Abstract
Our aim has been to characterize the molecular mechanisms regulating the expression of the channel-forming tight-junctional protein claudin-2 in response to the pro-inflammatory cytokine tumor necrosis factor-α (TNFα), which is elevated, for example, in active Crohn’s disease. TNFα caused an 89% decrease of the paracellular resistance in colonic HT-29/B6 cells, whereas transcellular resistance was unaltered. The claudin-2 protein level was increased by TNFα without changes in subcellular tight-junctional protein localization as revealed by confocal laser scanning microscopy. Enhanced gene expression was identified as the source of this increase, since claudin-2-specific mRNA and promoter activity was elevated, whereas mRNA stability remained unaltered. Specific inhibitors and phospho-specific antibodies revealed that the increased gene expression of claudin-2 after TNFα treatment was mediated by the phosphatidylinositol-3-kinase pathway. Thus, the up-regulation of claudin-2 by TNFα is attributable to the regulation of the expression of the gene, as a result of which epithelial barrier function is disturbed, for example, during chronic intestinal inflammation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The paracellular barrier of epithelial cells is formed by the tight junction, a belt of strands surrounding the cells at the apical edge of their lateral membranes and dividing the cell membrane into an apical and a basolateral component. In this manner, the tight junction serves as a gate for paracellular transport and a hurdle against lateral protein diffusion within the cell membrane. The tight junction is composed of a number of integral membrane proteins including occludin (Furuse et al. 1993), junctional adhesion molecule (Martin-Padura et al. 1998), tricellulin (Ikenouchi et al. 2005), and the claudin family (Furuse et al. 1998), the last-mentioned consisting of at least 24 members (Tsukita et al. 2001). The function of a number of tight-junctional proteins has previously been characterized. In contrast to their structural similarity, the claudins fulfil different functions and can roughly be divided into barrier-forming and channel-forming claudins (for a review, see Van Itallie and Anderson 2006). Claudin-1 is crucial for the tightening of the epithelial barrier in monolayers of the Madin-Darby-canine kidney (MDCK) cell line and in native skin epithelium (Furuse et al. 2002; Inai et al. 1999). In contrast, the expression of claudin-2 markedly decreases the tightness of the epithelial barrier (Furuse et al. 2001), which can be explained by the induction of paracellular cation channels (Amasheh et al. 2002). Furthermore, the expression of claudins is variable in different tissues or even along the crypt-to-villus axis, as has been shown for claudin-2 (Rahner et al. 2001). This suggests that the differing leakiness of the various epithelia is a function of their tight-junctional protein composition.
Under pathophysiological conditions, the disturbance of the epithelial barrier allows contact or even penetration of noxious luminal agents such as antigens or bacteria into the intestinal immune system leading to, or at least sustaining, mucosal inflammation, e.g., in inflammatory bowel diseases (IBD). The latter are autoimmune disorders of the gastrointestinal tract accompanied by diarrhea and dysfunction of the intestinal epithelial barrier. In vitro studies of the human intestinal epithelial cell line HT-29/B6 (Kreusel et al. 1991) have shown pro-inflammatory effects on epithelial apoptosis, transepithelial resistance, and tight-junctional strands when the cells are incubated with tumor necrosis factor-α (TNFα; Gitter et al. 2000; Mankertz et al. 2000; Schmitz et al. 1999).
Claudin-2, a member of the claudin multigene family of transmembrane tight-junctional proteins has been shown to be upregulated in active Crohn’s disease and in the inflamed mucosa of patients with ulcerative colitis (Heller et al. 2005; Zeissig et al. 2007). Zeissig et al. (2007) also provide the claudin complement of normal colonic tight junctions, as based on a model of HT-29/B6 cells. Since claudin-2 forms cation-selective channels (Amasheh et al. 2002), its up-regulation in IBD has been proposed to contribute to altered barrier function. Whereas the Th2 cytokine interleukin-13 (IL-13) has turned out to be the key effector in ulcerative colitis with the ability to up-regulate claudin-2 expression and acting in concert with TNFα, mucosal inflammation in Crohn’s disease is a Th1-immune response (Fuss et al. 1996). In order to characterize the influence of TNFα on claudin-2 expression, we have analyzed the intracellular localization, protein and mRNA expression, promoter activity, and signaling pathways of claudin-2. We have employed intestinal HT-29/B6 cells, which represent an intermediate-tight intestinal epithelium of human colonic origin, and which have proven to be a highly suitable model system for studying pro-inflammatory cytokine effects.
Materials and methods
Cell culture and chemicals
HT-29/B6 cells, a subclone of the human intestinal cell line HT-29 (Kreusel et al. 1991), was maintained in RPMI1640 supplemented with 10% fetal calf serum and antibiotics (penicillin, streptomycin). Cells were seeded on Millicell PCF filters (Millipore, Eschborn, Germany) and grown to confluence before TNFα treatment. Recombinant human TNFα was purchased from Biomol (Hamburg, Germany; 108 U/mg) and Peprotech (Rocky Hill, USA; 2·107 U/mg). The phosphatidylinositol-3-kinase (PI3K) inhibitor (20 µM LY294002; Calbiochem, Darmstadt, Germany), the MAP-ERK kinase 1/2 (MEK1/2) inhibitor (5 µM U0126; Cell Signaling Technology, Frankfurt am Main, Germany), the c-Jun N-terminal kinase (JNK) inhibitor (5 µM SP600125; Calbiochem, Darmstadt, Germany), or the myosin light chain kinase (MLCK) inhibitor (5 µM ML-7; Calbiochem) were added 1 h prior to treatment with TNFα.
