Abstract
The worldwide prevalence of type 2 diabetes mellitus (T2DM) is constantly increasing, and it has become a major concern, with several implications for public health, economy, and social well-being. It is well-known that several factors such as lifestyle, increased intake of fat and sugar-rich foods, and host genetics can lead to T2DM. Some recent studies have suggested that the composition of the intestinal microbiota can trigger T2DM. Since then, considerable effort has been made to understand the link between the composition of intestinal microbiota and T2DM, as well as the role of probiotics in modulation of intestinal microbiota. This mini-review summarizes the major findings and discusses the close relationship between intestinal microbiota, probiotics, and T2DM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes is a chronic disease caused by inherited or acquired deficiency in the production of insulin by the pancreas or the inability of the body to make adequate use of the insulin produced (WHO 2016). Diabetes is a chronic condition, and affected individuals must routinely manage their lifestyle (ADA 2009; Ahola et al. 2017).
Diabetes affects more than 420 million people worldwide and this number may continue to increase in the future (WHO 2016). It is predicted that about 630 million people will be affected by the illness worldwide by the year 2045 (IDF 2017). In this context, diabetes is a global concern which strongly impacts public healthcare expenditures with an estimated cost of $827 billion worldwide (Seuring et al. 2015).
The three main types of diabetes are type 1, type 2, and gestational diabetes; however, there are some other types of diabetes such as autoimmune latent in adults (LADA), for example. Type 1 diabetes (also called insulin-dependent, juvenile, or childhood onset) is characterized by low insulin production and requires daily administration of this hormone. The cause of type 1 diabetes is still unknown and cannot be prevented with current knowledge (WHO 2016). Diabetes LADA is a special subtype of type 1 diabetes and it is characterized by slow β cell damage in the islets (Xiang et al. 2015). Therefore, patients with LADA usually show early signs that mimic type 2 diabetes, resulting in a non-negligible diagnostic rate. In fact, it has been estimated that the incidence of LADAs is about 6% among newly diagnosed patients with type 2 diabetes (Martinell et al. 2016).
Maturity-onset diabetes of the young (MODY) is a subtype of diabetes, characterized by early onset (usually under 25 years of age) and autosomal dominant transmission (determined in at least three generations). It corresponds to a primary defect in insulin secretion associated with pancreatic β cell dysfunction (Nyunt et al. 2009). Another type of diabetes, the gestational diabetes, is characterized by hyperglycemia (increased blood sugar) that appears during pregnancy and reaches values that, although higher than normal, are lower than those established for the diagnosis of diabetes. Women with gestational diabetes have a higher risk of complications during pregnancy and delivery. In addition, they and their children are at greater risk of developing type 2 diabetes in the future (WHO 2016).
The T2DM is a metabolic disorder characterized by high blood glucose that results from a combination of insufficient insulin secretion and insulin resistance (Asemi et al. 2013). Although T2DM is most commonly diagnosed in older adults, the incidence of this pathology has been increasingly observed in children, adolescents, and young adults due to increasing levels of obesity, physical inactivity, and poor diet (IDF 2017). The factors and mechanisms that trigger T2DM have been intensely discussed, and the major risk factors are genetic factors, high caloric intake, and physical inactivity (Lyssenko et al. 2008).
Obesity is one of the most important triggers for T2DM diabetes development. Obesity is a complex condition that is explained by risk factors, such as total energy expenditure, level of physical activity, food intake, genetics, socioeconomic status, or level of education, in addition to an unconventional but highly studied factor, which is the intestinal microbiota, which has been related to obesity (Dugas et al. 2018). There are differences between the composition of the intestinal microbiota of thin and obese individuals, so a correlation between obesity and the composition of the microbiota was observed and suggested (Peters et al. 2018). The majority of studies have found a higher proportion of Firmicutes phylum and a lower amount of Bacteroidetes in obese microbiota when compared to the lean (Schwiertz et al. 2010; Bervoets et al. 2013). However, according to recent revision about obesity and microbiota, the Firmicutes/Bacteroidetes ratio can change depending on the obese population under study, and therefore, a more detailed study of the intestinal microbiome, covering bacterial families, genera, and species, is required for a better understanding of the relationship between obesity and the gut microbiota (Bianchi et al. 2018).
Therefore, exercise is often prescribed for weight loss and maintenance. Some evidence suggests that chronic exercise usually causes partial, but incomplete, compensation for energy intake, and this is likely to be due to beneficial changes in appetite-regulating hormones (Stensel 2010). It is worth noting that the type of physical activity has a different impact on the intestinal microbiota, it has been reported that some moderate intensity exercises reduce intestinal transit (time) and increase the diversity of the microbiota (Evans et al. 2014; Campbell et al. 2016), while strenuous (prolonged) exercises may increase bowel permeability resulting in bacterial translocation of the colon, diarrhea, and gastrointestinal bleeding (Martin 2011). According to Matsumoto et al. (2008), he showed that voluntary physical exercise can stimulate species of butyrate-producing bacteria and, consequently, the production of AGCC (n-butyrate).
The intestinal microbiota profile may be associated with specific dietary patterns and responds to diet (David et al. 2014). Thus, beneficial microbes, such as probiotics and their metabolites, modify the microbiota profile and consequently influence metabolic parameters, such as the improvement of insulin sensitivity (Asemi et al. 2013). Probiotics are living microorganisms that, when administered in adequate amounts, confer benefits to an individual’s health (Hill et al. 2014). Two of the main probiotic strains that are advantageous to health include Lactobacillus and Bifidobacterium. However, others microorganisms, such as yeast, can be used as probiotic. The good example is S. cerevisiae also known as S. cerevisiae var. boulardii (Edwards-Ingram et al. 2007; Bernaola et al. 2010).
