Abstract
Purpose of the Review
Diabetes mellitus is a major cause of kidney disease [chronic kidney disease (CKD) and end-stage renal disease (ESRD)] and are both characterized by an increased risk of cardiovascular events. Diabetes and kidney disease are also commonly associated with a chronic inflammatory state, which is now considered a non-traditional risk factor for atherosclerosis. In the case of type 2 diabetes mellitus (T2DM), inflammation is mainly a consequence of visceral obesity, while in the case of CKD or ESRD patients on dialysis, inflammation is caused by multiple factors, classically grouped as dialysis-related and non-dialysis-related. More recently, a key role has been credited to the intestinal microbiota in the pathogenesis of chronic inflammation present in both disease states. While many recent data on the intestinal microbiota and its relationship to chronic inflammation are available for CKD patients, very little is known regarding T2DM and patients with diabetic nephropathy. The aim of this review is to summarize and discuss the main pathophysiological issues of intestinal microbiota in patients with T2DM and CKD/ESRD.
Recent Findings
The presence of intestinal dysbiosis, along with increased intestinal permeability and high circulating levels of lipopolysaccharides, a condition known as “endotoxemia,” characterize T2DM, CKD, and ESRD on dialysis. The hallmark of intestinal dysbiosis is a reduction of saccharolytic microbes mainly producing short-chain fatty acids (SCFA) and, in the case of CKD/ESRD, an increase in proteolytic microbes that produce different substances possibly related to uremic toxicity.
Summary
Dysbiosis is associated with endotoxemia and chronic inflammation, with disruption of the intestinal barrier and depletion of beneficial bacteria producing SCFAs. T2DM and CKD/ESRD, whose coexistence is increasingly found in clinical practice, share similar negative effects on both intestinal microbiota and function. More studies are needed to characterize specific alterations of the intestinal microbiota in diabetic nephropathy and to assess possible effects of probiotic and prebiotic treatments in this setting.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Type 2 diabetes mellitus (T2DM) accounts for at least 90% of all diabetes cases in the adult population [1]. In the last two decades, a true epidemic of T2DM has been observed, with more than 300 million people being affected globally [2]. It is estimated that more than 80% of patients with T2DM are overweight or have obesity, which is now considered the main cause of the disease [3]. Diabetes mellitus is also the leading cause of chronic kidney disease (CKD) and end-stage renal disease (ESRD), non-traumatic limb amputation, and blindness among adults [1]. In the USA, T2DM is the primary cause of ESRD, being responsible for 44% of all new cases in 2011 [4].
CKD is a global health issue since 6–10% of the whole adult population can be diagnosed with the disease according to the most recent classification [5, 6]. In this clinical setting, the most frequent cause of death is cardiovascular disease (CVD), which is attributable to the coexistence of traditional (e.g., hypertension, diabetes, and dyslipidemia) and non-conventional risk factors [7]. Among the latter, persistent low-grade inflammation has received increasing attention, and it is now regarded as a major catalyst for CVD in CKD [7]. Chronic inflammation is usually defined as a persistent inflammatory response by a causative stimulus. In the case of CKD, and especially ESRD on dialysis, increased production and decreased renal clearance contribute to the accumulation of cytokines [7]. The evidence concerning the use of inflammatory markers to diagnose chronic inflammation in the course of ESRD is vast [8] and suggests interleukin 6 (IL-6) as an important marker of inflammation, also representing the best outcome predictor in advanced CKD and ESRD [9]. However, since IL-6 measurement is not easily available in the clinical practice, the assessment of C-reactive protein (CRP), a marker of systemic inflammation and predictor of cardiovascular risk, is now the standard of care in the clinical setting because of its reliability, low cost, and wide availability [10].