Immunoblotting
HT-29/B6 cells were washed with phosphate-buffered saline (PBS) and lysed in buffer containing 2 mM TRIS-HCl, 5 mM MgCl2, 1 mM EDTA, and 0.6 mM EGTA supplemented with protease inhibitors (pH 7.4). The lysate was frozen and thawed three times in liquid nitrogen and homogenized through 20-G and 26-G needles (ten times each). After centrifugation for 5 min at 200 g, the supernatant was centrifuged for a further 30 min at 43,000g. The pellet containing the membrane protein fraction was resuspended in lysis buffer. For whole-cell lysates, cells were washed with PBS and scraped with phospho-lysis buffer (20 mM TRIS-HCl pH 7.5, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM β-glycerolphosphate, 1 mM sodium orthovanadate, 1 µg/ml leupeptin, 1 mM phenylmethane sulfonyl-fluoride, complete protease inhibitor mixture w/o EDTA; Sigma, Taufkirchen, Germany). After being frozen at -20°C, the lysate was centrifuged (15,000g, 5 min, 4°C), and the supernatant was used as the whole-cell extract.
Protein concentration was estimated by a standard BCA protein assay (Pierce, Rockford, USA). Aliquots representing 5-40 µg protein were separated by SDS-polyacrylamide gel electrophoresis and transferred to a nylon membrane (PolyScreen polyvinylidene difluoride, NEN, Rodgau-Jugesheim, Germany). After a blocking step, the membrane was incubated with anti-claudin-2 antibodies (for membrane lysates: rabbit anti-claudin-2, 1:1,000, directed against a peptide derived from the C-terminus of human claudin-2, Invitrogen, Carlsbad, USA; for whole cell lysates: rabbit anti-claudin-2, 1:1,000, rabbit anti-claudin-1, −5, and −8, 1:2,000, mouse anti-claudin-4, 1:2,000). Analysis of signaling pathways was performed with commercial phospho-Akt and mitogen-activated protein kinase (MAPK) family antibody sampler kits (Cell Signaling Technology, Frankfurt am Main, Germany). Anti-β-actin antibodies (Sigma or Thermo Fisher Scientific, Fremont, USA) were used for loading controls. Antibody binding was detected with the Lumi-lightPLUS Western Blotting Kit (Roche, Mannheim, Germany) and visualized by employing a LAS-1000 imaging system (Fuji, Japan).
Two-path impedance spectroscopy
Two-path impedance spectroscopy was performed by means of a similar method to that described by Reiter et al. (2006). The epithelial resistance (Re) consisting of two parallel resistors, viz., the paracellular resistance (Rpara) caused by the barrier properties of the tight junction and the transcellular resistance (Rtrans) as determined by the cell membranes, are in series to the subepithelial resistance (Rsub) caused by the filter support. In this circuit, the apical and the basolateral membranes of the confluent cell layer are represented by resistors and capacitors in parallel (Ra, Ca and Rb, Cb, respectively). Ra and Rb add up to Rtrans. After application of alternating current (35 µA/cm2, frequency range 1.3 Hz to 65 kHz), voltage changes were detected by phase-sensitive amplifiers (402 frequency response analyzer, Beran Instruments, Glen Allen, Va., USA; 1286 electrochemical interface; Solartron Schlumberger, Atlanta, Ga., USA). Complex impedance (Zreal, Zimaginary) values were calculated and plotted in a Nyquist diagram, which yields a circular arc as long as the apical and basolateral membranes do not clearly differ (Ra≈Rb and Ca≈Cb); this was true for the experiments described here. Rtrans and Rpara were determined from experiments in which impedance spectra and fluxes of a paracellular marker substance, fluorescein, were obtained before and after chelating extracellular Ca2+ with EGTA. This caused the tight junctions of the system to open partially and to increase the fluorescein flux in an inversely proportional manner to Re changes.
Quantification of claudin-2 mRNA and analysis of mRNA stability
RNA isolation, reverse transcription, and real-time polymerase chain reaction (PCR) was performed as described earlier (Heller et al. 2005). For analysis of mRNA stability, resting HT-29/B6 cells were cultured for 24 h in the presence or absence of TNFα, and RNA transcription was blocked by the addition of 5 µg/ml actinomycin D (Sigma, Schnelldorf, Germany). RNA was isolated 0, 1, 2, 4, and 8 h after actinomycin D addition, reversely transcribed, and quantified by real-time PCR. The amount of claudin-2-specific copies was plotted against time after actinomycin D addition on a logarithmic scale. The half-life (T1/2) of mRNA was calculated from the slope (m) of the linear smoothing function between 2 and 8 h after actinomycin D addition by the equeation T1/2=0.693/λ, with the decay constant λ=2.303 m.