In this context, the impact of probiotic microorganisms on T2DM acquires special interest, since strategies aimed at the use of probiotics can alter the microbial balance in favor of the host. Thus, the goal of this review is to discuss the use of probiotics and their impact on the microbiota and on major biomarkers as a strategy for the prevention of T2DM as well as to prescribe probiotic use for the amelioration of illness progression.
Relationship of intestinal microbiota with T2DM
The intestinal microbiota, often referred to as a hidden organ harboring trillions of microorganisms, are arguably as important to the metabolic health of the host as the organs that sustain them (Patterson et al. 2016). The adult intestine has approximately 500–1000 different bacterial species and may have 1012–1014 microorganisms with a mass weight of about 1–2 kg (Blaut and Clavel 2007). Metagenomic studies have revealed that approximately 90% of the bacterial species present in the adult intestine belong to the phyla of Bacteroidetes and Firmicutes. In addition, other phyla such as Actinobacteria, Proteobacteria, and Verrucomicrobia are found in low abundance (HUMAN MICROBIOME PROJECT C 2012a, 2012b; Kalinkovich and Livshits 2019). Depending on the anatomy, abiotic environment, and diverse functions of different parts of the intestine, the microbial composition may vary (Blaut and Clavel 2007). The gut microbiota is characterized by a significant interpersonal variability and depends on differences attributed to genetics, diet, lifestyle, health status, and hygiene (Kalinkovich and Livshits 2019).
A healthy gut commensal microbiota is beneficial to the host, and it is linked with vital activities, such as digestion, harvesting energy from food components, xenobiotic degradation, production of water-soluble vitamins, and production of metabolites. These vital activities can promote intestinal barrier integrity, support the functional capacity of the gut epithelium, and provide protection from pathogens (van de Wiele et al. 2016).
On the other hand, dysbiosis is a disruption of the host-microbiota equilibrium due to gut inflammation, use of antibiotics, stress, menopause, toxin, and others triggers (Hegde et al. 2018). In addition, dysbiosis has been linked with a range of disorders such as cardiovascular (Battson et al. 2018) and autoimmune disorders (Opazo et al. 2018), autism (Sgritta et al. 2019), obesity (Bianchi et al. 2018), and T2DM (Karlsson et al. 2013), among others.
T2DM may be linked to the composition of the intestinal microbiota and is directly responsible for the induction of low-grade inflammation. Further, the composition of the intestinal microbiota plays a significant role in the development of pre-diabetic conditions, such as insulin resistance (Roager et al. 2017). In this context, studies on the characterization of the intestinal microbiota of individuals with T2DM as well as the evaluation of possible correlations between the abundance of certain microorganisms and metabolic aspects are fundamental to clarify and strengthen the role of the microbiota in this clinical condition (Sabatino et al. 2017).
According to Sabatino et al. (2017), the main characteristics of the microbiota of T2DM patients are reduced butyrate-producing bacteria (especially Roseburia intestinalis and Faecalibacterium prausnitzii); moderate dysbiosis; pro-inflammatory environment with increased expression of microbial genes involved in oxidative stress, reduced expression of genes involved in vitamin synthesis, and increased serum LPS concentration; and increased intestinal permeability.
In addition, the major alterations in the intestinal microbiota that are associated with T2DM include a significantly lower prevalence of Firmicutes and an enrichment of Bacteroidetes and Proteobacteria (Roager et al. 2017). In terms of marker species in the microbiota of T2DM patients, some studies have observed a high number of opportunistic pathogens, such as Clostridium clostridioforme, Bacteroides caccae, Clostridium hathewayi, Clostridium ramosum, Clostridium symbiosum, Eggerthella sp., and Escherichia coli (Larsen et al. 2010; Karlsson et al. 2013). Larsen et al. (2010) showed that Betaproteobacteria family was highly enriched in T2DM patients compared to non-diabetic individuals.
Dysbiosis in T2DM patients, caused by the interaction of the intestinal microbiota with environmental and genetic factors, leads to increased intestinal permeability and altered mucosal immune response, which may result in the development or worsening of T2DM (Razmpoosh et al. 2018). It is important to highlight the interactions between the microbiota and the immune system since factors hampering these interactions can lead to metabolic disturbances. The lipopolysaccharide (LPS) of Gram-negative bacteria can stimulate the inactive immune system by activating toll-like receptors and inducing the release of inflammatory cytokines. Further, LPS promotes the activation of the nuclear factor kappa-B and c-Jun N-terminal kinase pathways, both of which are linked to the development of insulin resistance and the deficiency of insulin signaling in the muscle, adipose tissue, liver, and hypothalamus (White 2002; Caricilli and Saad 2013; Newsholme et al. 2016).
It is important to highlight that the gut microbiota is also responsible to produce and contribute to energy by short chain fat acids (SCFA) production, which involves the anaerobic breakdown of dietary fiber, protein, and peptides. The most produced by colonic bacteria are acetate, propionate, and butyrate (Baxter et al. 2019). Acetate and propionate are mostly produced by Bacteroidetes phylum, while butyrate is produced by the Firmicutes phylum. When the gut microbiota is in dysbiosis is directly related with alteration of SCFA production (Alexander et al. 2019). According to Gao et al. (2009), SCFA, particularly butyrate, improves insulin sensitivity and secretion by stimulating the secretion of peptide 1 like glucagon (GLP-1) and reducing the inflammation of adipocytes (Ríos-Covián et al. 2016; Tolhurst et al. 2012; Wang et al. 2015). Further, Qin et al. (2012) showed that Chinese patients with T2DM demonstrated a decrease in SCFA-producing bacteria, mainly butyrate-producing bacteria (Clostridiales sp. SS3/4, Eubacterium rectale, F. prausnitzii, and R. intestinalis, among others). These studies suggest that factors that are able to increase levels of SCFA, especially butyrate, are important for relieving T2DM symptoms. In additional, dietary butyrate supplementation has been associated with decrease of weight gain in animals fed high-fat diets (HFD). Although numerous bacterial strains have been analyzed for their butyrate-producing capacities, such as Faecalibacterium prausnitzii (a member of Clostridium cluster IV) and Eubacterium rectale/Roseburia (Clostridium cluster XIVa) (Lu et al. 2016).
