplification was performed using 24 cycles of denaturation at 94 °C for 30 s; annealing at 64 °C for 30 s, which was decreased by 1 °C every 3 cycles; and extension at 72 °C for 30 s. Subsequently, ten additional cycles of denaturation at 94 °C for 20 s, annealing at 56 °C for 30 s and extension at 72 °C for 20 s were performed, with a final extension at 72 °C for 5 min. Concentrations were measured using a NanoDrop ND-1000 spectrophotometer (Thermo Electron Corporation). All amplified products were stored at −20 °C before DGGE analysis.
Denaturing gradient gel electrophoresis (DGGE)
DGGE analysis was performed using the DCode universal mutation detection system (Bio-Rad, Hercules, CA, USA) with 16 cm × 18 cm × 1.5 mm gels. For each sample, 20 μl of PCR fragment was loaded onto the gels. An identical standard reference (a randomly selected sample) was applied in the middle and at both ends of each gel for digital gel normalization and for comparison between gels. Sequence-specific separation of the PCR fragments was obtained in 8 % (wt/vol) polyacrylamide gels in 1 × TAE buffer. Electrophoresis was performed for 16 h at 60 °C and 70 V in a linear 35 to 75 % denaturant gradient (100 % denaturant is defined as 7 M urea and 40 % deionized formamide). After electrophoresis, the gels were stained using SYBR green I (Sigma-Aldrich, Castle Hill, Australia) and photographed.
Comparative analyses of the DGGE profiles
DGGE profiles were digitally processed using BioNumerics software version 6.01 (Applied Maths, St-Martens-Latem, Belgium) in a multistep procedure following the manufacturer’s instructions. All profiles were compared using the band-matching tool, and uncertain bands were included in the position tolerance settings. Parameters for allocating band classes were chosen according to Joossens et al. (2011). A cluster analysis of DGGE pattern profiles used the unweighted pair group method with the arithmetic means (UPGMA) method based on the Dice similarity coefficient (band based) (Vanhoutte et al. 2004). Multidimensional scaling (MDS) and principal components analysis (PCA) were performed according to the BioNumerics software manuals. MDS is an optimized three-dimensional representation of the similarity matrix, and the Euclidean distance between two points (lanes in the gel profiles) reflects the similarity between them, providing a convenient visual interpretation. PCA is another method to visualize the relationships among lanes using the lane data (band classes) set itself, which generates new variables called principal components (linear components of the original variables) that explain the highest dispersion of the samples (Ma and Dai 2011).
Sequencing of DGGE bands
Predominant bands were excised from the DGGE gels, washed three times in TE buffer (10 mM Tris–HCl and 1 mM EDTA, pH 8.0), disrupted, and incubated in 50 μl of TE buffer for 30 min at 80 °C (Lu et al. 2013). Subsequently, 5 μl of the supernatant from each sample was used as a template for PCR reamplification using the primers described above. Cloned PCR products were subsequently verified and sequenced (Invitrogen, Shanghai, China). Homology searches were performed using BLAST. Reference sequences of phylogenetic neighbor species (up to 98 % similarity) were introduced for clustering analysis using multiple sequence alignment with the K-Lite Mega Codec Pack 5.05 (Tamura et al. 2011).
Histopathology
Samples from the left lobe of the liver and from the terminal ileum were immediately fixed in 10 % neutral buffered formalin at the time of death. Paraffin-embedded samples were cut into 4-μm-thick sections. Subsequently, the sections were stained using hematoxylin and eosin and analyzed by a pathologist who was blind to the groups. At least three slides and 20 random microscopic fields were studied from each specimen. To grade the degree of liver injury, the amount of tissues damaged by necrosis or inflammation was semiquantitatively assessed on a scale of 0 (absent), 1 (mild), 2 (moderate), and 3 (extensive) (Deutschman et al. 2006). To grade the degree of terminal ileum injury, the amount of goblet cell depletion or decreased mucus accumulation (score, 0–3), mucosa thickening (score, 0–3), destruction, or loss of crypts (score, 0–3) was also semiquantitatively assessed (Johansson et al. 2013).