Confocal laser scanning microscopy
Cells were washed after incubation with or without cytokines twice with PBS and fixed for 10 min in ice-cold methanol at −20°C. Subsequently, cells were washed in PBS, permeabilized for 7 min with 0.5% Triton X-100 in PBS, and finally washed in PBS. For the immunodetection of zonula occludens protein 1 (ZO-1), cells were incubated first for 45 min with 2% goat serum in PBS, followed by incubation with a mouse monoclonal anti-ZO-1 antibody (BD Transduction, Heidelberg, Germany) diluted 1:100 in 2% goat serum for 1 h at 37°C. For the immunodetection of claudin-2, a rabbit polyclonal anti-claudin-2 antibody directed against the C-terminus of claudin-2 (Invitrogen, Carlsbad, USA; diluted 1:50,) was used. Cells were washed with 2% goat serum and incubated for 1 h at 37°C with goat anti-mouse IgG conjugated to Alexa-Fluor-488 and goat anti-rabbit IgG conjugated to Alexa-Fluor-594 (Invitrogen, Karlsruhe, Germany; diluted 1:500 in blocking solution). Cells were washed with 2% goat serum, with water, and with 80% ethanol and mounted with ProTaqs MountFluor (Biocyc, Luckenwalde, Germany). Fluorescence images were obtained with a confocal laser scanning microscope (LSM 510 Meta, Carl Zeiss, Jena, Germany).
Generation of stable cell lines and promoter analysis
A 264-bp human genomic DNA fragment encompassing the predicted transcription start point for claudin-2 mRNA was cloned into pGL3-Basic as described previously (Mankertz et al. 2004). Sequences containing the promoter in either the original or inverted direction in combination with the luciferase gene were subcloned into pcDNA5/FRT by using MluI and BamHI, thereby deleting the CMV promoter region. The resulting plasmids were transfected into HT-29/B6 cells prepared for site-directed integration with the Flp-In recombinase system (Invitrogen, Carlsbad, USA). Cells were selected with hygromycin. They were grown to confluence on Millicell-PCF filter supports (4.2 cm2; Millipore, Eschborn, Germany) and analyzed for luciferase expression in the presence or absence of TNFα.
Statistics
Values are given as means ± SEM. The unpaired two-tailed t-test was used to determine the significance of differences unless otherwise noted; P<0.05 was considered significant. The Bonferroni-Holm correction was applied in multiple testing.
Results
Time-dependent action of TNFα on claudin-2 protein expression
To quantify the amount of claudin-2 in TNFα-treated cells, crude membrane fractions were prepared and subjected to immunoblotting with claudin-2 specific antibodies followed by densitometric analysis. Incubation of HT-29/B6 cell monolayers with TNFα (10,000 U/ml) induced an increase in claudin-2 protein, 24 h after cytokine addition (Fig. 1a). During this incubation period, an additional 10-kDa band accumulated, which represented the C-terminal sequences of the claudin-2 protein against which the antibody was raised (Fig. 1a). In densitometric analysis, only the full length 22-kDa protein was considered to represent the functionally active tight-junctional strand component. Densitometry revealed an increase of claudin-2 in HT-29/B6 cells to 159% after 24 h and to 197% after 72 h of treatment with TNFα (n=9 and n=5, respectively, P<0.05 each, Fig. 1b). Parallel measurements confirmed the known (time-dependent) reduction in Re after treatment with TNFα in this set of data (data not shown here, since the functional response to TNFα is given in more detail below by using two-path impedance spectroscopy).
Dose-dependent action of TNFα on claudin-2 expression
TNFα increased claudin-2 expression in a dose-dependent manner (Fig. 2a). In addition to the 22-kDa claudin-2 signal, a 10-kDa cleavage product was induced, which was also observed, after the addition of interferon-γ (IFNγ; Fig. 2a). The induction of bands with identical molecular weights by TNFα and IFNγ is direct evidence that both cytokines induce the same cleavage product. The 10-kDa cleavage product was previously characterized by Willemsen et al. (2005) and interpreted as being the result of epithelial apoptosis (Bojarski et al. 2004; see also Discussion). The appearance of this 10-kDa fragment was also dose-dependent. However, this cleavage product was not induced at the lower TNFα dose of 500 U/ml, whereas the up-regulation of the full length 22-kDa claudin-2 signal persisted (Fig. 2b). This allowed us to discriminate between the functional consequences of both TNFα-dependent phenomena, namely to perform a functional analysis restricted to the changes in the up-regulation of the full-length 22-kDa claudin-2 signal (Fig. 2c).
Effect of TNFα and LY294002 on paracellular resistance of HT-29/B6 cells
To examine the effect of TNFα on components of the transepithelial electrical resistance in more detail, two-path impedance spectroscopy was applied, which also allowed us to determine the paracellular (tight-junctional) resistance (Fig. 3). A decrease of about three-fold in epithelial resistance was observed after treatment with 500 U/ml TNFα (Re; control: 389±19 Ω·cm2, n=6; TNFα: 123±12 Ω·cm2, n=5; P<0.001). This had its origin in a nine-fold decrease of the paracellular resistance (Rpara; control: 1824±546 Ω·cm2; TNFα: 204±37 Ω·cm2; P<0.05), whereas no significant change was observed in the transcellular resistance (Rtrans; control: 549±28 Ω·cm2; TNFα: 381±60 Ω·cm2). At 500 U/ml, TNFα no longer induced apoptosis, as judged from TUNEL assays (data not shown).