Besides insulin resistance/sensitivity, the intestinal microbiota and its metabolites can affect other factors involved in T2DM, such as body weight, pro-inflammatory status, and the modulation of intestinal hormones. In this sense, the modulation of intestinal microbiota composition and metabolites using beneficial microorganisms, such as probiotics, can have advantageous effects on glucose metabolism and insulin resistance. The physiological functions of probiotics might lead to modulation of intestinal microbiota and can affect appetite, food intake, body weight, and metabolic functions of the body by means of gastrointestinal pathways (Rad et al. 2017; Kobyliak et al. 2016).
The use of probiotics for the management of T2DM
Probiotics were recognized for conferring health benefits; however, based on a large number of well-designed clinical trials, it was agreed that certain health beneficial effects of various strains of various well-studied microbial species can be attributed to probiotics as a general class (Hill et al. 2014; Fijan 2014). Several species of the genera Bifidobacterium and Lactobacillus claim to have a major benefit in healthy intestinal microbiota, creating a favorable intestinal environment. In addition, strain-specific probiotics support positive health outcomes, including the maintenance of a healthy immune system (Hill et al. 2014; McFarland et al. 2018). Probiotics are considered as complementary and alternative medicine, along with vitamins, minerals, and other food supplements (April et al. 2012).
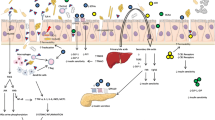
In this context, some studies have suggested that probiotics, as good intervention options in metabolic diseases, can positively alter intestinal microbiota safely and effectively and as a consequence a positive response in diseases (Reyes et al. 2016; Dahiya et al. 2017; Bianchi et al. 2018). In general, probiotics have shown beneficial effects, and various mechanisms have been proposed for T2DM therapy (Panwar et al. 2013). The possible relationship between probiotics, gut microbiota composition, and reduction of T2DM symptoms is shown in Fig. 1. After ingestion of probiotics, an improvement in T2DM symptoms is usually observed, such as improved intestinal integrity, decreased systemic LPS levels, decreased endoplasmic reticulum stress, and improved peripheral insulin sensitivity (Park et al. 2015; Balakumar et al. 2018; Lim et al. 2016).
Intestinal microbiota in homeostasis and dysbiosis promoted by type 2 diabetes and consequent impact on the development or prevention of T2DM. Intake of probiotics can positively modulate the intestinal microbiota, resulting in increased production of saccharolytic fermentation, short chain fatty acids (SCFA), and improved function of the intestinal barrier. Increased SCFAs are implicated in the release of glucagonal peptide-1 (GLP-1), which have an important impact on satiety, hunger, insulin sensitivity, and also improve intestinal barrier function. Consequently, increased bowel barrier function may reduce translocation of bacteria and liposaccharide (LPS), and thus reduce pro-inflammatory markers (interleukin-6 (IL-6), tumor necrosis factor (TNF)), and increase anti-inflammatory markers (interleukin-10 (IL-10)), as well as increase glycosylated hemoglobin A1c (HbA1c)
The levels of LPS are closely related to intestinal integrity. Thus, it is known that the translocation of LPS from the intestinal lumen to the circulatory system is prevented by the intestinal barrier during homeostasis (Vera et al. 2018). This barrier possesses an intestinal permeability that is usually regulated by tight junction proteins and adhesion between the epithelial cells of the intestine, which create a barrier that prevents bacteria, toxins, and intestinal lumen products from reaching circulation (Vancamelbeke and Vermeire 2018). The translocation of LPS into circulation due to disruption of the intestinal barrier might trigger inflammation, leading to the development of various diseases, such as obesity (von Scholten et al. 2013), atherosclerosis (Wiesner et al. 2010), and diabetes (Creely et al. 2007). One hypothesis is that the T2DM can be improved by decreasing the concentration of LPS in the blood (Trøseid et al. 2013). Amar et al. (2011) showed, using an animal model for insulin resistance and T2DM, that treatment with the probiotic Bifidobacterium animalis subsp. lactis 420 for 6 weeks can reduce metabolic endotoxemia, inflammation, and translocation of LPS and improve the overall metabolism.
Different probiotic strains showed a beneficial impact on T2DM both for clinical models and for animal models (Table 1 and Table 2) such as reduction in plasma lipids and pro-inflammatory genes (TNF-α, IL-6, IL-β) and increase production of short chain fatty acids (SCFA). In addition, some studies have shown that a mixture of different probiotic strains has a better and broader impact on human health when used individually (Kobyliak et al. 2018; Razmpoosh et al. 2018; Ejtahed et al. 2012; Bagarolli et al. 2017).
According to Bagarolli et al. (2017), the treatment with the probiotics L. rhamnosus, L. acidophilus, and B. bifidum in animal model increase of Bacteroidetes and decrease of Firmicutes abundance. In addition, the authors showed that a high-calorie diet promotes changes in gut microbiota that are reflected in increased intestinal permeability, translocation of the SPL, and low-grade systemic inflammation, resulting in decreased glucose tolerance. In addition, according to Firouzi et al. (2016) and O’Connor et al. (2017), probiotics may modulate intestinal microbiota, which, through cascading metabolic processes, may result in improved lipid profiles (decreased LDL, CT normalization, increased HDL), reduced fasting glucose levels, hemoglobin A1c, fasting insulin levels, and levels of C-reactive protein (CRP).