Statistics
The obtained data were analyzed using SPSS 16.0 software. The data were first tested for homogeneity of variances using one-way ANOVA. If the variances were homogeneous (p > 0.1), the data were evaluated using one-way ANOVA followed by the Student–Newman–Keuls method. The values are presented as the means ± SEM. Otherwise, the data were analyzed using Kruskal–Wallis one-way ANOVA on ranks followed by the Student–Newman–Keuls method. Probability levels of <0.05 were considered significant. The values are presented as the median (25th and 75th percentiles). Probability levels of <0.05 were considered significant (Osman et al. 2007).
Results
L. salivarius LI01 or P. pentosaceus LI05 reduces elevated levels of both aspartate transaminase and alanine transaminase during d-GalN-induced acute liver injury
Twenty-four hours after the induction of liver injury, no mortality was observed in the different experimental groups. Serum levels of AST, ALT, and ALP were profoundly increased in the liver injury control group (Table 1). Pretreatment with L. salivarius LI01, L. salivarius LI02, L. plantarum LI04, or P. pentosaceus LI05 significantly lowered the increase of serum AST induced by d-GalN, but pretreatment with L. paracasei LI03 aggravated the increase of serum AST under identical conditions. Pretreatment with L. salivarius LI01 or P. pentosaceus LI05 relieved the increase of serum ALT, but pretreatment with L. plantarum LI04, L. salivarius LI02, or L. paracasei LI03 did not demonstrate significant effects. These results indicate that pretreatment with either L. salivarius LI01 or P. pentosaceus LI05 reduces the inflammation or damage to the liver induced by d-GalN because higher than normal amounts of cellular substances, including liver enzymes, typically leaked from inflamed or injured liver cells into the bloodstream. None of the five evaluated bacterial strains prevented the increase of serum ALP in d-GalN-induced acute liver injury, which likely indicates that the five bacterial strains do not aid in the protection against the destruction of mucosal cells that line the bile system of the liver and serve as the source of ALP.
L. salivarius LI01 or P. pentosaceus LI05 prevents the increase of bilirubin during d-GalN-induced acute liver injury
As shown in Table 1, the total bilirubin level of the groups supplemented with either L. salivarius LI01 or P. pentosaceus LI05 was not significantly different from that of the normal control group and, therefore, was significantly lower than that of the liver injury control group. However, pretreatment with L. paracasei LI03, L. plantarum LI04, or L. salivarius LI02 did not significantly prevent this increase in total bilirubin, which indicates that only L. salivarius LI01 or P. pentosaceus LI05 protects the ability of the liver to take up, process, and secrete bilirubin into the bile from destruction by d-GalN. Compared with the normal control, the serum albumin level decreased in all of the other groups except that supplemented with L. plantarum LI04. Changes in the level of albumin were minor in all groups (date not shown), which relates to the relatively long half-life of albumin that can maintain normal concentrations even in the early stages of severe acute liver disease. None of the five microbes prevented the decrease in serum globulin that is induced by d-GalN.
L. salivarius LI01 or P. pentosaceus LI05 reduces d-GalN-induced histological abnormalities of both the liver and the terminal ileum
Twenty-four hours after d-GalN administration, all groups, except the normal group, demonstrated histological abnormalities of the liver and terminal ileum. Damaged liver tissues exhibited a loss of hepatocytes, microabscesses, and extensive neutrophil and macrophage accumulation in the parenchyma (Fig. 1b–g). Damaged terminal ileum exhibited the rupture and absence of villi, disintegration of crypts, and perforation of the mucosa (Fig. 2b–g). All strains, except L. paracasei LI03, significantly reduced the histological abnormalities of d-GalN-induced liver injury (Fig. 1, Table 2). However, only pretreatment with L. salivarius LI01 or P. pentosaceus LI05 reduced the disintegration of the mucosa, villi, and crypts (Fig. 2, Table 2). This result, together with the improved liver function after L. salivarius LI01 or P. pentosaceus LI05 administration in d-GalN-induced liver injury, indicates that a reduction of the destruction of the intestinal barrier plays a critical role in relieving liver injury.