Pre-treatment with the PI3K inhibitor LY294002 (n=7) caused an increase in Rpara (Ly+TNFα: 464±90 Ω·cm2; P<0.05), which could only be identified with two-path impedance spectroscopy, whereas the TNFα treatment did not significantly affect Re (Ly+TNFα: 142±16 Ω·cm2). Treatment of HT-29/B6 cells with LY294002 alone (n=9) resulted in a strong increase of Rpara (LY: 7422±1769 Ω·cm2; P<0.05), again without altering Re (LY: 486±51 Ω·cm2).
Distribution of claudin-2 protein in TNFα-treated HT-29/B6 cells
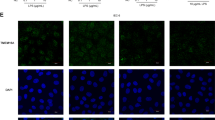
To characterize the effect of TNFα on epithelial tight junctions, HT-29/B6 cells were examined by confocal laser scanning microscopy (CLSM) with antibodies against the channel-forming tight-junctional protein claudin-2 and against ZO-1, a tight-junction-associated protein that interacts as a scaffold protein with claudins and the cellular cytoskeleton (PDZ domain protein). CLSM Z-scans allowing the characterization of the localization of a protein along the apical to basal axis of the cell were performed to analyze the distribution of tight-junctional proteins. In Z-scans of HT-29/B6 control cells, both ZO-1 and claudin-2 were exclusively localized at tight junctions (Fig. 4a). Thus, no change in subcellular protein localization was detected after treatment with TNFα (Fig. 4b).
Cytokine-dependent expression and stability of claudin-2-specific mRNA
For assessing transcriptional regulation, claudin-2-specific mRNA was quantified by real-time PCR. In HT-29/B6 cell monolayers, TNFα treatment led to an increase of claudin-2-specific mRNA (163±14%, n=8, P<0.01, Fig. 5a), which correlated with the elevation of claudin-2 protein (Fig. 1b). In order to elucidate whether the rise in claudin-2 mRNA in TNFα-treated cells depended on enhanced transcription rates and/or improved mRNA stability, the mRNA half-life was determined in the absence or presence of TNFα. For this purpose, transcription was blocked at different time points with actinomycin D, and claudin-2 mRNA was quantified by real-time PCR. However, the half-life was not significantly changed in TNFα-treated HT-29/B6 cells (2.1±0.2 h, n=3) when compared with that of untreated control cells (2.6±0.5 h, n=3, Fig. 5b). Thus, the elevation of claudin-2-specific mRNA after TNFα treatment was attributable to increased transcriptional activity and was not caused by altered mRNA stability.
Influence of TNFα on claudin-2 promoter activity
Since the analysis of mRNA expression had indicated transcriptional up-regulation by TNFα, claudin-2 promoter activity was subsequently investigated by means of reporter gene assays. Claudin-2 promoter sequences in either the regular or inverse orientation to the luciferase reporter gene were stably transfected into HT-29/B6 cells by site-specific recombination to ensure identical integration (Fig. 6a). Quantification of luciferase enzyme activity in extracts from these cells showed much greater luciferase expression when the promoter was cloned in the regular orientation compared with the inverse orientation indicating promoter specificity. Promoter activity was enhanced after treatment with TNFα (Fig. 6b).
Influence of TNFα on claudin-2 promoter activity. a Claudin-2 promoter sequence inserted in either the regular (P) or inverse (d) orientation to the luciferase reporter gene (luc). Orientation is indicated by arrows (CLDN2+ regular, CLDN2- inverse). b Quantification of luciferase enzyme activity as an indication of claudin-2 promoter activity. **P<0.05, ***P<0.001
Signaling pathways involved in TNFα-dependent claudin-2 expression
The increase of claudin-2 protein expression after treatment with TNFα was inhibited by pre-incubation with LY294002, an inhibitor of PI3K (28±6% compared with TNFα-treated cells, n=4, P<0.01, Fig. 7a; for densitometry, see Fig. 7e). This inhibitory action of LY294002 was absent or much less pronounced for other claudins. The induction of PI3K signaling was confirmed by the time-dependent phosphorylation of Akt (a downstream component of the PI3K pathway), which was also blocked by LY294002 (Fig. 7b). In contrast, ML-7, an inhibitor of MLCK signaling, was not able to prevent TNFα-dependent claudin-2 expression (83±6% compared with TNFα-treated cells, n=4, not significant, Fig. 7a. Moreover, the inhibition of MEK1/2 and JNK, which play a central role in MAPK signaling cascades, also did not prevent the induction of claudin-2 expression by TNFα (73±6%, n=4, not significant, and 173±27%, n=4, not significant, respectively, compared with TNFα-treated cells, Fig. 7c; for densitometry, see Fig. 7e). As far as the extracellular signal-regulated kinase 1/2 (ERK1/2) and JNK are concerned, activation by TNFα and the specificity of inhibition could be confirmed by the phosphorylation status of the kinases (Fig. 7d).