In addition, the elegant study realized by Brandão et al. (2018) showed for first the time that administration of Saccharomyces Boulardii is strongly associated with glycemic control, cardiovascular protection, and improvement of inflammatory profile in animal model.
The strengths and distinctions of this review include the inclusion of the specific medium (animal or clinical) dose and duration of each probiotic intervention, providing insight into the probiotic that may be clinically relevant and beneficial for blood glucose. However, the specific relationship between microbiota-probiotic-T2DM has not been clarified. There are few experimental studies on microbiota modulation by probiotic and DM2 ingestion and we found many studies (clinical or animal) on probiotic ingestion and biochemical parameters. Finally, further studies are needed to identify which specific microorganisms and mechanisms of action are involved in preventing DM2 and probiotic ingestion.
Conclusions and future prospects
T2DM is an important and widespread chronic disease. This mini-review shows that gut microbiota composition is essential for understanding the mechanisms involved in the pathology of T2DM. Compared to various other means of controlling T2DM, the consumption of probiotics is a promising strategy with a beneficial impact on the intestinal microbiota. Several different probiotic strains, especially those belonging to Lactobacillus and Bifidobacterium spp., have demonstrated the ability to improve parameters related to T2DM, highlighting the importance of studying probiotics for diabetes prevention, progression, and symptom amelioration. With the emergence of molecular biology and the “omic” era, a better understanding of the mechanisms involving T2DM and gut microbiota is expected, making it possible to advance our knowledge of the relationship between microbiota composition and diabetes with more determination and detail. Finally, the intestinal microbiota can be key to the management of this chronic disease.
References
Ahola AJ, Harjutsalo V, Forsblom C, Freese R, Makimattila S, Groop PH (2017) The self-reported use of probiotics is associated with better glycaemic control and lower odds of metabolic syndrome and its components in type 1 diabetes. J Prob Health 5(4):188. https://doi.org/10.4172/2329-8901.1000188
Alexander C, Swanson KS, Fahey GC, Garleb KA (2019) Perspective: physiologic importance of short-chain fatty acids from nondigestible carbohydrate fermentation. Adv Nutr 1;10(4):576–589. https://doi.org/10.1093/advances/nmz004
Amar J, Chabo C, Waget A, Klopp P, Vachoux C, Bermúdez-Humarán LG, Smirnova N, Bergé M, Sulpice T, Lahtinen S, Ouwehand A, Langella P, Rautonen N, Sansonetti PJ, Burcelin R (2011) Intestinal mucosal adherence and translocation of commensal bacteria at the early onset of type 2 diabetes: molecular mechanisms and probiotic treatment. EMBO Mol Med 3(9):559–572. https://doi.org/10.1002/emmm.201100159
American Diabetes Association (ADA) (2009) Diagnosis and classification of Diabetes mellitus. Diabetes Care 32(Suppl 1):62–67. https://doi.org/10.2337/dc09-S062
April K, Moher D, Stinson J, Byrne A, White M, Boon H, Duffy CM, Rader T, Vohra S, Tugwell P (2012) Measurement properties of questionnaires assessing complementary and alternative medicine use in pediatrics: A systematic review. PLoS ONE. 7:e39611. https://doi.org/10.1371/journal.pone.0039611
Asemi Z, Zare Z, Shakeri H, Sabihi S-S, Esmaillzadeh A (2013) Effect of multispecies probiotic supplements on metabolic profiles, hs-CRP, and oxidative stress in patients with type 2 diabetes. Ann Nutr Metab 63(1–2):1–9. https://doi.org/10.1159/000349922
Bagarolli RA, Tobar N, Oliveira AG, Araújo TG, Carvalho BM, Rocha GZ, Vecina JF, Calisto K, Guadagnini D, Prada PO, SantosA SSTO, Saad MJ (2017) Probiotics modulate gut microbiota and improve insulin sensitivity in DIO mice. J Nutr Biochem 50:16–25. https://doi.org/10.1016/j.jnutbio.2017.08.006
Balakumar M, Prabhu D, Sathishkumar C, Prabu P, Rokana N, Kumar R, Raghavan S, Soundarajan A, Grover S, Batish VK, Mohan V, Balasubramanyam M (2018) Improvement in glucose tolerance and insulin sensitivity by probiotic strains of Indian gut origin in high-fat diet-fed C57BL/6J mice. Eur J Nutr 1:279–295. https://doi.org/10.1007/s00394-016-1317-7
Battson ML, Lee DM, Weir TL, Gentile CL (2018) The gut microbiota as a novel regulator of cardiovascular function and disease. J Nutr Biochem 56:1–15. https://doi.org/10.1016/j.jnutbio.2017.12.010
Baxter NT, Lesniak NA, Sinani H, Schloss PD, Koropatkin NM (2019) The glucoamylase inhibitor acarbose has a diet-dependent and reversible effect on the murine gut microbiome. mSphere 4:e00528–e00518. https://doi.org/10.1128/mSphere.00528-18