Chronic inflammation is common in patients with T2DM, CKD, and ESRD [7,12,13,14,15,16,17,18,, 11–19]. In the case of T2DM, inflammation is mainly considered a consequence of obesity, in particular visceral obesity [2, 20, 21]. As for CKD and ESRD, many dialysis-related and non-dialysis-related factors are thought to contribute to the chronic inflammatory state [10, 22], and recent research has also highlighted a key role of the intestinal microbiota. Two main pathophysiological mechanisms are likely to be involved. Firstly, the low-grade inflammation typical of T2DM and CKD and ESRD can be potentiated by translocation, from the gut lumen to the blood, of bacteria and bacterial products (e.g., lipopolysaccharides, LPS) caused by an increase in intestinal permeability (“leaky gut syndrome”) [12,24,25,26,, 13, 23–27]. Secondly, modifications in the intestinal microbiota in terms of species richness, diversity, composition, and function may have a profound impact on host physiology, through changes in nutrient utilization and synthesis of bioactive metabolites [11, 27]. While abundant evidence has been accrued recently in patients with CKD or ESRD, information on this issue in T2DM is scarce. Thus, this review is aimed at summarizing and discussing the main pathophysiology of the intestinal microbiota in the presence of T2DM and CKD and ESRD.
Intestinal Microbiota in Healthy Subjects
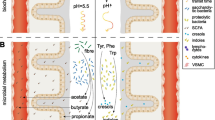
In humans, microbial cells from different bacterial species outnumber human cells by 10-fold, with the gastrointestinal (GI) tract being the habitat for greater than 70% of this microbial population [28•]. The amount of microbes changes along the intestine, being highest in its distal tract, where the environment is poor in oxygen and rich in molecules that these micro-organisms can utilize as nutrients [28•]. The intestinal microbiota exerts important trophic and protective functions that are not limited to the intestine but can affect the whole organism (Table 1). The composition, function, and structure of the intestinal microbiota is generally stable, but it is also very adaptive depending on the biochemical environment of the GI tract and changes in nutrient availability, which represents the most important regulator of bacterial metabolism [28•]. Normal or “healthy” intestinal microbiota consists of the bacterial phyla Firmicutes and Bacteroidetes (>90%), followed by Actinobacteria and Verrucomicrobia; usually few (0.1%) pathogenic and opportunistic species are present [29,30,31].
The two main nutrients utilized by intestinal bacteria are carbohydrates and proteins. The ratio between these two macronutrients significantly impacts on the predominance of different species. In the presence of adequate amounts of undigested complex carbohydrates (especially dietary fibers), saccharolytic bacteria are favored, and proteins are used for bacterial growth, while carbohydrates are used for energy production through bacterial anaerobic metabolism (fermentation). As a consequence of carbohydrate fermentation, methane, hydrogen, and short-chain fatty acids (SCFA) are produced as end-products [27]. However, in the absence of undigested carbohydrates, also proteins and other nitrogen compounds can be fermented by proteolytic bacteria in order to increase energy availability, with parallel production of potentially toxic end-products, such as ammonia, amines, thiols, phenols, and indoles [27].
Derangements of the intestinal milieu and related changes in the composition of the intestinal microbiota represent a condition referred to as “intestinal dysbiosis” that may trigger a systemic inflammatory response [28•, 32•]. Many external (e.g., antibiotics and nutrient intake) and internal (e.g., host genotype, extra-intestinal non-communicable diseases, and inflammatory bowel diseases) factors may contribute to the pathogenesis of intestinal dysbiosis and to the overgrowth of pathobionts (microbes with pathogenic potential) [32•].
Normally, the intestinal barrier prevents the translocation of substances and microbes from the lumen to the bloodstream. The intestinal barrier is formed by different structures/systems: tight junctions, enterocyte membranes, mucus secretion, and immunologic defense mechanisms in the intestinal wall [28•, 33••]. Particularly, tight junctions are a very efficient mechanical protection against the translocation of substances and bacteria along para-cellular pathways from the gut to the bloodstream; in fact, they bind together with epithelial cells and are capable of adjusting their tightness according to physiological needs [34].
Intestinal Microbiota in Type 2 Diabetes
Obesity-induced insulin resistance is the dominant underlying pathophysiological factor for the development of T2DM [3]. Obesity is a state of chronic low-grade systemic inflammation, which is a well-known cause of insulin resistance [35].
