Abstract
The progression and exacerbation of liver fibrosis are closely related to the gut microbiome. It is hypothesized that some probiotics may slow the progression of liver fibrosis. In human stool analysis [healthy group (n = 44) and cirrhosis group (n = 18)], difference in Lactobacillus genus between healthy group and cirrhosis group was observed. Based on human data, preventive and therapeutic effect of probiotics Lactobacillus lactis and L. rhamnosus was evaluated by using four mice fibrosis models. L. lactis and L. rhamnosus were supplied to 3,5-diethoxycarbonyl-1,4-dihydrocollidine or carbon tetrachloride-induced liver fibrosis C57BL/6 mouse model. Serum biochemical measurements, tissue staining, and mRNA expression in the liver were evaluated. The microbiome was analyzed in mouse cecal contents. In the mouse model, the effects of Lactobacillus in preventing and treating liver fibrosis were different for each microbe species. In case of L. lactis, all models showed preventive and therapeutic effects against liver fibrosis. In microbiome analysis in mouse models administered Lactobacillus, migration and changes in the ratio and composition of the gut microbial community were confirmed. L. lactis and L. rhamnosus showed preventive and therapeutic effects on the progression of liver fibrosis, suggesting that Lactobacillus intake may be a useful strategy for prevention and treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The gut has approximately 100 trillion microbes that promote normal gastrointestinal tract function, protect the body from infection, and regulate metabolism and the mucosal immune system (Belkaid & Hand, 2014). Irregular lifestyle habits and stress in modern society cause an imbalance of intestinal microbes (Zhang et al., 2015). Recently, probiotics have been shown to modulate the gut microbiome by increasing beneficial bacteria and reducing harmful bacteria in the gut (Azad et al., 2018). Microbes have been found to play important roles in human health and disease (Turnbaugh & Stintzi, 2011). Gut microbiome have been linked to a variety of disorders, from bowel diseases such as colorectal cancer and inflammatory bowel disease to more systemic disease, such as diabetes, metabolic syndrome, and atopy (Walker & Lawley, 2013). The gut microbiota also affects patients with obesity-induced diabetes and related complications (Ortega et al., 2020).
Liver fibrosis is an excessive accumulation of extracellular matrix proteins, including collagen, that occurs in most chronic liver diseases (Bataller & Brenner, 2005). The main causes of liver fibrosis in developed countries are alcohol abuse, chronic hepatitis viral infection, and nonalcoholic fatty hepatitis. Fibrosis is the final and common pathological consequence of many chronic inflammatory diseases (Wynn, 2008). Collagen deposition is essential and is usually a reversible part of wound healing, but if tissue damage is severe or repetitive or if the wound healing response itself becomes dysregulated, normal tissue repair can progressively develop into an irreversible fibrosis reaction (Wynn & Ramalingam, 2012). Liver fibrosis can progress into more severe stages, such as cirrhosis and further to liver cancer (Yanguas et al., 2016). Controlling inflammation can prevent fibrosis progression. The goal of developing antifibrotic medication is to suppress or reverse the progression of fibrosis in chronic liver disease (Trautwein et al., 2015).
Liver and biliary tract disease refer to a related cause of chronic liver disease associated with severe complications (Arroyo et al., 2016). The intake of 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC) recreates the main pathological features of human liver and biliary tract diseases, such as fibrosis and inflammatory infiltration (Pose et al., 2019). The toxicity of carbon tetrachloride (CCl4) can induce the physiological changes observed in cirrhosis and liver fibrosis. CCl4 can be inhaled; administered orally or intragastrically; or subcutaneously or intraperitoneally injected, and its effects can be induced more quickly if added to drinking water (Dong et al., 2016).
Probiotics can live by forming colonies in the gut through various mechanisms in the stomach and intestines. The beneficial effect of lactic acid bacteria via the gut-liver axis is extended to liver function in cirrhosis, nonalcoholic fatty liver diseases and alcoholic liver disease (Sharma et al., 2013). A previous study showed that the administration of beneficial bacterial strains helps to improve harmful interactions and liver disease (Lee et al., 2020a, 2020b). In addition, many studies have shown that probiotics can play a role in controlling harmful bacteria in the body (Kim et al., 2019). To take advantage of the beneficial properties of these probiotics, it is important to determine the effects of microbes in the digestive tract of humans and animals (Markowiak & Slizewska, 2017). Moreover, some studies have reported that the use of probiotics may be an alternative to antibiotic treatments. Therefore, probiotics that restore or improve the gut microbiota can be expected to have a role in the development of treatments for modern diseases (Hemarajata & Versalovic, 2013).
Antifibrotic therapy is aimed at inhibiting the accumulation of fibrotic cells or preventing the deposition of extracellular matrix proteins (Ghiassi-Nejad & Friedman, 2008). Recent advances in microbiome research have shown that the gut microbiome modulates barrier function and affects dysbiosis (Fukui, 2019; Lee et al., 2020a, 2020b). In previous studies, probiotics have been shown to reduce weight and body mass index, improve liver function, lower glucose levels in plasma, relieve inflammation, and restore liver fat penetration. Therefore, these findings suggest a potential role for probiotics in the prevention or treatment of liver disease. This study evaluated preventive and therapeutic role in liver fibrosis of probiotics L. lactis and L. rhamnosus.