Bernaola AG, Bada MCA, Carreazo NY, Rojas GRA (2010) Probiotics for treating persistent diarrhoea in children. Cochrane Database Syst Rev (11):CD007401. https://doi.org/10.1002/14651858.CD007401.pub2
Bervoets L, Hoorenbeeck KV, Kortleven I, van-Noten C, Hens N, Vael C, Goossens H, Desager KN, Vankerckhoven V (2013) Differences in gut microbiota composition between obese and lean children: a cross-sectional study. Gut Pathog. 5:10. https://doi.org/10.1186/1757-4749-5-10
Bianchi F, Duque ALRF, Saad SMI, Sivieri K (2018) Gut microbiome approaches to treat obesity in humans. Appl Microbiol Biotechnol 103(3):1081–1094. https://doi.org/10.1007/s00253-018-9570-8
Blaut M, Clavel T (2007) Metabolic diversity of the intestinal microbiota: implications for health and disease. J Nutr 137:751S–755S. https://doi.org/10.1093/jn/137.3.751S
Brandão BPA, Abreu IC, Aimbire F, Higa ME, Casali A, Ferreira FG, Albuquerque RCM, Santos LB, Irigoyen MCC, Casali KR, Cunha ST (2018) Saccharomyces Boulardii attenuates autonomic cardiovascular dysfunction and modulates Inflammatory cytokines in diabetic mice. Diabetes 67(Supplement 1). https://doi.org/10.2337/db18-2365-PUB
Campbell SC, Wisniewski PJ, Noji M, McGuinness LR, Häggblom MM, Lightfoot SA, Joseph LB, Kerkhof LJ (2016) The Effect of Diet and Exercise on Intestinal Integrity and Microbial Diversity in Mice. PLoS ONE 11(3):e0150502. https://doi.org/10.1371/journal.pone.0150502
Caricilli AM, Saad MJA (2013) The Role of Gut Microbiota on Insulin Resistance. Nutrients 5:829–851. https://doi.org/10.3390/nu5030829
Creely SJ, McTernan PG, Kusminski CM, Fisher fM, Da Silva NF, Khanolkar M, Evans M, Harte AL, Kumar S (2007) Lipopolysaccharide activates an innate immune system response in human adipose tissue in obesity and type 2 diabetes. Am J Physiol Endocrinol Metab 292(3):E740–E747. https://doi.org/10.1152/ajpendo.00302.2006
Dahiya DK, Renuka PM, Shandilya UK, Dhewa T, Kumar N, Kumar S, Puniya AK, Shukla P (2017) Gut microbiota modulation and its relationship with obesity using prebiotic fibers and probiotics: a review. Front Microbiol 8:563. https://doi.org/10.3389/fmicb.2017.00563
Dang F, Jiang Y, Pan R, Zhou Y, Wu S, Wang R, Zhuang K, Zhang W, Li T, Man C (2018) Administration of Lactobacillus paracasei ameliorates type 2 diabetes in mice. Food Funct 9(7):3630–3639. https://doi.org/10.1039/c8fo00081f
David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, Biddinger SB, Dutton RJ, Turnbaugh PJ (2014) Diet rapidly and reproducibly alters the human gut microbiome. Nature 505:559–563. https://doi.org/10.1038/nature12820
Dugas LR, Lie L, Plange-Rhule J, Bedu-Addo K, Bovet P, Lambert EV, Forrester TE, Luke A, Gilbert JA, Layden BT (2018) Gut microbiota, short chain fatty acids, and obesity across the epidemiologic transition: the METS-Microbiome study protocol. BMC Public Health. 18:978. https://doi.org/10.1186/s12889-018-5879-6
Edwards-Ingram L, Gitsham P, Burton N, Warhurst G, Clarke I, Hoyle D, Oliver SG, Stateva L (2007) Genotypic and physiological characterization of Saccharomyces boulardii, the probiotic strain of Saccharomyces cerevisiae. Appl Environ Microbiol. 73:2458–2467. https://doi.org/10.1128/AEM.02201-06
Ejtahed HS, Mohtadi-Nia J, Homayouni-Rad A, Niafar M, Asghari-Jafarabadi M, Mofid V (2012) Probiotic yogurt improves antioxidant status in type 2 diabetic patients. Nutrition 5:539–543. https://doi.org/10.1016/j.nut.2011.08.013
Evans CC, LePard KJ, Kwak JW, Stancukas MC, Laskowski S, Dougherty J, Moulton L, Glawe A, Wang Y, Leone V, Antonopoulos DA, Smith D, Chang EB, Ciancio MJ (2014) Exercise prevents weight gain and alters the gut microbiota in a mouse model of high fat diet-induced obesity. PLOS ONE. 9(3):e92193. https://doi.org/10.1371/journal.pone.0092193
Fijan S (2014) Microorganisms with claimed probiotic properties: an overview of recent literature. Int J Environ Res Public Health. b 11(5):4745–4767. https://doi.org/10.3390/ijerph110504745
Firouzi S, Majid HA, Ismail A, Kamaruddin NA, Barakatun-Nisak MY (2016) Effect of multi-strain probiotics (multi-strain microbial cell preparation) on glycemic control and other diabetes-related outcomes in people with type 2 diabetes: a randomized controlled trial. Eur J Nutrition 56(4):1535–1550. https://doi.org/10.1007/s00394-016-1199-8
Gao Z, Yin J, Zhang J, Ward RE, Martin RJ, Lefevre M, Cefalu WT, Ye J (2009) Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes 58(7):1509–1517. https://doi.org/10.2337/db08-1637
Hegde S, Lin Y-M, Golovko G, Khanipov K, Cong Y, Savidge T, Fofanov Y, Shi XZ (2018) Microbiota dysbiosis and its pathophysiological significance in bowel obstruction. Sci Rep 8:13044. https://doi.org/10.1038/s41598-018-31033-0
Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, Calder PC, Sanders ME (2014) Expert consensus document. The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 11(8):506–514. https://doi.org/10.1038/nrgastro.2014.66
HUMAN MICROBIOME PROJECT C (2012a) A framework for human microbiome research. Nature 486:215–221. https://doi.org/10.1038/nature11209
HUMAN MICROBIOME PROJECT C (2012b) Structure, function and diversity of the healthy human microbiome. Nature 486:207–214. https://doi.org/10.1038/nature11234
International Diabetes Federation (IDF) (2017) Diabetes Atlas. Eighth Edition. https://diabetesatlas.org
Kalinkovich A, Livshits G (2019) A cross talk between dysbiosis and gut-associated immune system governs the development of inflammatory arthropathies. Semin Arthritis Rheum (19):30170–30172. https://doi.org/10.1016/j.semarthrit.2019.05.007
Karlsson FH, Tremaroli V, Nookaew I, Bergström G, Behre CJ, Fagerberg B, Nielsen J, Bäckhed F (2013) Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature 498(7452):99–103. https://doi.org/10.1038/nature12198
Khalili L, Alipour B, Asghari Jafar-Abadi M, Faraji I, Hassanalilou T, Mesgari Abbasi M, Vaghef-Mehrabany E, Alizadeh SM (2019) The effects of Lactobacillus casei on glycemic response, serum sirtuin1 and fetuin-a levels in patients with Type 2 Diabetes Mellitus: A Randomized Controlled Trial. Iran Biomed J 1:68–77. https://doi.org/10.29252/.23.1.68
Kobyliak N, Conte C, Cammarota G, Haley AP, Styriak I, Gaspar L, Fusek J, Rodrigo L, Kruzliak P (2016) Probiotics in prevention and treatment of obesity: a critical view. Nutr Metab (Lond) 13:14. https://doi.org/10.1186/s12986-016-0067-0
Kobyliak N, Falalyeyeva T, Mykhalchyshyn G, Kyriienko D, Komissarenko I (2018) Effect of alive probiotic on insulin resistance in type 2 diabetes patients: randomized clinical trial. Diabetes Metab Syndr 12:617–624. https://doi.org/10.1016/j.dsx.2018.04.015
Larsen N, Vogensen FK, van den Berg FW, Nielsen DS, Andreasen AS, Pedersen BK, Al-Soud WA, Sørensen SJ, Hansen LH, Jakobsen M (2010) Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS One 5(2):e9085. https://doi.org/10.1371/journal.pone.0009085
Lee E, Jung RA, Lee SY, Lee NK, Paik HD, Lim SII (2018) Lactobacillus plantarum strain Ln4 attenuates diet-induced obesity, insulin resistance, and changes in hepatic mRNA levels associated with glucose and lipid metabolism. Nutrients (5):643. https://doi.org/10.3390/nu10050643
Li X, Wang E, Yin B, Fang D, Chen P, Wang G, Zhao J, Zhang H, Chen W (2017) Effects of Lactobacillus casei CCFM419 on insulin resistance and gut microbiota in type 2 diabetic mice. Benef Microbes 3:421–432. https://doi.org/10.3920/BM2016.0167
Lim S, Jeong JJ, Woo KH, Han MJ, Kim DH (2016) Lactobacillus sakei OK67 ameliorates high-fat diet-induced blood glucose intolerance and obesity in mice by inhibiting gut microbiota lipopolysaccharide production and inducing colon tight junction protein expression. Nutr Res 36(4):337–348. https://doi.org/10.1016/j.nutres.2015.12.001
Lu Y, Fan C, Li P, Lu Y, Chang X, Qi K (2016) Short chain fatty acids prevent high-fat-diet-induced obesity in mice by regulating g protein-coupled receptors and gut microbiota. Sci Rep 6:37589. https://doi.org/10.1038/srep37589
Lyssenko V, Jonsson A, Almgren P, Pulizzi N, Isomaa B, Tuomi T, Berglund G, Altshuler D, Nilsson P, Groop L (2008) Clinical risk factors, DNA variants, and the development of Type 2 Diabetes. N Engl J Med 359:2220–2232. https://doi.org/10.1056/NEJMoa0801869
Martin D (2011) Physical activity benefits and risks on the gastrointestinal system. South Med J 104:831–837. https://doi.org/10.1097/SMJ.0b013e318236c263
Martinell M, Dorkhan M, Stålhammar J, Storm P, Groop L, Gustavsson C (2016) Prevalence and risk factors for diabetic retinopathy at diagnosis (DRAD) in patients recently diagnosed with type 2 diabetes (T2D) or latent autoimmune diabetes in the adult (LADA). J Diabetes Complications 30:1456–1461. https://doi.org/10.1016/j.jdiacomp.2016.08.009
Matsumoto M, Inoue R, Tsukahara T, Ushida K, Chiji H, Matsubara N, Hara H (2008) Voluntary running exercise alters microbiota composition and increases n-butyrate concentration in the rat cecum. Biosci Biotechnol Biochem 72:572–576. https://doi.org/10.1271/bbb.70474
McFarland LV, Evans CT, Goldstein EJC (2018) Strain-specificity and disease-specificity of probiotic efficacy: a systematic review and meta-analysis. Front Med (Lausanne) 5:124. https://doi.org/10.3389/fmed.2018.00124
Mobini R, Tremaroli V, Ståhlman M, Karlsson F, Levin M, Ljungberg M, Sohlin M, Bertéus Forslund H, Perkins R, Bäckhed F, Jansson PA (2017) Metabolic effects of Lactobacillus reuteri DSM 17938 in people with type 2 diabetes: A randomized controlled trial. Diabetes Obes Metab 4:579–589. https://doi.org/10.1111/dom.12861