In mouse models of obesity, dysbiosis is usually present [17, 36, 37]. Specifically, a decrease in the Bacteroidetes/Firmicutes ratio is associated with the obese state. Germ-free mice are resistant to obesity induced by a high-fat diet [36], and colonization of germ-free mice with the microbiota of obese female humans caused obesity in the colonized mouse [38], indicating that the composition of the microbiota can predispose to the development of obesity. In addition, cohousing the colonized mice with lean mice and giving them a low-fat and high-fiber diet prevented further increase in adiposity, while in lean animals no changes toward obesity were found, suggesting that in the end, diet is responsible for phenotype development [39].
A recent human study demonstrated that poor diversity of intestinal microbiota (defined as low gene count, LGC) is also associated with obesity, insulin resistance, hepatic steatosis, and low-grade inflammation [11]. In this study, LGC subjects had a more pro-inflammatory microbial profile, characterized by a reduction of butyrate-producing bacteria and increased mucus degradation and oxidative stress [11].
Similar results were found in the two largest metagenome studies in T2DM [13, 40]. A moderate dysbiosis was demonstrated, characterized by a microbiota with decreased butyrate synthesis capacity [13, 40]. Dysbiosis promoted enrichment in membrane transport of sugars and branched chain amino acids, and increase in oxidative stress response and sulfate reduction [13]. Table 2 summarizes the major findings from studies in T2DM patients. Earlier studies in humans and in mice models of T2DM and obesity reported that obesity and impaired glucose metabolism were associated with an altered microbiota in comparison to healthy subjects [12, 41, 42]. Particularly, a proliferation of Gram-negative bacteria might explain the increase in serum LPS levels in obese and T2DM patients, likely triggering the low-grade inflammation state typical of these two conditions [12, 13].
Indeed, endotoxemia is known to induce the secretion of pro-inflammatory cytokines [14]. Studies on animal models and humans have demonstrated that a high-fat diet is able to modulate intestinal microbiota and to increase serum levels of LPS. The mechanisms involved in this endotoxemia state are probably related to an increased uptake of LPS in chylomicrons secreted from enterocytes and an increased intestinal permeability, known as “leaky gut” [14,15,16,17]. Circulating LPS are recognized by Toll-like receptors and activate the innate immune system and pro-inflammatory pathways.
Glucose and energy metabolism are also influenced by the microbial production of SCFA. Butyrate is the main source of energy to the intestinal epithelium and also seems to have an effect on insulin sensitivity and energy balance [43], while acetate and propionate are mainly substrates for gluconeogenesis and lipogenesis in the liver. In addition, butyrate has been demonstrated to increase the secretion of GLP1 and PYY from L cells in the colon [43, 44] and to increase the intestinal transit time [45]. Furthermore, GLP-1 and the activation of the complex GLP-1/GLP-1 receptor have been demonstrated to ameliorate the early effects of diabetes on the kidney in part by attenuating proximal tubular hyper-reabsorption and growth [46].
To summarize, present evidence suggests that dysbiosis may result in a “leaky gut syndrome,” with increased permeability that might activate the innate immune system, altering signaling pathways that affect lipid and glucose metabolism and triggering low-grade inflammation, eventually leading to insulin resistance and possibly T2DM. However, studies in human are yet to fully ascertain whether dysbiosis is a cause or consequence of T2DM [47].
Intestinal Microbiota in CKD and ESRD
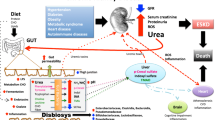
The cause–consequence relationship between the alterations of intestinal microbiota and kidney disease is complex and difficult to dissect. Indeed, it is reasonable to hypothesize that both factors may influence each other. A dysbiotic microbiota seems to represent a susceptibility factor for the development of kidney disease following injury or in predisposed individuals [48]. In addition, it is known that the progressive loss of kidney function significantly contributes to worsen the intestinal dysbiosis found in CKD/ESRD patients [49]. Different mechanisms are involved due to derangement of the intestinal barrier and modifications of microbiota composition (Table 3).