Effects of pretreatment with different lactic acid bacteria on liver histological alterations during d-GalN-induced acute liver injury. a Saline + saline; b GalN + saline; c GalN + L. paracasei LI03; d GalN + L. plantarum LI04; e GalN + L. salivarius LI01; f GalN + P. pentosaceus LI05; g GalN + L. salivarius LI02. Circles, cells with a clear nucleus; stars, lipid vacuoles; black arrows, microabscesses; white arrows, neutrophil and macrophage accumulation
Effects of pretreatment with different lactic acid bacteria on histological alterations in the distal ileum during d-GalN-induced acute liver injury. a Saline + saline; b GalN + saline; c GalN + L. paracasei LI03; d GalN + L. plantarum LI04; e GalN + L. salivarius LI01; f GalN + P. pentosaceus LI05; g GalN + L. salivarius LI02. Stars, mucosa; triangles, crypts; black arrows, villi
L. salivarius LI01 or P. pentosaceus LI05 increases the serum IL-10 or IFN-γ level during d-GalN-induced acute liver injury
As shown in Fig. 3a, the serum level of TNF-α in the group supplemented with L. salivarius LI02 was higher than that in the liver injury control group. No significant difference in serum TNF-α (Fig. 3a), IL-1β (Fig. 3b), or IL-6 (data not shown) was detected among all the other groups supplemented with microbes and the liver injury control group. This finding results from the typical burst of the proinflammatory cytokines TNF-α, IL-1β, and IL-6 in the early stage of d-GalN treatment. Pretreatment with L. salivarius LI01 or P. pentosaceus LI05 significantly increases the serum level of IL-10 in rats with liver injury (Fig. 3c), which partially explains the minor liver injury observed in the groups supplemented with these two microbes. Importantly, pretreatment with L. plantarum LI04 or P. pentosaceus LI05 greatly elevated the serum level of IFN-γ in d-GalN-induced liver injury (Fig. 3d), which is beneficial for innate and adaptive immunity against viral and intracellular bacterial infections.
L. salivarius LI01, P. pentosaceus LI05, or L. paracasei LI03 decreases the incidence of bacterial translocation during d-GalN-induced acute liver injury
As shown in Table 3, only pretreatment with L. salivarius LI01 decreased the incidence of bacterial translocation (BT) in all evaluated tissues. Pretreatment with P. pentosaceus LI05 decreased the BT incidence in the portal blood, liver, and MLN. Pretreatment with L. paracasei LI03 decreased the BT incidence in the arterial blood, portal blood, and liver. Pretreatment with L. plantarum LI04 decreased the BT incidence in arterial blood and portal blood but increased the BT incidence in the MLN. Pretreatment with L. salivarius LI02 decreased the BT incidence in the MLN arterial blood and portal blood but increased the BT incidence in the liver. Consequently, L. salivarius LI01, L. paracasei LI03, and P. pentosaceus LI05 efficiently prevented bacterial translocation.
Supplementation with L. salivarius LI01 or P. pentosaceus LI05 results in a different cecal microbiome compared with that in liver injury
Cecal contents were collected from sacrificed rats and were examined using PCR-denaturing gradient gel electrophoresis (DGGE) of the V3 variable regions of total community 16S rDNA (Fig. 4). Multidimensional scaling (MDS) and principal component analysis (PCA) of the DGGE fingerprinting data were conducted using BioNumerics software version 6.01 to demonstrate the uniqueness and stability of the predominant cecal microbiome composition. Both cluster analysis of samples and the MDS plot (an ordination method that reduces the complex DGGE patterns to one point per sample) indicate that the cecal microbiome composition in the group supplemented with either L. salivarius LI01 (Figs. 4 and 5d) or P. pentosaceus LI05 (Figs. 4 and 5e) was clearly distinct from that in the liver injury group. Similar results for these two groups were observed using a PCA plot, which is a method based on a linear response model. However, for the groups supplemented with L. paracasei LI03 (Fig. 5b), L. plantarum LI04 (Fig. 5c), or L. salivarius LI02 (Fig. 5f), the cecal microbiome compositions could not be clearly distinguished from that in the liver injury control using either MDS or PCA.