Newsholme P, Cruzat VF, Keane KN, Carlessi BPI (2016) Molecular mechanisms of ROS production and oxidative stress in diabetes. Biochem J 473:4527–4550. https://doi.org/10.1042/BCJ20160503C
Nyunt O, Wu JY, McGown IN, Harris M, Huynh T, Leong GM, Cowley DM, Cotterill AM (2009) Investigating maturity onset diabetes of the young. Clin Biochem Rev 30:67–74
O’Connor S, Chouinard-Castonguay S, Gagnon C, Rudkowska I (2017) Prebiotics in the management of components of the metabolic syndrome. Maturitas 104:11–18. https://doi.org/10.1016/j.maturitas.2017.07.005
Opazo MC, Ortega-Rocha EM, Coronado-Arrázola I, Bonifaz LC, Boudin H, Neunlist M, Bueno SM, Kalergis AM, Riedel CA (2018) intestinal microbiota influences non-intestinal related autoimmune diseases. Front Microbiol 9:432. https://doi.org/10.3389/fmicb.2018.00432
Panwar H, Rashmi HM, Batish VK, Grover S (2013) Probiotics as potential biotherapeutics in the management of type 2 diabetes: Prospects and perspectives. Diabetes Metab Res Rev 29(2):103–112. https://doi.org/10.1002/dmrr.2376
Park KY, Kim B, Hyun CK (2015) Lactobacillus rhamnosus GG improves glucose tolerance through alleviating ER stress and suppressing macrophage activation in db/db mice. J Clin Biochem Nutr 56(3):240–246. https://doi.org/10.3164/jcbn.14-116
Patterson E, Ryan PM, Cryan JF, Dinan TG, Ross RP, Fitzgerald GF, Catherine S (2016) Gut microbiota, obesity and diabetes. Post Med J 92:286–300. https://doi.org/10.1136/postgradmedj-2015-133285
Peters BA, Shapiro JA, Church TR, Miller G, Trinh-Shevrin C, Yuen E, Friedlander C, Hayes RB, Ahn J (2018) A taxonomic signature of obesity in a large study of American adults. Scientific Reports 8:9749–9713. https://doi.org/10.1038/s41598-018-28126-1
Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang J, Liang S, Zhang W, Guan Y, Shen D, Peng Y, Zhang D, Jie Z, Wu W, Qin Y, Xue W, Li J, Han L, Lu D, Wu P, Dai Y, Sun X, Li Z, Tang A, Zhong S, Li X, Chen W, Xu R, Wang M, Feng Q, Gong M, Yu J, Zhang Y, Zhang M, Hansen T, Sanchez G, Raes J, Falony G, Okuda S, Almeida M, LeChatelier E, Renault P, Pons N, Batto JM, Zhang Z, Chen H, Yang R, Zheng W, Li S, Yang H, Wang J, Ehrlich SD, Nielsen R, Pedersen O, Kristiansen K, Wang J (2012) A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 490:55–60. https://doi.org/10.1038/nature11450
Rad AH, Abbasalizadeh S, Vazifekhah S, Abbasalizadeh F, Hassanalilou T, Bastani P, Ejtahed HS, Soroush AR, Javadi M, Mortazavian AM, Khalili L (2017) The future of diabetes management by healthy probiotic microorganisms. Cur Diabetes Rev 13(6):582–589. https://doi.org/10.2174/1573399812666161014112515
Razmpoosh E, Javadi A, Ejtahed HS, Mirmiran P, Javadi M, Yousefinejad A (2018) The effect of probiotic supplementation on glycemic control and lipid profile in patients with type 2 diabetes: A randomized placebo-controlled trial. Diabetes Met Synd. https://doi.org/10.1016/j.dsx.2018.08.008
Razmpoosh E, Javadi A, Ejtahed HS, Mirmiran P, Javadi M, Yousefinejad A.(2019) The effect of probiotic supplementation on glycemic control and lipid profile in patients with type 2 diabetes: A randomized placebo controlled trial. Diabetes Metab Syndr. 13(1):175-182. https://doi.org/10.1007/s00253-019-10156-y10.1016/j.dsx.2018.08.008
Reyes LM, Vázquez RG, Arroyo SMC, Avalos AM, Castillo PAR, Pérez DAC, Terrones IR, Ibáñez NR, Magallanes MMR, Langella P, Humarán LB, Espinosa AA (2016) Correlation between diet and gut bacteria in a population of young adults. Int J Food Sci Nutr 67:470–478. https://doi.org/10.3109/09637486.2016.1162770
Ríos-Covián D, Ruas-Madiedo P, Margolles A, Gueimonde M, de los Reyes-Gavilán CG, Salazar N (2016) Intestinal short chain fatty acids and their link with diet and human health. Front Microbiol 7:185. https://doi.org/10.3389/fmicb.2016.00185
Ritchie ML, Romanuk TN (2012) A meta-analysis of probiotic efficacy for gastrointestinal diseases. PLoS One. 7(4):e34938. https://doi.org/10.1371/journal.pone.0034938
Roager HM, Vogt JK, Kristensen M, LBS H, Ibrugger S, Maerkedahl RB, Bahl MI, Lind MV, Nielsen RL, Frøkiaer H, Gøbel RJ, Landberg R, Ross AB, Brix S, Holck J, Meyer AS, Sparholt MH, Christensen AF, Carvalho V, Hartmann B, Holst JJ, Rumessen JJ, Linneberg A, Sicheritz-Pontén T, Dalgaard MD, Blennow A, Frandsen HL, Villas-Bôas S, Kristiansen K, Vestergaard H, Hansen T, Ekstrøm CT, Ritz C, Nielsen HB, Pedersen OB, Gupta R, Lauritzen L, Licht TR (2017) Whole grain-rich diet reduces body weight and systemic low-grade inflammation without inducing major changes of the gut microbiome: A randomised cross-over trial. Gut. https://doi.org/10.1136/gutjnl-2017-314786
Sabatino A, Regolisti G, Cosola C, Gesualdo L, Fiaccadori E (2017) Intestinal Microbiota in Type 2 Diabetes and Chronic Kidney Disease. Curr Diab Rep 17(3):16. https://doi.org/10.1007/s11892-017-0841-z