Materials and Methods
Patients
This observational study was carried out between April 2018 and March 2021 (Table 1). A total of 62 patients comprising normal controls (n = 44) and alcoholic cirrhosis patients (n = 18) were enrolled. The patients underwent standard treatment for their disease. Stools of the normal control group were collected from the health center in the hospital. The diagnosis of cirrhosis was made through imaging findings, alcohol consumption history, and pathologic examination of liver biopsy. The exclusion criteria were as follows: patients with a history of viral hepatitis, nonalcoholic fatty liver disease, autoimmune disease, tumor presence, or drug-induced liver injury. This study was controlled in accordance with ethical guidelines from the 1975 Helsinki Declaration as reflected by prior approval by the institutional review notice for human research in the hospital participating in the trial (2016-134). Basic information has been registered on ClinicalTrials.gov for registration in the public trial registry (NCT04339725). Informed consent for enrollment was received from each participant.
A baseline evaluation was performed, including a complete blood count, liver function test, and assessment of viral markers. Patients underwent abdominal ultrasound or computed tomography imaging. aspartate amino transferase (AST), alanine amino transferase (ALT), total bilirubin (T-BIL), gamma-glutamyl transferase, albumin, prothrombin time, international normalized ratio creatinine, and α-fetoprotein were included in the serum biochemical parameters. Tests for hepatitis viruses and human immunodeficiency virus were conducted in all subjects. Enrolled patients and control patients underwent stool sampling and clinical analysis. Clinical data were simultaneously matched with metagenomics data. Fecal samples were obtained in a plastic collection kit at various times during the day. All samples were stored at -80 °C. Stool samples were collected from healthy controls at home and kept at -20 °C in a refrigerator. The patients then sent the stool box to the hospital, where the samples were kept at − 80 °C.
Microbiome Analysis
Metagenomic DNA was extracted using a QIAamp stool kit (Qiagen). After the first amplification of the V3-V4 region of the bacterial 16S rRNA gene, the second amplification was performed using Barcoded universal primers. An Agencourt AMPure XP system (Beckman) was used for the purification of amplicons. PicoGreen and quantitative PCR were utilized for quantification of the purified amplicons. After pooling of the barcoded amplicons, a MiSeq sequencer on an Illumina platform (CJ bioscience Inc.) was used for sequencing according to the manufacturer’s specifications.
The 16S-based Microbial Taxonomic Profiling platform of EzBioCloud Apps (CJ bioscience Inc.) was used for microbiome profiling. After taxonomic profiling of each sample, a comparative analysis of the samples was performed by comparison with the EzBioCloud database. CJ bioscience’s 16S rRNA database (DB ver. PKSSU4.0) (Yoon et al., 2017) was used for the taxonomic assignment of reads. OTU picking was achieved with UCLUST (Edgar, 2010) and CDHIT utilizing a 97% similarity cutoff (Xie et al., 2006). Beta-diversity, which includes PCoA and UPGMA clustering, was displayed in the comparative MTP analyzer.
Strain Preparation
Lactobacillus lactis and L. rhamnosus are lactic acid bacteria that have been isolated from various sources, including sour milk and newborn baby feces, respectively. Lactobacillus spp. were inoculated into a flask containing de Man, Rogosa and Sharpe medium (BD Difco). The strains were incubated under anaerobic conditions at 37 °C for 24 h. Stocks of each strain were prepared by mixing the culture broth with an equivalent 20% skim milk solution and then stored at -80 °C until use. The seed culture for Lactobacillus spp. was grown at 37 °C for 24 h in a flask containing MRS broth. Each culture was inoculated in an optimized medium in a fermenter (Bio Control & Science, MARADO-05D-PS, Daeduk). The fermentation was carried out at a constant pH of 5.5–6.0 via automatic addition of NaOH solution (25% w/v) under the conditions of 120 rpm agitation at 37 °C for 18–20 h. At the end of the fermentation, the cells were harvested by centrifugation at 6000 rpm for 10 min (Hanil, Supra R12, Hanil). Lyophilization of 40 × concentrated cells was accomplished in accordance with the Cooling & Heating System manual (Lab-Mast 10). After lyophilization, the colony-forming units (CFU) per gram of each probiotic powder were measured using a serial dilution method. Probiotics were suspended in 0.1 M PBS and adjusted to a density of 109 CFU/ml prior to use. The baseline characteristics of the probiotic strains are reported in Table 2.
Animal Study
Five weeks old pathogen free male C57BL/6J mice were sourced from Doo Yeol Biotech. All mice were housed in individual steel micro isolator cages maintained at 22 °C ± 2 °C having a 12/12-h light/dark cycle. Throughout the experiment, mice had free access to water and food, and were monitored daily. The experiment design included an adaptation period for all groups, during which mice were fed a normal diet for a week for adaptation period. Mice were treated humanely, and all aspects of the animal study was performed in accordance with National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. This experiment was designed by selecting a candidate for the treatment of liver disease among the probiotics that approved by Ministry of Food and Drug Safety, Korean. All the procedures were licensed by the Institutional Animal Care and Use Committee of the College of Medicine, Hallym University R1(2019-04).
DDC diet (2018S, Doo Yeol Biotech) was prepared using 0.1% of DDC (Sigma-Aldrich) reagent. In the prevention and treatment model, DDC diet was supplied as a chew diet every day for 23 days. CCl4 was mixed 1:4 ratios with corn oil (Sigma-Aldrich) and intraperitoneal injection with 100 µl twice in a week. CCL4 was injected for 9 weeks in prevention and treatment model.
Strains were suspended in distilled water or binge that maintaining concentration of 109 CFU/g. Weight was recorded daily from the first day of the experiment. The mice were eventually sacrificed via overdose of inhalation anaesthesia by isoflurane (Hana Pharm) at the conclusion of treatment period. Mice were sacrificed after 1 day starvation at the last day. Following the weighing of the mouse blood, liver, cecum, and small intestine were collected. The collected blood by cardiac puncture was placed overnight at 4 °C and centrifuged to collect serum. Serum was collected via centrifugation (5 min for 19,000×g) from whole blood (800 μl). The liver and cecum were excised and subsequently stored at − 80 °C. Cecal stool was collected for the microbiota analysis and stored at − 80 °C.