Effects of pretreatment with different lactic acid bacteria on the composition profiles of the cecal microbiome during d-GalN-induced acute liver injury. MDS multidimensional scaling analysis, PCA principal component analysis. Composition profiles of the cecal microbiome in the liver injury control group were compared with those in the groups supplemented with saline + saline (a), GalN + L. paracasei LI03 (b), GalN + L. plantarum LI04 (c), GalN + L. salivarius LI01 (d), GalN + P. pentosaceus LI05 (e), or GalN + L. salivarius LI02 (f)
A total of 38 predominant bands were sequenced after excision from the DGGE gels and reamplification (Fig. 4). The closest matches (and percentages of similarity) for the sequences retrieved from each band were determined using BLAST searches. The intensity of each band in every group was compared with that in the liver injury control group. As shown in Fig. 6, an uncultured Bacteroidetes (band class 66, GenBank accession no. GU959627.1) increased in all groups except the group supplemented with L. plantarum LI04, but Clostridium sp. (band class 8.6, GenBank accession no. AB809064.1) only increased in the group supplemented with L. plantarum LI04. Clostridiales bacterium (band class 51.4, GenBank accession no. AB702927.1) and an uncultured rumen bacterium (band class 25, GenBank accession no. JX218354.1) increased in the group supplemented with L. salivarius LI01 or P. pentosaceus LI05. Lactobacillus reuteri (band class 62.2, GenBank accession no. KF267448.1), an uncultured Bacteroidetes (band class 62.2, GenBank accession no. GU959253.1), and Bacteroides chinchilla (band class 6.4, GenBank accession no. AB547637.1) increased in the group supplemented with L. salivarius LI02. An uncultured bacterium (band class 81.9, GenBank accession no. JF245950.1) and another uncultured bacterium (band class 58.2, GenBank accession no. JF794942.1) increased in the group supplemented with L. salivarius LI01 or L. salivarius LI02. Corynebacterium casei (band class 62.7, GenBank accession no. JX966460.1) and an uncultured bacterium (band class 62.7, GenBank accession no. FJ835427.1) increased in the group supplemented with P. pentosaceus LI05 or L. salivarius LI02. An uncultured bacterium (band class 39.4, GenBank accession no. HQ320396.1) increased, but another uncultured bacterium (band class 46.4, GenBank accession no. JF794942.1) decreased in the normal control. The other 27 bands were not significantly different between the liver injury control group and each of the other groups. These results offer potential directions for further studies on the impact of specific microbes on liver injury.
Effects of pretreatment with different lactic acid bacteria on the predominant bacteria of the cecal microbiome during d-GalN-induced acute liver injury. The phylogenetic tree was based on the sequences of the V3 variable regions of 16S rDNA of selected bands. *p < 0.05 compared with the liver injury control; **p < 0.01 compared with the liver injury control (GalN + saline)
Discussion
Acute liver failure is often accompanied by certain complications, such as sepsis, bacterial peritonitis, and hepatic encephalopathy, that are related to the gut microbiome (Tranah et al. 2013). However, the molecular mechanism for the interaction between acute liver failure and the gut microbiome, particularly the roles of most gut microbes during this process, is not fully understood. Furthermore, although modulations of the gut microbiome with probiotics or prebiotics such as lactulose have been applied in treatment, no probiotics have been developed for the clinical prevention and treatment of liver failure (Kirpich and McClain 2012). Lactic acid bacteria are excellent sources of probiotics (Capozzi et al. 2012; Hynonen and Palva 2013; Tsai et al. 2012). In this study, we examined the effects of five lactic acid bacteria from healthy people on acute liver failure using a d-galactosamine-induced rat model. All strains exhibited tolerance property to bile, acid, and strong antimicrobial activity against tested enteropathogens (data not shown). To the best of our knowledge, three important species—L. salivarius, P. pentosaceus, and L. paracasei—have not been previously investigated in this field. L. salivarius has been frequently isolated from the mammalian digestive tract and has been studied as a candidate probiotic (Messaoudi et al. 2013). In addition to mammalian sources, L. paracasei is naturally found in fermented products and natural dairy products such as raw milk, and P. pentosaceus is used as a starter culture to ferment foods such as various meats, vegetables, and cheeses (Bengmark 2009). We found that L. salivarius LI01 and P. pentosaceus LI05 were beneficial in the prevention of acute liver failure. L. plantarum LI04 and L. salivarius LI02 did not demonstrate significant beneficial effects. L. paracasei LI03 aggravated liver injury. Two strains from the same species, L. salivarius LI01 and L. salivarius LI02, demonstrated differential effects. Our L. plantarum LI04 did not prevent acute liver injury, as was reported for L. plantarum 9843 (Adawi et al. 1998; Kasravi et al. 1997; Osman et al. 2007). These conflicting findings may be explained by confounding variables but are likely caused by different sources of lactic acid bacteria and differences in their characterization.