Sato J, Kanazawa A, Azuma K, Ikeda F, Goto H, Komiya K, Kanno R, Tamura Y, Asahara T, Takahashi T, Nomoto K, Yamashiro Y, Watada H (2017) Probiotic reduces bacterial translocation in type 2 diabetes mellitus: A randomised controlled study. Sci Rep 7(1):12115. https://doi.org/10.1038/s41598-017-12535-9
Schwiertz A, Taras D, Schafer K, Beijer S, Bos NA, Donus C, Hardt PD (2010) Microbiota and SCFA in lean and overweight healthy subjects. Obesity (Silver Spring) 18:190–195. https://doi.org/10.1038/oby.2009.167
Seuring T, Archangelidi O, Suhrcke M (2015) The economic costs of type 2 diabetes: A global systematic review. Pharmacoeconomics. 33(8):811–831. https://doi.org/10.1007/s40273-015-0268-9
Sgritta M, Dooling SW, Buffington SA, Momin EN, Francis MB, Britton RA, Costa-Mattioli M (2019) Mechanisms underlying microbial-mediated changes in social behavior in mouse models of autism spectrum disorder. Neuron 101(2):246–259.e6. https://doi.org/10.1016/j.neuron.2018.11.018
Stensel D (2010) Exercise, appetite and appetite-regulating hormones: implications for food intake and weight control. Ann Nutr Metab. 57(suppl 2):36–42. https://doi.org/10.1159/000322702
Tolhurst G, Heffron H, Lam YS, Parker HE, Habib AM, Diakogiannaki E, Cameron J, Grosse J, Reimann F, Gribble FM (2012) Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via theg-protein–coupled receptor ffar2. Diabetes 61:364–371. https://doi.org/10.2337/db11-1019
Trøseid M, Nestvold TK, Rudi K, Thoresen H, Nielsen EW, Lappegård KT (2013) Plasma lipopolysaccharide is closely associated with glycemic control and abdominal obesity: evidence from bariatric surgery. Diabetes Care 36(11):3627–3632. https://doi.org/10.2337/dc13-0451
van De Wiele T, Boon N, Possemiers S, Jacobs H, Verstraete W (2004) Prebiotic effects of chicory inulin in the simulator of the human intestinal microbial ecosystem. FEMS Microbiol Ecol 51:143–153. https://doi.org/10.1016/j.femsec.2004.07.014
Van de Wiele T, Van Praet JT, Marzorati M, Dreannan M, Elewaut E. (2016) How the microbiota shapes rheumatic diseases. Nat. Rev. Rheumatol.12:398–411. https://doi.org/10.1038/nrrheum.2016.85
Vancamelbeke M, Vermeire S (2018) The intestinal barrier: a fundamental role in health and disease. Expert Rev Gastroenterol Hepatol. 11(9):821–834. https://doi.org/10.1080/17474124.2017.1343143
Vera IM, Tapia MS, Noriega-López L, Granados-Portillo O, Guevara-Cruz M, Flores-López A, Avila-Nava A, Fernández ML, Tovar AR, Torres N (2018) A dietary intervention with functional foods reduces metabolic endotoxaemia and attenuates biochemical abnormalities by modifying faecal microbiota in people with type 2 diabetes. Diabetes Metab 2:122–131. https://doi.org/10.1016/j.diabet.2018.09.004
von Scholten BJ, Andresen EN, Sørensen TI, Jess T (2013) Aetiological factors behind adipose tissue inflammation: an unexplored research area. Public Health Nutr. 16(1):27–35. https://doi.org/10.1017/S1368980012000894
Wang X, He G, Peng Y, Zhong W, Wang Y, Zhang B (2015) Sodium butyrate alleviates adipocyte inflammationby inhibiting nlrp3 pathway. Sci Rep 5:12676. https://doi.org/10.1038/srep12676
Wang G, Li X, Zhao J, Zhang H, Chen W (2017) Lactobacillus casei CCFM419 attenuates type 2 diabetes via a gut microbiota dependent mechanism. Food Funct. 8(9):3155–3164. https://doi.org/10.1039/c7fo00593h
White MF (2002) IRS proteins and the common path to diabetes. Am J Physiol Endocrinol Metab 283:E413–E422. https://doi.org/10.1152/ajpendo.00514.2001
Wiesner P, Choi SH, Almazan F, Benner C, Huang W, Diehl CJ, Gonen A, Butler S, Witztum JL, Glass CK, Miller YI (2010) Low doses of lipopolysaccharide and minimally oxidized low-density lipoprotein cooperatively activate macrophages via nuclear factor kappa B and activator protein-1: possible mechanism for acceleration of atherosclerosis by subclinical endotoxemia. Circ Res. 107(1):56–65. https://doi.org/10.1161/CIRCRESAHA.110.218420
World Health Organization (2016) Global Reports on Diabetes. World Health Organization. WHO Press, Geneva
Xiang Y, Huang G, Shan A, Pan L, Luo S, Yang L, Shi L, Li Q, Leslie RD, Zhou Z (2015) Glutamic acid decarboxylase autoantibodies are dominant but insufficient to identify most Chinese with adult-onset non-insulin requiring autoimmune diabetes: LADA China study 5. Acta Diabetol. 52:1121–1127. https://doi.org/10.1007/s00592-015-0799-8
Acknowledgments
The authors wish to thank Lucas A.R. Tannuri for drawing and editing the figure shown in this article.
Funding
This work was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Salgaço, M.K., Oliveira, L.G.S., Costa, G.N. et al. Relationship between gut microbiota, probiotics, and type 2 diabetes mellitus. Appl Microbiol Biotechnol 103, 9229–9238 (2019). https://doi.org/10.1007/s00253-019-10156-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-019-10156-y