Signaling pathways involved in TNFα-dependent claudin-2 expression. a Expression of various claudins nd actin after treatment with TNFα but without (ctrl control) or with pre-incubation with LY294002 (Ly), an inhibitor of PI3K. The inhibitory action of LY294002 is absent or much less pronounced for claudins other than for claudin-2. ML-7 (ML), an inhibitor of myosin light chain kinase signaling, had no effect on TNFα-dependent claudin-2 expression. b Confirmation of induction of PI3K signaling by time-dependent phosphorylation of Akt after treatment with TNFα. Note the inhibition of Akt phosphorylation by LY294002 (Ly). c The MEK1/2 inhibitor (U0126) and the c-Jun N-terminal kinase inhibitor (SP600125) had no effect on the induction of claudin-2 expression by TNFα. d Phosphorylation status of extracellular signal-regulated kinase (ERK1/2) and c-Jun N-terminal kinase (JNK) after activation by TNFα in the presence and absence of the inhibitors U0126 and SP600125. e Densitometry showing inhibition of TNFα-induced increase of claudin-2 protein expression by pre-incubation with LY294002. However, inhibition of MEK1/2 by U0126 does not prevent induction of claudin-2 expression by TNFα, although U0126 does slightly inhibit claudin-2 expression in the absence of TNFα. ### P<0.001 compared with control, ***P<0.01 compared with TNFα-treated cells, ## P<0.01 compared with TNFα-treated cells
Discussion
TNFα increases claudin-2 expression in epithelial cells and disturbs epithelial barrier function
Our first relevant finding is that TNFα time- and dose-dependently increases the claudin-2 protein level. However, we have recently shown that this might also be provoked by the Th2-cytokine IL-13 (Heller et al. 2005) suggesting a role for both cytokines in the regulation of claudin-2 expression during intestinal inflammation.
In addition to changing the expression of the full-length 22-kDa claudin-2, treatment of HT-29/B6 cells with higher TNFα doses (e.g., 10,000 U/ml) leads to the generation of a 10-kDa fragment, as detected by an antibody directed against the C-terminus of claudin-2. In a study of T84 cells, the generation of a claudin-2-specific subfragment after incubation with IFNγ has been attributed to the intracellular cleavage of claudin-2 (Willemsen et al. 2005). Since this fragment is functionally not competent, it has not been included in the quantitative analysis of claudin-2 immunoreactivity in our study here. Interestingly, we have previously shown, in MDCK cells, that TNFα can induce the cleavage of claudin-2 and of another tight-junctional protein, namely of occludin (Bojarski et al. 2004). This can be reduced by caspase and metalloproteinase inhibitors indicating an involvement of cytokine-dependent apoptotic pathways. We therefore hypothesize that TNFα at higher doses not only induces claudin-2, but also epithelial apoptosis or at least cell damage with the consequent appearance of the 10-kDa claudin-2 cleavage product.
In general, TNFα treatment is accompanied by a decrease in transepithelial resistance (Mankertz et al. 2000; Schmitz et al. 1999); this can be explained by transcellular and/or paracellular mechanisms including the induction of epithelial apoptoses (Gitter et al. 2000). One possible explanation for this emerges from the increased expression of claudin-2, which forms cation-selective channels in the tight junction (Amasheh et al. 2002). Thus, without further information, the decrease in transepithelial resistance after TNFα treatment cannot be assigned a priori to up-regulated claudin-2 expression.
TNFα decreases the paracellular resistance of HT-29/B6 cells
Two-path impedance spectroscopy was applied to characterize functional changes in response to TNFα in more detail. This revealed that the TNFα-induced change in epithelial resistance was attributable to a dramatic decrease in paracellular resistance (Rpara) and hence to resistance changes of the tight junction. Therefore, we can reasonably conclude that the increased expression of claudin-2 observed in the present study contributes to the decrease in Rpara presented in Fig. 3.
Since TNFα is known to activate the PI3K pathway with its downstream target Akt (Ozes et al. 1999), additional measurements for TNFα effects were performed after a 2-h treatment with the PI3K inhibitor LY294002. Two-path impedance spectroscopy revealed that the decrease in paracellular resistance (Rpara) induced by TNFα was attenuated by LY294002. Re did not parallel the increase in Rpara (Fig. 3), a result that is probably caused by the induction of cell toxicity/apoptosis by LY294002 (Leverrier et al. 1999).
TNFα does not alter subcellular claudin-2 localization
In addition to a change in the claudin-2 expression level that might affect barrier function, tight-junctional molecules can also undergo specific functional regulation by changes in their subcellular distribution. Another proinflammatory cytokine, IFNγ, has been shown to induce protein sorting of the tight junction toward endosomal compartments in T84 cells (Bruewer et al. 2005). The internalization of tight-junctional proteins after exposure to IFNγ relies on a macropinocytosis-like process and results in the formation of vacuolar apical compartments (VACs) possibly driven by myosin-II-mediated contraction. VAC formation can be prevented by the inhibition of Rho-associated protein kinase (ROCK), indicating a role of Rho/ROCK signaling in IFNγ-induced VAC formation (Utech et al. 2005). Another study has suggested that the disorganization of the tight junction and the increase in paracellular permeability after IFNγ treatment of T84 cells is attributable to a perturbation of apical actin organization and a decrease of ZO-1 expression (Youakim and Ahdieh 1999). In addition, evidence has been provided, in Caco-2 cells, for a barrier effect of TNFα and IFNγ by transcriptional activation and phosphorylation of MLCK via TNF receptor type 2 leading to cytoskeletal tight-junction dysregulation (Wang et al. 2006).
Therefore, we extended our study by analyzing the subcellular localization of claudin-2 after exposure to TNFα with respect to the tight-junction domain by using immunohistochemistry and CLSM. Importantly, the subcellular localization of claudin-2 was not altered by TNFα, i.e., the claudin-2 signal was exclusively detected in the tight junction and not intracellularly. This suggests that the TNFα-induced up-regulation of expression leads subsequently to an enhanced recruitment of claudin-2 into the tight-junction domain.