In this context, specific associations have been recently shown between certain intestinal [50] and salivary [51] microbial phyla/families and the condition of IgA nephropathy in comparison to healthy controls, with a further discrimination between progressor and non-progressor patients. This evidence suggests an active involvement of microbiota in the etiology and/or progression of this particular kidney disease, characterized by the key involvement of the immune system, which is known to be importantly influenced and modulated by the microbiota [21]. In this complicated framework, one additional variable to be considered is the diet, known to substantially contribute to the modifications of intestinal microbiota composition and metabolism [52]. It is by now ascertained that typical dietary restrictions, used to be considered as mandatory for the conservative treatment of CKD, are responsible of the worsening of the intestinal dysbiosis already occurring in this clinical setting. Dietary fiber is the primary substrate for colonic bacterial fermentation; however, patients with CKD often have a low intake of dietary fibers, mainly because of the need to control potassium intake. The main sources of potassium and fiber in the diet are fruits and vegetables, which are reportedly low in CKD patients’ diet [53]. Reduced intake of fibers, in addition to other factors related to CKD treatment (dialysis modality, phosphate binders, low fluid intake), lifestyle (inactivity), and comorbidities (diabetes, malnutrition, heart failure, and cerebrovascular diseases), may prolong the GI transit time. Prolonged transit time may lead to increased CHO fermentation in the proximal segments of the intestine [54], thus reducing CHO availability to the colonic bacteria. In addition, protein digestion and absorption seem to be impaired in CKD patients [55] due to alterations in the GI tract motility, hypochlorhydria, and bacterial overgrowth in the small bowel, thus increasing the amount of intact protein available for proteolytic bacteria in the colon [54, 56, 57].
Recent concepts about the nutritional management of CKD are expanding the vision from the focus on a single nutrient to the concept of “food matrices,” namely complex associations of different food categories, often nutraceuticals (antioxidants, fibers, proteins, vitamins, etc.) found together in the same food. In particular, food matrices traditionally belonging to the Mediterranean Diet are particularly rich in nutraceutical components so that this dietary scheme is being reconsidered as suitable for this category of patients [58].
As discussed earlier, the intestinal microbiota can be highly adaptable to changes in the biochemical environment. That is why relevant quantitative and qualitative changes in the bacterial population of CKD patients have been demonstrated, also in earlier stages of the syndrome [59]. Recent studies have demonstrated increased counts of aerobic and anaerobic bacteria in the small bowel of CKD patients as well as overgrowth of Proteobacteria, Actinobacteria, and Firmicutes in the colon [49]. In particular, an expansion of bacterial species with urease, uricase, and indole- and p-cresol-forming enzymes has been documented [60]. Urea is now considered a key factor in the pathogenesis of increased permeability of the intestinal barrier. The increased influx of urea into the intestinal lumen as a consequence of uremia may foster bacterial species that produce urease. These bacterial species hydrolyze urea to ammonia and increase intestinal pH, leading to mucosal irritation and structural damage [61, 62]. In addition, intestinal excretion of both uric acid and oxalate is also increased in CKD [63, 64]. Because of the wide availability of nitrogen waste products in the intestine, the overgrowth of microbes capable of utilizing these substrates is thus favored [60].
CKD is characterized by the progressive accumulation of many substances and solutes, such as electrolytes, hormones, and other solutes. Some of these compounds, termed uremic toxins, may interfere with many biological functions and may have important effects on inflammatory status and CVD risk [7, 65, 66]. The identity of such toxins remains an active area of study [67, 68]. Their precursors are formed in the GI tract during bacterial protein fermentation. The two most widely studied of these compounds are p-cresol (the precursor of p-cresyl-sulfate and p-cresyl-glucuronide) and indole (the precursor of indoxyl sulfate), generated respectively from the fermentation of amino acids tyrosine and tryptophan. In healthy subjects, the kidney excretes these molecules by active tubular secretion, while in CKD increased blood concentration of p-cresyl-sulfate and indoxyl sulfate follows the reduction of renal function [69]. In ESRD patients on dialysis, the clearance of p-cresyl-sulfate and indoxyl sulfate is less than 10% of that of healthy subjects [70], and their increased blood concentration correlates with poor outcomes [71,72,73,74,75,76,77]. These protein-bound compounds negatively affect endothelial function and repair by several mechanisms, including inflammation, oxidative stress, impaired nitric oxide production, and inhibition of endothelial proliferation and healing [73,74,75,76,77,78].