Serum Biochemistry Analysis
From animal serum, AST, ALT and T-BIL were quantified utilizing a biochemical blood analyzer (KoneLab 20, Thermo Fisher Scientific).
Pathology Analysis
10% formalin was used in fixation of specimens, which were embedded in paraffin. The tissue sections underwent staining with Hematoxylin and Eosin, Masson trichrome, and Sirius red stain. The liver was categorized in accordance with the clinical research network scoring system for lobular inflammation and necrosis from grade 0 to 4 (0, none; (1) minimal and patchy; (2) mild and involving some or all portal tracts; (3) involving all portal tracts; (4) severe may have bridging necrosis) and criteria from stage 0 to 4 (0, no fibrosis; 1, portal fibrosis; 2, periportal fibrosis; 3, septal fibrosis; 4, cirrhosis). All these biopsy specimen analyses were performed by one pathologist (SHH) who was blinded to the experimental conditions.
RNA Extraction and Quantitative Real-Time Reverse-Transcription Polymerase Chain Reaction
Liver tissue samples were homogenized in TRIzol reagent (Invitrogen). High Pure RNA Isolation Kit (Roche) was used to isolate RNA from liver tissue. Total RNA isolation from tissue was used a cDNA reverse transcription kit (Applied Biosystems), aliquots of total RNA (2 μg) were transformed into cDNA. The cDNA subsequently underwent amplification for quantitative PCR utilizing Luna® Universal Probe qPCR Master Mix (New England Biolabs Beverly) and target-specific probe-primer (Applied Biosystems).
For the evaluation of fibrosis severity, we used molecular marker of liver fibrosis/injury (Collagen, type I, alpha 1 [Col1a1, major component of type I collagen], tissue inhibitor of metalloproteinases1 [Timp1, degradation of the extracellular matrix], and transforming growth factor beta [TGF-β, multifunctional cytokine]).
Statistical Analysis
Continuous variables were expressed as means and standard deviations. One-way ANOVA and independent sample T-test were performed for body weight, liver function test, and histology analyses. For additional statistical analysis, data underwent normalization based on MSTUS18 which is implemented in NOREVA (http://idrb.zju.edu.cn/noreva). Multiple Experiment Viewer (MeV) was employed for hierarchical clustering analysis (HCA) and analysis of variance (ANOVA) with a post hoc test. P value < 0.05 was statistically significant. All statistical analyses were performed via SPSS software (ver. 19, SPSS Inc.).
Results
Baseline Characteristics of Patients
There was a significant difference in the mean levels of AST (P < 0.001) and ALT (P < 0.004) between the groups, with higher levels in the cirrhosis patient group than in the normal group. The mean cholesterol level was significantly (P < 0.001) higher in the normal group than in the cirrhosis patient group. The mean level of γGT was significantly (P < 0.001) higher in the cirrhosis patient group than in the normal group. There was no significant difference in triglyceride, High-density lipoprotein, or low-density lipoprotein levels (Table 1).
Human Stool Microbiome Analysis
We performed a taxonomic composition comparison between groups at the phylum level. The proportion of Bacteroidetes was decreased in the cirrhosis patient group (24.6%) compared to the normal control group (50.9%), and Proteobacteria abundance was increased in the cirrhosis group (35.8%) compared to the normal control group (11.8%) (Fig. 1A). The alpha diversity indices were significantly different between the groups. The ACE and Chao1 indices, which are species richness indices, were significantly decreased (P < 0.01) in the cirrhosis patient group compared to the normal group, and a significant decrease (P < 0.01) in the cirrhosis patient group was also confirmed by the Shannon index, which is a diversity index (Fig. 1B). A comparative analysis of beta diversity between groups was performed. For principal coordinates analysis (PCoA), UniFrac distances were used to check the differences between groups, and the change in movement between groups was confirmed through region discrimination (Fig. 1C).
The human gut microbiome analysis. A Phylum composition of normal control and cirrhosis patients group. B Alpha diversity in normal control and cirrhosis patients group. C Beta diversity: PCoA in normal control and cirrhosis patients group. D The relative abundance of important taxa in human gut. E LEfSe analysis between normal control and cirrhosis patients group. *P < 0.05, **P < 0.01
We compared and analyzed the relative abundance of specific taxa in the normal control group and cirrhosis patient group (Fig. 1D). Bacteroidetes and Firmicutes are the dominant taxa accounting for the largest proportion of the intestinal microbiota, and the ratio of Bacteroidetes and Firmicutes can be used as an indicator of various diseases. The relative abundance of Bacteroidetes was significantly decreased (P < 0.01) in the cirrhosis patient group compared with the normal control group. There was no significant difference in Firmicutes. However, the Firmicutes/Bacteroidetes (F/B) ratio was significantly increased (P < 0.05) in the cirrhosis patient group compared to the normal control group. We analyzed the relative abundance between groups of major taxa known to be important in the human gut. Bacteroides, Blautia, Christensenellaceae, Faecalibacterium, Ruminococcaceae, Lachnospiraceae and Alistipes were significantly decreased (P < 0.01) in the cirrhosis patient group compared to the normal control group. Enterobacteriaceae, Proteobacteria and Lactobacillus were significantly increased (P < 0.01) in the cirrhosis patient group compared to the normal control group. There was no significant difference between the two groups in Bifidobacterium.