TNFα induces genomic regulation of claudin-2 gene expression
As the key finding of the present study in HT-29/B6 cells, the mechanism of claudin-2 up-regulation by TNFα can be attributed to transcriptional activation, since claudin-2-specific mRNA levels increase during TNFα treatment with no difference in mRNA stability. To investigate cytokine effects on claudin-2 gene expression under the same conditions as those employed for the analysis of protein and mRNA expression, reporter gene assays were performed in HT-29/B6 cells stably transfected with the claudin-2 promoter joined to the luciferase gene. In accordance to the results from protein and mRNA analysis, gene expression mediated by the claudin-2 promoter increased after incubation with TNFα indicating the genomic regulation of claudin-2 expression.
Signaling pathways for claudin-2 expression
A number of signal transduction pathways have been reported to be involved in the genomic regulation of claudin-2. These include factors that control tissue-specific cell differentiation via GATA-4, hepatocyte nuclear factor-1α, and caudal-related homeodomain (Cdx) proteins (Escaffit et al. 2005; Sakaguchi et al. 2002). Moreover, functional crosstalk between Cdx-related transcriptional activation and Wnt-signaling has been reported by our group in the regulation of gene expression mediated by the claudin-2 promoter (Mankertz et al. 2004).
However, less information is available on the influence of pro-inflammatory cytokines on claudin-2 expression. In a recent study, the down-regulation of claudin-2 protein expression and altered tight-junction protein localization after the treatment of MDCK CCL-34 cells with a combination of TNFα and IFNγ has been attributed to the activation of ERK1/2, essential components of the MAPK signaling pathway (Patrick et al. 2006). This has been corroborated by investigating MDCK II cells, which form a “leaky” epithelium, and which have endogenously low ERK1/2 activity. Constitutive activation of ERK1/2 inhibits claudin-2 expression and increases transepithelial electrical resistance (Lipschutz et al. 2005). In contrast, our data indicate an up-regulation, rather than a down-regulation, of claudin-2 expression by TNFα in human intestinal HT-29/B6 cells, which form a moderately tight epithelium; this response also represents the scenario seen in chronically inflamed colonic mucosa (Prasad et al. 2005; Zeissig et al. 2007). Most probably, this discrepancy reflects different claudin-2 regulation patterns in the kidney and intestine.
Our analysis of other signaling pathways has revealed that neither inhibitors of MEK1/2 and JNK, which are essential components of MAPK signaling, nor inhibition of MLCK, which has also been described to be involved in TNFα-dependent barrier regulation (Clayburgh et al. 2006; Ye et al. 2006), can prevent claudin-2 up-regulation in HT-29/B6 cells indicating that MAPK or MLCK signaling is less relevant for the transcriptional regulation of claudin-2. However, Kinugasa and coworkers (2000) have described that MAPK signaling via ERK can increase claudin-2 mRNA levels in T84 cells. We should mention at this point that we have also observed a tendency toward a reduction of claudin-2 expression by ERK1/2 inhibition with U0126 in our cell model. To avoid unspecific effects, the U0126 concentration used in our study was only 5 mM and thus lower than the 10 mM used by Reinecker’s group (Kinugasa et al. 2000). Taken together, an additional influence of ERK signaling is possible, although this effect seems to be inferior to the inhibitory effect of PI3K/Akt signaling on claudin-2 expression, since the up-regulation of claudin-2 is completely blockable by LY294002 in HT-29/B6 cells.
The PI3K/Akt pathway is known to be important for various cellular functions including intestinal cell proliferation and survival. Furthermore, there is growing evidence that PI3K and its downstream target Akt regulate immune responses and inflammation in response to TNFα (Ozes et al. 1999). In particular, the epithelial tight junction can also be affected by the PI3K signaling pathway (for a review, see Gonzalez-Mariscal et al. 2008). The involvement of PI3K/Akt signaling in TNFα-dependent tight-junction regulation via claudin-2 expression is thus another novel finding of our study. Interestingly, a recent paper has shown that toll-like receptor 2 (TLR2) controls mucosal inflammation by regulating epithelial barrier function, and that stimulation of TLR2 protects tight-junction-associated barrier assembly in intestinal epithelial cells by functional activation of the PI3K/Akt pathway (Cario et al. 2007). Thus, different stimuli can lead to the PI3K/Akt-mediated regulation of epithelial barrier properties further supporting the importance of this regulatory pathway for intestinal barrier function.
Functional implications of claudin-2 expression regulation
Enhanced claudin-2 expression represents a protective mechanism by which the epithelium can help to rinse off noxious luminal agents including bacteria and antigens from its surface. This is assumed to be the consequence of increased claudin-2-mediated passive (paracellular) ion permeability, which facilitates the flow of ions and water from subepithelial capillaries to the intestinal lumen. On the other hand, this phenomenon might also contribute to diarrhea by a leak flux mechanism caused by an impaired epithelial barrier and an increased back-leak of ions and water into the intestinal lumen. The latter mechanism has, for example, been identified as being responsible for the diarrhea induced by zonula occludens toxin, thereby leading to a reversible impairment of tight-junctional structure and function (Fasano et al. 1991).