Targeting the Intestinal Microbiota to Modulate Intestinal Barrier Dysfunction and Dysbiosis in T2DM and CKD/ESRD
Probiotics and Prebiotics in T2DM
Probiotics are “viable organisms that, when ingested in sufficient amounts, exert positive health effects” [79]. Among the many claimed health benefits associated with probiotic bacteria, particularly important is the (transient) modulation of the intestinal microbiota and the capability to interact with the immune system. The term prebiotic refers to “a selectively fermented ingredient that allows specific changes, both in the composition and/or activity in the gastrointestinal microbiota that confer benefits” [80]. Prebiotics promote the growth of bacterial species that stabilize the mucosal barrier function, reduce the abundance of pathogenic bacteria by intestinal lumen acidification, overcome the competition for nutrients, and produce antimicrobial substances [81]. Some characteristics must be present in the food ingredient to be classified as a prebiotic, such as resistance to digestion and absorption in the upper gastrointestinal tract, easy fermentability by the intestinal microbiota, and capability of selective stimulation of growth and/or activity of beneficial bacteria potentially associated with health and well-being [79, 80]. Finally, when probiotics are administered along with prebiotics, the combination is referred to as “synbiotics.”
It remains to be fully ascertained whether probiotic administration may represent an efficacious treatment for T2DM. Recent literature has focused on targeting bacterial strains and increased intestinal permeability by SCFAs. SCFAs have been shown to modulate intestinal hormones and to have important effects in metabolic health since they affect intestinal permeability, satiety, gastric emptying, and food intake [82, 83]. It appears that butyrate plays a pivotal role in the correction of endotoxemia, by improving intestinal wall barrier function, with proliferation of colonic epithelial cells and tight-junction tightness [84]. In a landmark study performed in 18 obese subjects with metabolic syndrome and insulin resistance, fecal transplantation from lean donors, but not autologous transplantation, improved insulin sensitivity and increased the microbiota diversity, in particular butyrate-producing bacteria such as Roseburia intestinalis, Faecalibacterium prausnitzii, and Eubacterium hallii [85]. There is also evidence that classical probiotics can stop weight gain and improve glucose tolerance in mice with T2DM [86].
Research focusing on the intestinal barrier function demonstrated that supplementation with 2 × 108 CFU/day of Akkermansia muciniphila, a bacterium found in the mucus layer of the intestinal wall, reduced serum LPS in mice fed a high-fat diet [87]. However, data on A. muciniphila are still controversial since some studies reported increased concentrations of this bacterial strain in some disease states or during high-fat diet [13, 88].
Regarding the use of prebiotics, this approach was associated with favorable changes in the microbiota and improvement of metabolic markers of obese mice [89]. In humans, administration of prebiotics improved insulin sensitivity in subjects without T2DM [90]. However, not all humans respond to prebiotic treatment in the same manner, and lower bacterial diversity is related to no response [91, 92]. More specifically, in a recent study, the Prevotella/Bacteroides ratio of the intestinal microbiota of healthy subjects allowed the identification of responders and non-responders [92]. In this study, glucose metabolism of germ-free mice that received the microbiota of responder subjects improved, while nothing happened to germ-free mice that received the microbiota of non-responders. More studies are needed to assess the role of prebiotics and probiotics in the treatment of T2DM.
Probiotics and Prebiotics in CKD and ESRD
Currently, nutritional strategies aimed to modulate intestinal microbiota in CKD and to reduce the serum levels of uremic toxins p-cresol and indoxyl sulfate are a promising area of research [93,94,95,96,97,98,99,100,101,102]. Preliminary data already demonstrated the ability of a functional food rich in prebiotic fibers (barley beta-glucans) to modulate intestinal microbiota composition and metabolome in a clinical trial involving healthy volunteers [103]. Moreover, beta-glucans were able to increase fecal SCFA levels [103] and to reduce circulating p-cresyl sulfate levels [104], demonstrating their ability to induce a shift toward an intestinal metabolism driven by saccharolytic bacteria.