We analyzed the difference between the normal control group and the cirrhosis patient group using linear discriminant analysis effect size (LEfSe) analysis in the gut microbial community at the genus level (Fig. 1E). In the cirrhosis patient group, at the genus level, taxa belonging to the Enterobacteriaceae family, such as the Enterobacteriaceae_g, Escherichia, Enterobacter, and Enterobacteriaceae_uc, and Lactobacillus and Streptococcus genera, were dominant. In the normal control group, the predominance of the Bacteroides, Faecalibacterium, Megamonas, Alisipes, Oscillibacter and Roseburia genera was confirmed.
Preventive Effect in Fibrosis Mouse Model
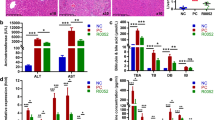
To confirm a preventive effect, Lactobacillus spp. were administered while liver fibrosis was induced by feeding with a DDC diet for 3 weeks (Fig. 2A). Body weight decreased in all the groups due to the DDC diet feeding but not in the normal diet group (Fig. 2B). There was no significant difference in the body and liver weights after 3 weeks in the probiotics groups compared with the DDC group. In serum biochemical analysis, the strain-administered groups showed a tendency toward decreased AST compared to the DDC diet group, and AST was significantly decreased in the L. rhamnosus group (P < 0.05) (Fig. 2C). There was no significant difference in AST and T-BIL due to strain administration (Fig. S1A). A significant decrease in the fibrotic area (P < 0.01) was confirmed in the group administered L. lactis and L. rhamnosus, as shown by Sirius red staining (Fig. 2D). In the measurement of fibrosis-related gene expression levels, L. lactis administration significantly reduced Col1a, Timp1, and TGF-β expression levels (P < 0.05) (Fig. 2E).
Prevention effect of Lactobacillus in DDC diet-induced liver fibrosis model. A Flow chart of the DDC diet-induced liver fibrosis model. B The body weight change of DDC diet-induced liver fibrosis model for 4 weeks. C The body weight, Liver weight, Liver/Body weight ratio and serum biochemistry AST. D Effects of strain on the liver tissue: H&E. MT, and Sirius red staining. × 200. E mRNA expression of the liver tissue. *P < 0.05, **P < 0.01
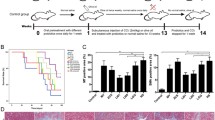
To confirm the preventive effect of Lactobacillus in the liver fibrosis, it was induced by CCl4 injection for 9 weeks. CCl4 was diluted 1:4 with corn oil and injected by intraperitoneal injection (Fig. 3A). For 9 weeks, L. lactis group was protect-ed from weight loss, but the L. rhamnosus group was not (Fig. 3B). There was no significant difference in weight and L/B ratio between groups (Fig. 3C). In blood biochemical analysis, AST and ALP were not significantly different between the CCl4 group and the probiotics groups (Figs. 3C, S1B). However, T-BIL was significantly decreased in the L. lactis group (P < 0.05) and L. rhamnosus group (P < 0.01) compared with the CCl4 group (Fig. S1B). There was a significant decrease (P < 0.01) in fibrosis area by probiotics administration (Fig. 3D). The L. lactis reduced the expression of Col1a and Timp1 significantly in the liver tissue (P < 0.01) (Fig. 3E).
Prevention effect of Lactobacillus in CCl4 injection-induced liver fibrosis model. A Flow chart of the CCl4 injection-induced liver fibrosis model. B The body weight change of CCl4-induced liver fibrosis model for 10 weeks. C The body weight, Liver weight, Liver/Body weight ratio and serum biochemistry AST. D Effects of strain on the liver tissue: H&E. MT, and Sirius red staining. × 200. F mRNA expression of the liver tissue. *P < 0.05, **P < 0.01
Therapeutic Effect on Fibrosis Model
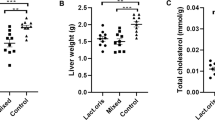
To confirm the therapeutic effect of Lactobacillus in the DDC diet-induced liver fibrosis model, a 1 week intake period of Lactobacillus was added after liver fibrosis induction for 3 weeks with the DDC diet (Fig. 4A). After being fed the DDC diet, the body weight of the mice in all groups decreased and then increased again when the mice were switched to a normal diet (Fig. 4B). However, weights decreased again in the DDC diet group. With the additional intake of Lactobacillus after induction of liver fibrosis, mice in the L. lactis (25 ± 1.1 g) and L. rhamnosus (23.5 ± 1 g) groups recovered body weight compared with mice in the DDC diet group (20.3 ± 1.2) (P < 0.01) (Fig. 4C). In addition, in all groups, L. lactis (92 ± 26.4) and L. rhamnosus (92 ± 15) significantly reduced AST levels compared to levels in the DDC diet group (164 ± 14.3) (P < 0.01) (Fig. 4C). In both the L. lactis group and L. rhamnosus group, the fibrotic area was significantly reduced compared to that in the DDC group (P < 0.01) (Fig. 4D). The L. rhamnosus group showed significantly reduced expression of Col1a (P < 0.05) and Timp1 (P < 0.01) compared to the DDC group. The L. lactis group exhibited significantly reduced expression of Timp1 (P < 0.05) compared to the DDC group (Fig. 4E). The TGF-β level in all groups recovered to a normal level after the DDC diet was stopped.