The levels of AST and ALT typically indicate hepatocyte damage or liver inflammation. Supplementation with the two lactic acid bacteria—L. salivarius LI01 or P. pentosaceus LI05—significantly reduced elevated levels of both AST and ALT during d-GalN-induced acute liver injury. It was reported that pretreatment with L. plantarum DSM 9843 (Adawi et al. 1997, 1998; Kasravi et al. 1997), L. rhamnosus ATCC 53103 (Adawi et al. 2001; Osman et al. 2007), or L. casei Zhang (Wang et al. 2013) decreased both AST and ALT levels. Pretreatment with Bifidobacterium infantis DSM 15159 (=CURE21) (Osman et al. 2007) or L. casei CRL 431 (Haro et al. 2009) decreased the levels of ALT but not those of AST. Pretreatment with Lactobacillus acidophilus NM1 decreased ALT levels (Adawi et al. 2001), whereas the effect of this strain on AST has not been reported. However, pretreatment with L. reuteri R2LC (Adawi et al. 1997; Kasravi et al. 1997), L. rhamnosus DSM 6594 (Adawi et al. 1997), L. fermentum 8704:3 (Adawi et al. 1997), L. reuteri 108 (Adawi et al. 1997), Bifidobacterium animalis NM2 (Adawi et al. 2001), or L. plantarum DSM 15313 (Osman et al. 2007) did not demonstrate significant effects.
Bilirubin, ALP, albumin, and globulin are important factors that reflect liver function. None of the five evaluated microbes significantly decreased elevated ALP levels in d-GalN-induced liver injury, which is consistent with other studies under identical conditions (Adawi et al. 2001; Kasravi et al. 1997; Wang et al. 2013). This finding likely indicates that pretreatment with probiotics does not aid in the protection against the d-GalN-induced destruction of mucosal cells of the liver and biliary system. The administration of d-GalN decreased the serum level of albumin and globulin, thereby decreasing the total serum protein levels. Only pretreatment with L. plantarum LI04 slightly prevented the decrease in albumin and total protein. No significant modifications in the serum concentrations of total proteins were observed after the administration of 800 mg/kg body weight d-GalN (Haro et al. 2009). However, most studies have not reported the effects on serum proteins. Different results on the serum total protein level are most likely caused by different doses of d-GalN. Both L. salivarius LI01 and P. pentosaceus LI05 significantly reduced elevated total bilirubin in liver injury, which indicates the ability of these two probiotics to improve liver function. In other reports, L. plantarum DSM 9843 (Adawi et al. 1997, 1998), L. plantarum DSM 15313 (Osman et al. 2007), and B. infantis DSM 15159 (Osman et al. 2007) also significantly decreased total bilirubin levels.
Cytokines are critical pathogenic factors involved in liver injury and can be broadly divided into proinflammatory cytokines, such as TNF-α, IL-1β, and IL-6, and anti-inflammatory cytokines, such as IL-4, IL-10, and IL-13. Compared with the liver injury control, the serum level of TNF-α was higher in the group supplemented with L. salivarius LI02, but serum TNF-α levels in other groups and serum levels of IL-1β or IL-6 in all groups were not significantly different. Although the evaluated strains did not decrease the levels of proinflammatory cytokines, this result likely reflects the typical increase in TNF-α, IL-1β, or IL-6 in the early stage of d-GalN-induced liver injury, which then quickly decreases. Therefore, a combination of d-galactosamine and endotoxin has also been widely used to induce liver failure in rats, which triggers high levels of proinflammatory cytokines (Tunon et al. 2009). The increase of these cytokines varied greatly with the dose of d-GalN, the type of animals, and other drugs such as endotoxin that were used together with d-GalN. Because some probiotics function by reducing the endotoxin of Gram-negative bacteria in the gut, we did not use endotoxin in this study. In a rat model induced by 1,100 mg/kg body weight d-GalN together with 10 μl of endotoxin, B. infantis DSM 15159 (=CURE21) (Osman et al. 2007) or Lactobacillus plantarum DSM 15313 (Osman et al. 2007) decreased TNF-α and IL-1β levels in liver tissue. When 800 mg/kg body weight d-galactosamine (Haro et al. 2009) or 50 μg/kg body weight endotoxin together with 300 mg/kg body weight d-GalN (Wang et al. 2013) was used, L. casei Zhang decreased serum TNF-α levels. In the present study, we also found that the level of the anti-inflammatory cytokine IL-10 increased in the group supplemented with L. salivarius LI01 or P. pentosaceus LI05, which partially explains the reduction of liver injury caused by these two strains. Pretreatment with L. plantarum LI04 or P. pentosaceus LI05 significantly increased IFN-γ, which indicates the activation of circulating T cells against infection. Other studies using d-GalN-induced rat models supplemented with probiotics have not reported on the effects on IL-10 and IFN-γ.