Implications for intestinal inflammation
Several recent studies have focused on altered barrier function during active IBD (for a review, see Mankertz and Schulzke 2007). In Crohn’s disease, barrier dysfunction is associated with a release of TNFα and IFNγ, whereas in ulcerative colitis, the Th2-cytokine IL-13 (acting in concert with TNFα) represents the key effector cytokine. In both Crohn’s disease and to an even greater extent in ulcerative colitis, barrier dysfunction is accompanied by an up-regulation of claudin-2 expression (Heller et al. 2005; Zeissig et al. 2007). The increase of claudin-2 protein can be provoked by TNFα. However, a similar effect has also been shown for IL-13 (Heller et al. 2005). The 3.5-fold higher claudin-2 expression in ulcerative colitis than in Crohn’s disease (Zeissig et al. 2007) on the other hand might be the result of a TNFα synergism with IL-13 in ulcerative colitis and a TNFα antagonism with IFNγ in Crohn’s disease.
References
Amasheh S, Meiri N, Gitter AH, Schöneberg T, Mankertz J, Schulzke JD, Fromm M (2002) Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J Cell Sci 115:4969–4976
Bojarski C, Weiske J, Schöneberg T, Schröder W, Mankertz J, Schulzke JD, Florian P, Fromm M, Tauber R, Huber O (2004) The specific fates of tight junction proteins in apoptotic epithelial cells. J Cell Sci 117:2097–2107
Bruewer M, Utech M, Ivanov AI, Hopkins AM, Parkos CA, Nusrat A (2005) Interferon-γ induces internalization of epithelial tight junction proteins via a macropinocytosis-like process. FASEB J 19:923–933
Cario E, Gerken G, Podolsky DK (2007) Toll-like receptor 2 controls mucosal inflammation by regulating epithelial barrier function. Gastroenterology 132:1359–1374
Clayburgh DR, Musch MW, Leitges M, Fu YX, Turner JR (2006) Coordinated epithelial NHE3 inhibition and barrier dysfunction are required for TNF-mediated diarrhea in vivo. J Clin Invest 116:2682–2694
Escaffit F, Boudreau F, Beaulieu JF (2005) Differential expression of claudin-2 along the human intestine: implication of GATA-4 in the maintenance of claudin-2 in differentiating cells. J Cell Physiol 203:15–26
Fasano A, Baudry B, Pumplin DW, Wasserman SS, Tall BD, Ketley JM, Kaper JB (1991) Vibrio cholerae produces a second enterotoxin, which affects intestinal tight junctions. Proc Natl Acad Sci USA 88:5242–5246
Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S (1993) Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol 123:1777–1788
Furuse M, Fujita K, Hiiragi T, Fujimoto K, Tsukita S (1998) Claudin-1 and -2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J Cell Biol 141:1539–1550
Furuse M, Furuse K, Sasaki H, Tsukita S (2001) Conversion of zonulae occludentes from tight to leaky strand type by introducing claudin-2 into Madin-Darby canine kidney I cells. J Cell Biol 153:263–272
Furuse M, Hata M, Furuse K, Yoshida Y, Haratake A, Sugitani Y, Noda T, Kubo A, Tsukita S (2002) Claudin-based tight junctions are crucial for the mammalian epidermal barrier: a lesson from claudin-1-deficient mice. J Cell Biol 156:1099–1111
Fuss IJ, Neurath M, Boirivant M, Klein JS, de la Motte C, Strong SA, Fiocchi C, Strober W (1996) Disparate CD4+ lamina propria (LP) lymphokine secretion profiles in inflammatory bowel disease. Crohn’s disease LP cells manifest increased secretion of IFN-gamma, whereas ulcerative colitis LP cells manifest increased secretion of IL-5. J Immunol 157:1261–1270
Gitter AH, Bendfeldt K, Schulzke JD, Fromm M (2000) Leaks in the epithelial barrier caused by spontaneous and TNF-α-induced single-cell apoptosis. FASEB J 14:1749–1753
Gonzalez-Mariscal L, Tapia R, Chamorro D (2008) Crosstalk of tight junction components with signaling pathways. Biochim Biophys Acta 1778:729–756
Heller F, Florian P, Bojarski C, Richter J, Christ M, Hillenbrand B, Mankertz J, Gitter AH, Bürgel N, Fromm M, Zeitz M, Fuss I, Strober W, Schulzke JD (2005) Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology 129:550–564
Ikenouchi J, Furuse M, Furuse K, Sasaki H, Tsukita S (2005) Tricellulin constitutes a novel barrier at tricellular contacts of epithelial cells. J Cell Biol 171:939–945
Inai T, Kobayashi J, Shibata Y (1999) Claudin-1 contributes to the epithelial barrier function in MDCK cells. Eur J Cell Biol 78:849–855
Kinugasa T, Sakaguchi T, Gu X, Reinecker HC (2000) Claudins regulate the intestinal barrier in response to immune mediators. Gastroenterology 118:1001–1011
Kreusel KM, Fromm M, Schulzke JD, Hegel U (1991) Cl- secretion in epithelial monolayers of mucus-forming human colon cells (HT-29/B6). Am J Physiol 261:C574–C582
Leverrier Y, Thomas J, Mathieu AL, Low W, Blanquier B, Marvel J (1999) Role of PI3-kinase in Bcl-X induction and apoptosis inhibition mediated by IL-3 or IGF-1 in Baf-3 cells. Cell Death Differ 6:290–296