Treatment effect of Lactobacillus in DDC Diet-induced liver fibrosis model. A Flow chart of the DDC diet-induced liver fibrosis model. B The body weight change of DDC diet-induced liver fibrosis model for 4 weeks. C The body weight, Liver weight, Liver/Body weight ratio and serum biochemistry about AST. D Effects of strain on the liver tissue: H&E. MT, and Sirius red staining. × 200. F mRNA expression of the liver tissue. *P < 0.05, **P < 0.01
To confirm the therapeutic effect of Lactobacillus in the liver fibrosis model induced by CCl4 injection, a 1 week Lactobacillus intake period was added after induction of liver fibrosis for 9 weeks via CCl4 injection (Fig. S2A). Intake of L. lactis was not shown to protect against body weight loss or liver weight loss (Fig. S2C). There was no significant difference in the ALP level, but treatment with L. lactis intake increased the ALT concentration, and the Lactobacillus intake treatment increased the T-BIL concentration (Fig. S2D). Lactobacillus treatment led to a significant decrease in the fibrosis area, measured by Sirius red staining (Fig. S2E). L. lactis significantly decreased the expression of Col1a (P < 0.01) (Fig. S2F). In the L. rhamnosus group, the TGF-β level recovered to the normal control level within 1 week after the CCl4 injections were stopped.
Mouse Cecal Microbiome Analysis
At the phylum level, the microbiome composition in each mouse model group showed differences. In the DDC prevention model, a decrease in the proportion of Proteobacteria was confirmed in the Lactobacillus-treated group compared to the DDC diet group. Similarly, in the DDC treatment model, the proportion of Proteobacteria was decreased by treatment with Lactobacillus. There was no significant change in the ratio of Firmicutes, Bacteroidetes, and Proteobacteria in the CCl4 prevention model and the CCL4 treatment model. In the CCl4 treatment model, an increase in the rate of Verrucomicrobia (21.6%) through L. lactis administration was confirmed (Fig. 5A).
We analyzed alpha diversity by group in four types of mouse models, and no significant difference in the species richness index Chao1 and the Shannon diversity index were observed (Fig. 5B). We confirmed beta diversity by model through PCoA. As the movement in each group was clearly distinct in the DDC prevention model and the DDC treatment model, changes in the microbiome through Lactobacillus treatment were confirmed. In the CCl4 prevention model, there was a change in the distance in each group, but a clear distinction was not made. In particular, distinction and distance changes induced by L. lactis and L. rhamnosus treatment were insignificant in the CCl4 treatment model (Fig. 5C).
The genera showing differences in each group were selected and compared through a heatmap (Fig. 5D). In the DDC prevention group, the Eubacterium_g17, Eubacterium_g23 and Eubacterium_g6 genera were increased through Lactobacillus treatment. In the DDC treatment group, a marked increase in the Muribaculum, Eubacterium_g17 and Akkermansia genera was confirmed to be induced by Lactobacillus treatment. In the CCl4 prevention group, the change caused by L. lactis treatment was clear, and increases in the Alisipes, Muribaculum, Parabacteroides and Mucispirillum genera were confirmed. In the CCl4 treatment group, Lactobacillus treatment increased the Bacteroides genus, and in particular, L. lactis treatment increased the Eubacterium_g23 and Akkermansia genera. We confirmed the abundance of major Lactobacillus species that changed after L. lactis and L. rhamnosus administration (Fig. S3). When the mice were treated with L. lactis and L. rhamnosus, the abundance of the Lactobacillus reuteri, Lactobacillus murinus and Lactobacillus gasseri and Lactobacillus genera in the intestine, which were commonly distributed in the four models, was confirmed. In the DDC prevention model and the DDC treatment model, an increase in intestinal Lactobacillus abundance induced by Lactobacillus treatment was confirmed, but in the CCl4 prevention model and CCl4 treatment model, a change in intestinal Lactobacillus abundance induced by Lactobacillus treatment was not confirmed.
Discussion
Hepatic fibrosis is a response to chronic liver damage, in which some hepatocytes undergo apoptosis and progressive loss of liver function occurs (Schuppan & Afdhal, 2008). Cirrhosis patients have a high probability of progressing to liver cancer and liver failure in the late stage of liver fibrosis (Wiegand & Berg, 2013). There are several causes for the onset and progression of liver fibrosis, and recent studies on its association with the microbiome have been extensively conducted (Ray, 2017). Through portal vein connections, the liver and microbiome interact in a bidirectional manner, and dysbiosis of the gut microbiome may contribute to the early stages of the hepatic fibrosis phase (Schnabl & Brenner, 2014). In our human stool microbiome data, the differences between the normal and cirrhosis patient groups are evident, supporting a bidirectional relationship between the liver and the microbiome. Excessive proliferation of the Proteobacteria taxa containing many harmful bacteria and the Enterobacteriaceae (Acharya & Bajaj, 2019) taxa containing mainly ammonia-producing bacteria is consistent with previous cirrhotic microbiome studies, suggesting a negative effect through dysbiosis. The data we collected showing decreased microbial diversity and species richness and changes in Firmicutes/Bacteroidetes ratios in the microbiome of mice in the normal and cirrhotic groups indicate distinct signs of dysbiosis. In addition, the pattern of changes in the abundance of important taxa in the human gut observed in these results suggests a link between disease and dysbiosis.
Several studies have been conducted on the ability of probiotics to regulate the intestinal microbial community and suppress harmful bacteria in the gut (Mulaw et al., 2019). In addition, probiotics aid the growth of beneficial bacteria in the intestine, and an immune enhancing effect of the substances produced by these beneficial bacteria has also been observed (Famouri et al., 2017). Many studies have been conducted on the effects of lactic acid bacteria treatment on liver cirrhosis and liver fibrosis, and an inhibitory effect of certain probiotics on fibrosis has been observed (Liu et al., 2020; Shi et al., 2017). However, although probiotics are attracting attention as a promising option for treatment of liver disease, the mechanism of action remains unclear.