Bacterial translocation has been postulated as the major mechanism in the pathogenesis of acute liver failure. We found that L. salivarius LI01, L. paracasei LI03, and P. pentosaceus LI05 efficiently prevented bacterial translocation in liver injury. Pretreatment with L. plantarum DSM 9843 (Adawi et al. 1997, 1998; Kasravi et al. 1997), L. plantarum DSM 15313 (Osman et al. 2007), or L. reuteri 108 (Adawi et al. 1997; Kasravi et al. 1997) decreased BT in the liver and MLN. Pretreatment with L. acidophilus NM1 or L. rhamnosus ATCC 53103 decreased BT in portal blood, liver, and MLN (Adawi et al. 2001). Intestinal bacterial overgrowth (IBO), increased intestinal permeability, and impaired immunity are important factors in the development of BT (Schnabl 2013). Therefore, the mechanism of BT prevention by probiotics is very complex. In this study, pretreatment with L. salivarius LI01 protected the intestinal mucosa, crypts, and villi; pretreatment with P. pentosaceus LI05 protected the intestinal mucosa and crypts; and pretreatment with L. paracasei LI03 protected the intestinal mucosa. These results partially explain the ability of these three strains to decrease the incidence of BT. Moreover, MDS analysis indicated that the composition of the cecal microbiome in the groups supplemented with each of these three strains differed from that in the liver injury control, indicating that the microbiome plays an important role in preventing BT.
Because most probiotics were applied through the gastrointestinal tract, their effects on liver disease were intensively related to their modulation of gut flora. We studied the effects of the five microbes on gut flora using PCR-DGGE. In the groups supplemented with L. salivarius LI01 or P. pentosaceus LI05, the microbiome compositions were clearly distinct from that of the liver injury control using both MDS and PCA analysis. This result is consistent with the ability of the two strains to decrease BT, reduce liver injury, and improve liver function. In the group supplemented with L. paracasei LI03, the composition of the microbiome in the MDS analysis was distinct from that in the liver injury control, but the principal components of the microbiome in PCA analysis were not significantly different. Although BT decreased in this group, liver injury was more serious, indicating that the alterations of the gut microbiome caused by L. paracasei LI03 were most likely only beneficial in the prevention of BT. In the group supplemented with L. plantarum LI04 or L. salivarius LI02, the principal components of the microbiome in PCA analysis were different from those of the liver injury control, but the composition of the microbiome in MDS analysis was not clearly distinguished from the control. No improvements in liver injury or decreases in BT were observed in the two groups. This result indicates that alterations in the profile of the microbiome likely contribute more than changes in only its principal components. In other studies, a culture-dependent method to determine bacterial counts was used to analyze alterations in the microbiome. No significant differences were found in the counts of total aerobic, total anaerobic, Gram-negative anaerobic, Enterobacteriaceae, or Lactobacillus between groups without or rectally supplemented with 3 × 109 colony-forming units of L. plantarum 9843 once daily for 8 days in d-GalN-induced liver injury (Adawi et al. 1997). Oral pretreatment with 2.5–5.0 × 109 colony-forming units of L. plantarum 9843 for 7 days decreased Enterobacteriaceae counts and increased Lactobacillus counts in the cecum, but pretreatment with L. reuteri R2LC did not significantly influence any species in an identical liver injury model (Kasravi et al. 1997). When 3 × 109 colony-forming units of probiotics were daily rectally administered for 8 days and compared with the liver injury control, pretreatment with B. animalis NM2 decreased Enterobacteriaceae counts in both the cecum and colon, pretreatment with L. acidophilus NM1 or L. rhamnosus ATCC 53103 decreased Enterobacteriaceae counts in the colon, and pretreatment with L. rhamnosus ATCC 53103 increased Lactobacillus counts in the colon (Adawi et al. 2001). Pretreatment with 3 ml of probiotics at a concentration of approximately 108 cells/ml through an orogastric tube twice daily for 8 days decreased aerobic counts in the group with L. plantarum DSM 15313 or B. infantis DSM 15159 in comparison to the liver injury control (Osman et al. 2007). Although the determination of the intestinal microbiome by bacterial counts is cheap, rapid, and simple, this method is limited by the fact that most gut bacteria are uncultivable. A metagenomic approach will provide more accurate information, but it is expensive and not generally available. PCR-DGGE is less expensive and can briefly reflect the change of microbiota. In this study, we did not observe the increase of any of the five strains in cecal microbiome. This reflects resolution and sensitivity limitations of PCR-DGGE, but is most probably because of no significant increases in the amount of these strains in the experimental rat cecum.