In the context of CKD, several studies tried to modulate the intestinal environment and microbiota by using probiotics (i.e., Lactobacillus, Streptococcus, Bifidobacteria) [93,94,95,96, 105], prebiotics (i.e., arabic gum, oligofructose) [97,98,99], or synbiotics (i.e., Lactobacillus and Bifidobacterium combined with oligosaccharides) [101, 102]. In most cases, prebiotics and probiotics were administered to explore their effects on blood accumulation of blood urea nitrogen (BUN), p-cresyl sulfate, and/or indoxyl sulfate, which are the metabolic byproducts of nitrogen-containing compounds. In one study on probiotics, the use of a mix of bacteria (Lactobacillus acidophilus KB27, Bifidobacterium longum KB31, and Streptococcus thermophilus KB19) for 6 months reduced BUN and uric acid levels in stage 3–4 CKD patients [94]. In a more recent study [96], a 2-month treatment with a dairy product containing 16 × 109 CFU of Lactobacillus casei Shirota was able to reduce BUN concentration in CKD patients stages 3 and 4. Non-randomized studies on hemodialysis (HD) patients [93, 105] demonstrated a reduced excretion of p-cresol and indican (i.e., a precursor of indoxyl sulfate) and decreased serum levels of indoxyl sulfate [93, 105], probably owing to reduced intestinal production of these toxins. In CKD patients, the use of prebiotics also presented beneficial effects, such as BUN decrease [98], improved eGFR [99], higher fecal nitrogen excretion, and increased fecal saccharolytic bacteria [89]. These data suggest that the presence of prebiotics provides enough energy substrates for the intestinal microbiota, allowing saccharolytic bacteria to incorporate nitrogen for growth, thus reducing the production of uremic compounds. Other studies have shown a reduction of serum p-cresyl sulfate and p-cresyl sulfate generation rates in ESRD patients on hemodialysis when patients received prebiotics [98] or fiber-enriched food [99]. The use of synbiotics decreased serum p-cresol conjugate levels [100,101,102], normalized the amount and consistency of stool in HD patients [100], increased the counts of Bifidobacteria [101], and modified the stool microbiome of HD patients [102]. However, studies investigating the impact of probiotics on clinical endpoints (i.e., CVD and mortality) are lacking.
Conclusion
There is increased interest in the complex and bidirectional relationship between the host and its microbiota, especially regarding the role on the development of non-communicable disease such as obesity and diabetes. Since many studies are cross-sectional, it is not possible at the present time to establish a clear-cut causal relationship between intestinal dysbiosis and obesity or T2DM. However, we know that dysbiosis may cause endotoxemia and chronic inflammation, both through directly disrupting the intestinal barrier and by reducing the number of beneficial bacteria that produce SCFAs. T2DM and CKD, whose coexistence is increasingly found in clinical practice, share similar negative effects on both intestinal microbiota and the intestine itself. Thus, prospective studies are necessary to define causality, and further randomized controlled trials are needed in both T2DM and CKD to fully define the role of probiotic and prebiotic therapies.
References
Papers of interest, published recently, have been highlighted as: • Of importance •• Of major importance
WHO. Global status report on noncommunicable diseases 2014. World Health Organization. 2014; 176.
Johnson AMF, Olefsky JM. The origins and drivers of insulin resistance. Cell. 2013;152:673–84.
Tilg H, Moschen A. Inflammatory mechanisms in the regulation of insulin resistance. Mol Med. 2008;14:222–31.
American Diabetes Association. National Diabetes Statistics Report: 2014 Estimates of Diabetes and Its Burden in the Epidemiologic estimation methods. Natl Diabetes Stat Rep. 2014; 2009–2012.
Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, Jafar TH, Heerspink HJL, Mann JF, et al. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet. 2013;382:339–52.
2013 USRDS Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Am J Kidney Dis 2014; 63: supplement e1–e478.
Carrero JJ, Stenvinkel P. Persistent inflammation as a catalyst for other risk factors in chronic kidney disease: a hypothesis proposal. Clin J Am Soc Nephrol. 2009;4:S49–55.
Stenvinkel P, Carrero JJ, Axelsson J, Lindholm B, Heimburger O, Massy Z. Emerging biomarkers for evaluating cardiovascular risk in the chronic kidney disease patient: how do new pieces fit into the uremic puzzle? Clin J Am Soc Nephrol. 2008;3:505–21.