Based on previous research evidence, the experiments in the present study were performed with animal models, and the results suggest that lactobacillus strains might be a treatment option for liver fibrosis. A model of liver fibrosis caused by various factors was induced by a DDC diet and CCl4 intraperitoneal injection. In our study, when DDC diet-induced liver fibrosis was present, simultaneously ingested Lactobacillus, L. lactis showed the most effective preventive effect against liver fibrosis by lowering the expression of Col1a, Timp1, and TGF-β. When Lactobacillus was ingested even after the induction of liver fibrosis, body weight was recovered in all the L. lactis and L. rhamnosus groups compared to the DDC diet group. In addition, all groups showed significantly reduced AST levels in serum compared to the DDC diet group. Moreover, all groups had significantly reduced cirrhosis scores. In the DDC diet model, Lactobacillus showed a greater effect in the treatment of induced liver fibrosis.
Both Col1a and Timp1 mRNA expression was decreased, but the decrease was most significant in the L. rhamnosus group. In the case of TGF-β, all groups recovered to normal levels. However, the TGF-β level appears to have recovered due to cessation of the DDC diet. In a previous report, L. plantarum and L. brevis counteracted the TGF-β-induced fibrotic marker by modulating SMAD-assocoated TGF-β signaling (Kanmani & Kim, 2022). It is supposed that L. lactis and L. rhamnosus are not related with SMAD-assocoated TGF-β signaling.
In our previous study, L. lactis alleviated inflammation and steatosis in nonalcoholic fatty liver disease (NAFLD) through regulation of the gut microbiome (Lee et al., 2020a, 2020b). This L. lactis strain, which was the same strain used in the present study, induced an effective reduction in AST, ALT, and cholesterol levels in the NAFLD model. Additionally, in this study, L. lactis showed positive effects on liver fibrosis in both the DDC diet and CCl4 injection models. L. lactis showed a particularly prophylactic effect against liver fibrosis in both models.
Lactobacillus rhamnosus is considered a subspecies of L. casei but has now been identified as its own species (Huang et al., 2018). L. rhamnosus is a probiotic that has been extensively studied. In a recent study, L. rhamnosus GG was shown to prevent fibrosis in a liver cirrhosis model induced by bile duct ligation (Liu et al., 2020). Lactobacillus rhamnosus GG treatment significantly attenuated liver inflammation, damage, and fibrosis and led to a reduction in hepatic bile acids (BA) in BDL mice. Additionally, this treatment altered the gut microbiota, which was associated with increased BA deconjugation and increased fecal and urine BA excretion. Clinical studies have evaluated the safety and tolerability of L. rhamnosus GG in liver cirrhosis patients, have shown minimal hepatic encephalopathy and investigated the mechanism of gut microbial transformation (Bajaj et al., 2014). Endotoxin and TNF-α levels were reported to be reduced, and the gut microbiota was changed to reduced Enterobacteriaceae abundance and increased Clostridiales Incertae Sedis XIV and Lachnospiraceae relative abundance. Many studies have suggested that L. rhamnosus GG is effective in lowering AST and ALT levels in alcoholic liver disease models (Marotta et al., 2005; Segawa et al., 2008).
In animal models of liver fibrosis induced by DDC diet or CCl4 injection, dysbiosis was confirmed, similar to the microbiome analysis in the human cirrhosis patient group. Although it was not significant, a decrease in bacterial diversity occurred, and a change in the composition of the taxa was observed. No dramatic change in abundance or composition occurred due to Lactobacillus administration, but as confirmed by beta diversity analysis, strain migration and compensation of some taxa induced by Lactobacillus were observed. Compensation of lactic acid bacteria taxa was confirmed, and among the lactic acid bacteria taxa, changes in L. gasseri (Carroll et al., 2007), L. murinus (Pan et al., 2018), and L. reuteri (Wang et al., 2020), which have been confirmed to have an anti-inflammatory effect, were prominent. Thus, Lactobacillus administration can be expected to relieve intestinal inflammation and inhibit harmful bacteria in the body in the presence of liver fibrosis, but further research is needed.
Probiotics have been verified in many studies to prevent various diseases. Although the underlying mechanism has not been well elucidated, there are certain probiotics that act specifically on specific diseases. In our study, liver fibrosis was induced via two methods, and in each model, Lactobacillus showed different protective effects against liver fibrosis. These results suggest that certain microbial species may act specifically to protect against liver fibrosis, suggesting their potential as customized treatments. The Lactobacillus strain we used showed a protective effect against liver fibrosis in an induced liver fibrosis model. Although more research is needed, we propose that the Lactobacillus strain might be a preventive and therapeutic agent against liver fibrosis.
Data availability
Data available within the article or its supplementary materials.
References
Acharya, C., & Bajaj, J. S. (2019). Altered microbiome in patients with cirrhosis and complications. Clinical Gastroenterology and Hepatology, 17, 307–321.
Arroyo, V., Moreau, R., Kamath, P. S., Jalan, R., Gines, P., Nevens, F., Fernandez, J., To, U., Garcia-Tsao, G., & Schnabl, B. (2016). Acute-on-chronic liver failure in cirrhosis. Nature Reviews Disease Primers, 2, 16041.
Azad, M. A. K., Sarker, M., Li, T., & Yin, J. (2018). Probiotic species in the modulation of gut microbiota: An overview. BioMed Research International, 2018, 9478630.
Bajaj, J. S., Heuman, D. M., Hylemon, P. B., Sanyal, A. J., Puri, P., Sterling, R. K., Luketic, V., Stravitz, R. T., Siddiqui, M. S., Fuchs, M., Thacker, L. R., Wade, J. B., Daita, K., Sistrun, S., White, M. B., Noble, N. A., Thorpe, C., Kakiyama, G., Pandak, W. M., … Gillevet, P. M. (2014). Randomised clinical trial: Lactobacillus gg modulates gut microbiome, metabolome and endotoxemia in patients with cirrhosis. Alimentary Pharmacology & Therapeutics, 39, 1113–1125.