In conclusion, this study describes the effects of five microbes, which were isolated from healthy people, on d-GalN-induced liver injury in rats. Pretreatment with L. plantarum LI04 or L. salivarius LI02 demonstrated no significant effects during this process. Pretreatment with L. paracasei LI03 aggravated liver injury. Pretreatment with L. salivarius LI01 or P. pentosaceus LI05 significantly reduced elevated ALT and AST levels, prevented the increase in total bilirubin, reduced the histological abnormalities of both the liver and the terminal ileum, decreased bacterial translocation, increased the serum level of IL-10 and/or IFN-γ, and resulted in a cecal microbiome that differed from that of the liver injury control. To the best of our knowledge, the effects of the three species, L. paracasei, L. salivarius, and P. pentosaceus, on d-GalN-induced liver injury have not been previously reported. The excellent characteristics of L. salivarius LI01 and P. pentosaceus LI05 enable them to serve as potential probiotics in the prevention and treatment of acute liver failure.
References
Adawi D, Kasravi FB, Molin G, Jeppsson B (1997) Effect of Lactobacillus supplementation with and without arginine on liver damage and bacterial translocation in an acute liver injury model in the rat. Hepatology 25:642–647
Adawi D, Molin G, Jeppsson B (1998) Inhibition of nitric oxide production and the effects of arginine and Lactobacillus administration in an acute liver injury model. Ann Surg 228:748–755
Adawi D, Ahrne S, Molin G (2001) Effects of different probiotic strains of Lactobacillus and Bifidobacterium on bacterial translocation and liver injury in an acute liver injury model. Int J Food Microbiol 70:213–220
Bengmark S (2009) Bio-ecological control of chronic liver disease and encephalopathy. Metab Brain Dis 24:223–236
Bernal W, Auzinger G, Dhawan A, Wendon J (2010) Acute liver failure. Lancet 376:190–201
Capozzi V, Russo P, Duenas MT, Lopez P, Spano G (2012) Lactic acid bacteria producing B-group vitamins: a great potential for functional cereals products. Appl Microbiol Biotechnol 96:1383–1394
Deutschman CS, Cereda M, Ochroch EA, Raj NR (2006) Sepsis-induced cholestasis, steatosis, hepatocellular injury, and impaired hepatocellular regeneration are enhanced in interleukin-6 −/− mice. Crit Care Med 34:2613–2620
Du WB, Li LJ, Huang JR, Yang Q, Liu XL, Li J, Chen YM, Cao HC, Xu W, Fu SZ, Chen YG (2005) Effects of artificial liver support system on patients with acute or chronic liver failure. Transplant Proc 37:4359–4364
Festi D, Vestito A, Mazzella G, Roda E, Colecchia A (2006) Management of hepatic encephalopathy: focus on antibiotic therapy. Digestion 73(Suppl 1):94–101
Haro C, Zelaya H, Lazarte S, Alvarez S, Aguero G (2009) Lactobacillus casei: influence on the innate immune response and haemostatic alterations in a liver-injury model. Can J Microbiol 55:648–656
Hynonen U, Palva A (2013) Lactobacillus surface layer proteins: structure, function and applications. Appl Microbiol Biotechnol 97:5225–5243
Johansson ME, Gustafsson JK, Holmen-Larsson J, Jabbar KS, Xia L, Xu H, Ghishan FK, Carvalho FA, Gewirtz AT, Sjovall H, Hansson GC (2013) Bacteria penetrate the normally impenetrable inner colon mucus layer in both murine colitis models and patients with ulcerative colitis. Gut 63:281–291
Joossens M, Huys G, Cnockaert M, De Preter V, Verbeke K, Rutgeerts P, Vandamme P, Vermeire S (2011) Dysbiosis of the faecal microbiota in patients with Crohn’s disease and their unaffected relatives. Gut 60:631–637