Lipschutz JH, Li S, Arisco A, Balkovetz DF (2005) Extracellular signal-regulated kinases 1/2 control claudin-2 expression in Madin-Darby canine kidney strain I and II cells. J Biol Chem 280:3780–3788
Mankertz J, Schulzke JD (2007) Altered permeability in inflammatory bowel disease: pathophysiology and clinical implications. Curr Opin Gastroenterol 23:379–383
Mankertz J, Tavalali S, Schmitz H, Mankertz A, Riecken EO, Fromm M, Schulzke JD (2000) Expression from the human occludin promoter is affected by tumor necrosis factor alpha and interferon gamma. J Cell Sci 113:2085–2090
Mankertz J, Hillenbrand B, Tavalali S, Huber O, Fromm M, Schulzke JD (2004) Functional crosstalk between Wnt signaling and Cdx-related transcriptional activation in the regulation of the claudin-2 promoter activity. Biochem Biophys Res Commun 314:1001–1007
Martin-Padura I, Lostaglio S, Schneemann M, Williams L, Romano M, Fruscella P, Panzeri C, Stoppacciaro A, Ruco L, Villa A, Simmons D, Dejana E (1998) Junctional adhesion molecule, a novel member of the immunoglobulin superfamily that distributes at intercellular junctions and modulates monocyte transmigration. J Cell Biol 142:117–127
Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM, Donner DB (1999) NF-kappaB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature 401:82–85
Patrick DM, Leone AK, Shellenberger JJ, Dudowicz KA, King JM (2006) Proinflammatory cytokines tumor necrosis factor-alpha and interferon-gamma modulate epithelial barrier function in Madin-Darby canine kidney cells through mitogen activated protein kinase signaling. BMC Physiol 6:2
Prasad S, Minigrino R, Kaukinen K, Hayes KL, Powell RM, MacDonald TT, Collins JE (2005) Inflammatory processes have differential effects on claudins 2, 3 and 4 in colonic epithelial cells. Lab Invest 85:1139–1162
Rahner C, Mitic LL, Anderson JM (2001) Heterogeneity in expression and subcellular localization of claudins 2, 3, 4, and 5 in the rat liver, pancreas, and gut. Gastroenterology 120:411–422
Reiter B, Kraft R, Günzel D, Zeissig S, Schulzke JD, Fromm M, Harteneck C (2006) TRPV4-mediated regulation of epithelial permeability. FASEB J 20:1802–1812
Sakaguchi T, Gu X, Golden HM, Suh E, Rhoads DB, Reinecker HC (2002) Cloning of the human claudin-2 5′-flanking region revealed a TATA-less promoter with conserved binding sites in mouse and human for caudal-related homeodomain proteins and hepatocyte nuclear factor-1alpha. J Biol Chem 277:21361–21370
Schmitz H, Fromm M, Bentzel CJ, Scholz P, Detjen K, Mankertz J, Bode H, Epple HJ, Riecken EO, Schulzke JD (1999) Tumor necrosis factor-alpha (TNF α) regulates the epithelial barrier in the human intestinal cell line HT-29/B6. J Cell Sci 112:137–146
Tsukita S, Furuse M, Itoh M (2001) Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol 2:285–293
Utech M, Ivanov AI, Samarin SN, Bruewer M, Turner JR, Mrsny RJ, Parkos CA, Nusrat A (2005) Mechanism of IFN-γ-induced endocytosis of tight junction proteins: myosin II-dependent vacuolarization of the apical plasma membrane. Mol Biol Cell 16:5040–5052
Van Itallie CM, Anderson JM (2006) Claudins and epithelial paracellular transport. Annu Rev Physiol 68:403–429
Wang F, Schwarz BT, Graham WV, Wang Y, Su L, Clayburgh DR, Abraham C, Turner JR (2006) IFN-gamma-induced TNF receptor 2 expression is required for TNF-dependent intestinal barrier dysfunction. Gastroenterology 131:1153–1163
Willemsen LE, Hoetjes JP, Deventer SJ van, Tol EA van (2005) Abrogation of IFN-gamma mediated epithelial barrier disruption by serine protease inhibition. Clin Exp Immunol 142:275–284
Ye D, Ma I, Ma TY (2006) Molecular mechanism of tumor necrosis factor-alpha modulation of intestinal epithelial tight junction barrier. Am J Physiol Gastrointest Liver Physiol 290:G496–G504
Youakim A, Ahdieh M (1999) Interferon-gamma decreases barrier function in T84 cells by reducing ZO-1 levels and disrupting apical actin. Am J Physiol 276:G1279–G1288
Zeissig S, Bürgel N, Günzel D, Richter JF, Mankertz J, Wahnschaffe U, Kroesen AJ, Zeitz M, Fromm M, Schulzke JD (2007) Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn’s disease. Gut 56:61–72
Acknowledgements
We thank Nicole Held and Susanna Schön for excellent technical assistance. The support of Detlef Sorgenfrei (electronic engineer) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
J. Mankertz and M. Amasheh contributed equally to this work.
This work was supported by the Deutsche Forschungsgemeinschaft and the Sonnenfeld-Stiftung Berlin.
Rights and permissions
About this article
Cite this article
Mankertz, J., Amasheh, M., Krug, S.M. et al. TNFα up-regulates claudin-2 expression in epithelial HT-29/B6 cells via phosphatidylinositol-3-kinase signaling. Cell Tissue Res 336, 67–77 (2009). https://doi.org/10.1007/s00441-009-0751-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-009-0751-8