Bataller, R., & Brenner, D. A. (2005). Liver fibrosis. Journal of Clinical Investigation, 115, 209–218.
Belkaid, Y., & Hand, T. W. (2014). Role of the microbiota in immunity and inflammation. Cell, 157, 121–141.
Carroll, I. M., Andrus, J. M., Bruno-Barcena, J. M., Klaenhammer, T. R., Hassan, H. M., & Threadgill, D. S. (2007). Anti-inflammatory properties of Lactobacillusgasseri expressing manganese superoxide dismutase using the interleukin 10-deficient mouse model of colitis. American Journal of Physiology-Gastrointestinal and Liver Physiology, 293, G729-738.
Dong, S., Chen, Q. L., Song, Y. N., Sun, Y., Wei, B., Li, X. Y., Hu, Y. Y., Liu, P., & Su, S. B. (2016). Mechanisms of ccl4-induced liver fibrosis with combined transcriptomic and proteomic analysis. The Journal of Toxicological Sciences, 41, 561–572.
Edgar, R. C. (2010). Search and clustering orders of magnitude faster than blast. Bioinformatics, 26, 2460–2461.
Famouri, F., Shariat, Z., Hashemipour, M., Keikha, M., & Kelishadi, R. (2017). Effects of probiotics on nonalcoholic fatty liver disease in obese children and adolescents. Journal of Pediatric Gastroenterology and Nutrition, 64, 413–417.
Fukui, H. (2019). Role of gut dysbiosis in liver diseases: What have we learned so far? Diseases, 7, 58.
Ghiassi-Nejad, Z., & Friedman, S. L. (2008). Advances in antifibrotic therapy. Expert Review of Gastroenterology & Hepatology, 2, 803–816.
Hemarajata, P., & Versalovic, J. (2013). Effects of probiotics on gut microbiota: Mechanisms of intestinal immunomodulation and neuromodulation. Therapeutic Advances in Gastroenterology, 6, 39–51.
Huang, C. H., Li, S. W., Huang, L., & Watanabe, K. (2018). Identification and classification for the Lactobacilluscasei group. Frontiers in Microbiology, 9, 1974.
Kanmani, P., & Kim, H. (2022). Probiotics counteract the expression of hepatic profibrotic genes via the attenuation of tgf-beta/smad signaling and autophagy in hepatic stellate cells. PLoS ONE, 17, e0262767.
Kim, S. K., Guevarra, R. B., Kim, Y. T., Kwon, J., Kim, H., Cho, J. H., Kim, H. B., & Lee, J. H. (2019). Role of probiotics in human gut microbiome-associated diseases. Journal of Microbiology and Biotechnology, 29, 1335–1340.
Lee, N. Y., Joung, H. C., Kim, B. K., Kim, B. Y., Park, T. S., & Suk, K. T. (2020a). Lactobacillus lactis ckdb001 ameliorate progression of nonalcoholic fatty liver disease through of gut microbiome: Addendum. Gut Microbes, 12, 1829449.
Lee, N. Y., Yoon, S. J., Han, D. H., Gupta, H., Youn, G. S., Shin, M. J., Ham, Y. L., Kwak, M. J., Kim, B. Y., Yu, J. S., Lee, D. Y., Park, T. S., Park, S. H., Kim, B. K., Joung, H. C., Choi, I. S., Hong, J. T., Kim, D. J., Han, S. H., & Suk, K. T. (2020b). Lactobacillus and pediococcus ameliorate progression of non-alcoholic fatty liver disease through modulation of the gut microbiome. Gut Microbes, 11, 882–899.
Liu, Y., Chen, K., Li, F., Gu, Z., Liu, Q., He, L., Shao, T., Song, Q., Zhu, F., Zhang, L., Jiang, M., Zhou, Y., Barve, S., Zhang, X., McClain, C. J., & Feng, W. (2020). Probiotic Lactobacillusrhamnosus gg prevents liver fibrosis through inhibiting hepatic bile acid synthesis and enhancing bile acid excretion in mice. Hepatology, 71, 2050–2066.
Markowiak, P., & Slizewska, K. (2017). Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients, 9, 1–30.
Marotta, F., Barreto, R., Wu, C. C., Naito, Y., Gelosa, F., Lorenzetti, A., Yoshioka, M., & Fesce, E. (2005). Experimental acute alcohol pancreatitis-related liver damage and endotoxemia: Synbiotics but not metronidazole have a protective effect. Chinese Journal of Digestive Diseases, 6, 193–197.
Mulaw, G., SisayTessema, T., Muleta, D., & Tesfaye, A. (2019). In vitro evaluation of probiotic properties of lactic acid bacteria isolated from some traditionally fermented Ethiopian food products. International Journal of Microbiology, 2019, 7179514.
Ortega, M. A., Fraile-Martinez, O., Naya, I., Garcia-Honduvilla, N., Alvarez-Mon, M., Bujan, J., Asunsolo, A., & de la Torre, B. (2020). Type 2 diabetes mellitus associated with obesity (diabesity). The central role of gut microbiota and its translational applications. Nutrients, 12, 2749.
Pan, F., Zhang, L., Li, M., Hu, Y., Zeng, B., Yuan, H., Zhao, L., & Zhang, C. (2018). Predominant gut lactobacillus murinus strain mediates anti-inflammaging effects in calorie-restricted mice. Microbiome., 6, 54.
Pose, E., Sancho-Bru, P., & Coll, M. (2019). 3,5-diethoxycarbonyl-1,4-dihydrocollidine diet: A rodent model in cholestasis research. Methods in Molecular Biology, 1981, 249–257.