Kasravi FB, Adawi D, Molin G, Bengmark S, Jeppsson B (1997) Effect of oral supplementation of Lactobacilli on bacterial translocation in acute liver injury induced by D-galactosamine. J Hepatol 26:417–424
Kirpich IA, McClain CJ (2012) Probiotics in the treatment of the liver diseases. J Am Coll Nutr 31:14–23
Lu H, He J, Wu Z, Xu W, Zhang H, Ye P, Yang J, Zhen S, Li L (2013) Assessment of microbiome variation during the perioperative period in liver transplant patients: a retrospective analysis. Microb Ecol 65:781–791
Ma S, Dai Y (2011) Principal component analysis based methods in bioinformatics studies. Brief Bioinform 12:714–722
McGee RG, Bakens A, Wiley K, Riordan SM, Webster AC (2011) Probiotics for patients with hepatic encephalopathy. Cochrane Database Syst Rev 11, CD008716. doi:10.1002/14651858.CD008716
Messaoudi S, Manai M, Kergourlay G, Prevost H, Connil N, Chobert JM, Dousset X (2013) Lactobacillus salivarius: bacteriocin and probiotic activity. Food Microbiol 36:296–304
Muyzer G, de Waal EC, Uitterlinden AG (1993) Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl Environ Microbiol 59:695–700
O’Grady J (2012) Liver transplantation for acute liver failure. Best Pract Res Clin Gastroenterol 26:27–33
Osman N, Adawi D, Ahrne S, Jeppsson B, Molin G (2007) Endotoxin- and D-galactosamine-induced liver injury improved by the administration of Lactobacillus, Bifidobacterium and blueberry. Dig Liver Dis 39:849–856
Schnabl B (2013) Linking intestinal homeostasis and liver disease. Curr Opin Gastroenterol 29:264–270
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
Tranah TH, Vijay GK, Ryan JM, Shawcross DL (2013) Systemic inflammation and ammonia in hepatic encephalopathy. Metab Brain Dis 28:1–5
Tsai YT, Cheng PC, Pan TM (2012) The immunomodulatory effects of lactic acid bacteria for improving immune functions and benefits. Appl Microbiol Biotechnol 96:853–862
Tunon MJ, Alvarez M, Culebras JM, Gonzalez-Gallego J (2009) An overview of animal models for investigating the pathogenesis and therapeutic strategies in acute hepatic failure. World J Gastroenterol 15:3086–3098
Vanhoutte T, Huys G, Brandt E, Swings J (2004) Temporal stability analysis of the microbiota in human feces by denaturing gradient gel electrophoresis using universal and group-specific 16S rRNA gene primers. FEMS Microbiol Ecol 48:437–446
Wang Y, Li Y, Xie J, Zhang Y, Wang J, Sun X, Zhang H (2013) Protective effects of probiotic Lactobacillus casei Zhang against endotoxin- and d-galactosamine-induced liver injury in rats via anti-oxidative and anti-inflammatory capacities. Int Immunopharmacol 15:30–37
Wlodzimirow KA, Eslami S, Abu-Hanna A, Nieuwoudt M, Chamuleau RA (2012) Systematic review: acute liver failure—one disease, more than 40 definitions. Aliment Pharmacol Ther 35:1245–1256
Acknowledgments
This study was supported by the National Basic Research Program of China (973 program) (No. 2013CB531401), the Key Program of the National Natural Science Foundation of China (No. 81330011), and the Scientific Research Fund of the Department of Education of Zhejiang Province (Y200805384).
Author information
Authors and Affiliations
Corresponding author
Additional information
Long-Xian Lv, Xin-Jun Hu, and Gui-Rong Qian contributed equally to this work.
Rights and permissions
About this article
Cite this article
Lv, LX., Hu, XJ., Qian, GR. et al. Administration of Lactobacillus salivarius LI01 or Pediococcus pentosaceus LI05 improves acute liver injury induced by d-galactosamine in rats. Appl Microbiol Biotechnol 98, 5619–5632 (2014). https://doi.org/10.1007/s00253-014-5638-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-014-5638-2