Ray, K. (2017). Alcoholic liver disease: Gut-liver axis: Ppis, enterococcus and promotion of alcoholic liver disease. Nature Reviews Gastroenterology & Hepatology, 14, 689.
Schnabl, B., & Brenner, D. A. (2014). Interactions between the intestinal microbiome and liver diseases. Gastroenterology, 146, 1513–1524.
Schuppan, D., & Afdhal, N. H. (2008). Liver cirrhosis. The Lancet, 371, 838–851.
Segawa, S., Wakita, Y., Hirata, H., & Watari, J. (2008). Oral administration of heat-killed lactobacillus brevis sbc8803 ameliorates alcoholic liver disease in ethanol-containing diet-fed c57bl/6n mice. International Journal of Food Microbiology, 128, 371–377.
Sharma, V., Garg, S., & Aggarwal, S. (2013). Probiotics and liver disease. The Permanente Journal, 17, 62–67.
Shi, D., Lv, L., Fang, D., Wu, W., Hu, C., Xu, L., Chen, Y., Guo, J., Hu, X., Li, A., Guo, F., Ye, J., Li, Y., Andayani, D., & Li, L. (2017). Administration of Lactobacillus salivarius li01 or Pediococcus pentosaceus li05 prevents ccl4-induced liver cirrhosis by protecting the intestinal barrier in rats. Scientific Reports, 7, 6927.
Trautwein, C., Friedman, S. L., Schuppan, D., & Pinzani, M. (2015). Hepatic fibrosis: Concept to treatment. Journal of Hepatology, 62, S15-24.
Turnbaugh, P. J., & Stintzi, A. (2011). Human health and disease in a microbial world. Frontiers in Microbiology, 2, 190.
Walker, A. W., & Lawley, T. D. (2013). Therapeutic modulation of intestinal dysbiosis. Pharmacological Research, 69, 75–86.
Wang, H., Zhou, C., Huang, J., Kuai, X., & Shao, X. (2020). The potential therapeutic role of Lactobacillusreuteri for treatment of inflammatory bowel disease. American Journal of Translational Research, 12, 1569–1583.
Wiegand, J., & Berg, T. (2013). The etiology, diagnosis and prevention of liver cirrhosis: Part 1 of a series on liver cirrhosis. Deutsches Ärzteblatt International, 110, 85–91.
Wynn, T. A. (2008). Cellular and molecular mechanisms of fibrosis. Journal of Pathology, 214, 199–210.
Wynn, T. A., & Ramalingam, T. R. (2012). Mechanisms of fibrosis: Therapeutic translation for fibrotic disease. Nature Medicine, 18, 1028–1040.
Xie, J. T., Shao, Z. H., Vanden Hoek, T. L., Chang, W. T., Li, J., Mehendale, S., Wang, C. Z., Hsu, C. W., Becker, L. B., Yin, J. J., & Yuan, C. S. (2006). Antioxidant effects of ginsenoside re in cardiomyocytes. European Journal of Pharmacology, 532, 201–207.
Yanguas, S. C., Cogliati, B., Willebrords, J., Maes, M., Colle, I., van den Bossche, B., de Oliveira, C., Andraus, W., Alves, V. A. F., Leclercq, I., & Vinken, M. (2016). Experimental models of liver fibrosis. Archives of Toxicology, 90, 1025–1048.
Yoon, S. H., Ha, S. M., Kwon, S., Lim, J., Kim, Y., Seo, H., & Chun, J. (2017). Introducing ezbiocloud: A taxonomically united database of 16s rrna gene sequences and whole-genome assemblies. International Journal of Systematic and Evolutionary Microbiology, 67, 1613–1617.
Zhang, Y. J., Li, S., Gan, R. Y., Zhou, T., Xu, D. P., & Li, H. B. (2015). Impacts of gut bacteria on human health and diseases. International Journal of Molecular Sciences, 16, 7493–7519.
Acknowledgements
This research was supported by Hallym University Research Fund, Korea National Research Foundation (2020R1A6A1A03043026, and 2021M3A9I4021433), Bio Industrial Technology Development Program (20018494) funded by the Ministry of Trade, Industry and Energy (MOTIE, Korea), and the Promotion of Innovative Businesses for Regulation-Free Special Zones funded by the Ministry of SMEs and Startups (MSS, Korea) (P0020622).
Author information
Authors and Affiliations
Contributions
SMW, NYL, and SKK: analysis and interpretation of the data, collection and assembly of data, drafting of the article. SMW and KTS: conception and design, critical revision of the article for important intellectual content, final approval of the article. KKO, HG, SPS, KHK, BKK, HCJ, JJJ, RG, SHH, SJY, and DJK: critical revision of the article for important intellectual content and provision of study materials.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest, including relevant financial interests, activities, relationships, affiliations, and any other conflict of interest as explicitly and implicitly expressed in the Editorial Policies for Authors.
Ethical approval
This study was controlled in accordance with ethical guidelines from the 1975 Helsinki Declaration as reflected by prior approval by the institutional review notice for human research in the hospital participating in the trial (2016-134). Basic information has been registered on ClinicalTrials.gov for registration in the public trial registry (NCT04339725). Informed consent for enrollment was received from each participant. This experiment was designed by selecting a candidate for the treatment of liver disease among the probiotics that approved by Ministry of Food and Drug Safety, Korean. All the procedures were licensed by the Institutional Animal Care and Use Committee of the College of Medicine, Hallym University R1(2019-04).
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Won, S.M., Lee, N.Y., Oh, KK. et al. Gut Lactobacillus and Probiotics Lactobacillus lactis/rhamnosis Ameliorate Liver Fibrosis in Prevention and Treatment. J Microbiol. 61, 245–257 (2023). https://doi.org/10.1007/s12275-023-00014-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12275-023-00014-y