Honda H, Qureshi AR, Heimburger O, Barany P, Wang K, et al. Serum albumin, C-reactive protein, interleukin-6, and fetuin A as predictors of malnutrition, cardiovascular disease, and mortality in patients with ESRD. Am J Kidney Dis. 2006;47:139–48.
Carrero JJ, Yilmaz MI, Lindholm B, Stevinkel P. Cytokine dysregulation in chronic kidney disease: how can we treat it? Blood Purif. 2008;26:291–9.
Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, Falony G, et al. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500:541–6.
Larsen N, Vogensen FK, Van Den Berg FWJ, Nielsen DS, Andreasen AS, Pedersen BK, et al. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS One. 2010;5:e9085.
Wang J, Qin J, Li Y, Cai Z, Li S, Zhu J, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490:55–60.
Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–72.
Erridge C, Attina T, Spickett CM, Webb DJ. A high-fat meal induces low-grade endotoxemia: evidence of a novel mechanism of postprandial inflammation. Am J Clin Nutr. 2007;86:1286–92.
Harte AL, Varma MC, Tripathi G, Mcgee KC, Al-Daghri NM, Al-Attas OS, et al. High fat intake leads to acute postprandial exposure to circulating endotoxin in type 2 diabetic subjects. Diabetes Care. 2012;35:375–82.
Hildebrandt M, Hoffman C, Sherrill-mix S, Keilbaugh S, Chen Y, Knight R, et al. High-fat diet determines the composition of the murine gut microbiome independently of the obesity. Gastroenterology. 2009;137:1716–24.
Stenvinkel P. Inflammation in end-stage renal failure: could it be treated? Nephrol Dial Transpl. 2002;17:S33–8.
Kalantar-Zadeh K, Brennan ML, Hazen SL. Serum myeloperoxidase and mortality in maintenance hemodialysis patients. Am J Kidney Dis. 2006;48:59–68.
Moller DE, Kaufman KD. Metabolic syndrome: a clinical and molecular perspective. Annu Rev Med. 2005;56:45–62.
Kau AL, Ahern PP, Griffin NW, Goodman AL, Jeffrey I. Human nutrition, the gut microbiome, and immune system: envisioning the future. Nature. 2012;474:327–36.
Carrero JJ, Stenvinkel P, Cuppari L, Ikizler TA, Kalantar-Zadeh K, Kaysen G, et al. Etiology of the protein-energy wasting syndrome in chronic kidney disease: a consensus statement from the International Society of Renal Nutrition and Metabolism (ISRNM). J Ren Nutr. 2013;23:77–90.
Cerf-Bensussan N, Eberl G. The dialog between microbiota and the immune system: shaping the partners through development and evolution. Semin Immunol. 2012;24:1–2.
Szeto CC, Kwan BC, Chow KM, Lai KB, Chung KY, Leung CB, et al. Endotoxemia Is related to systemic inflammation and atherosclerosis in peritoneal dialysis patients. Clin J Am Soc Nephrol. 2008;3:431–6.
Gonçalves S, Pecoits-Filho R, Perreto S, Barberato SH, Stinghen AEM, Lima EGA, et al. Associations between renal function, volume status and endotoxaemia in chronic kidney disease patients. Nephrol Dial Transplant. 2006;21:2788–94.
Anders H-J, Andersen K, Stecher B. The intestinal microbiota, a leaky gut, and abnormal immunity in kidney disease. Kidney Int. 2013;83:1010–6.
Evenepoel P, Meijers BKI, Bammens BRM, Verbeke K. Uremic toxins originating from colonic microbial metabolism. Kidney Int. 2009;76:S12–9.
• Aron-Wisnewsky J, Clément K. The gut microbiome, diet and links to cardiometabolic and chronic disorders. Nat Rev Nephrol. 2016;12:169–81. This review is important because summarizes data suggesting a link between the intestinal microbiota and derived metabolites with food intake patterns, metabolic alterations and chronic cardiometabolic diseases.
Pflughoeft KJ, Versalovic J. Human microbiome in health and disease. Annu Rev Pathol. 2012;7:99–122.
Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–14.
Hollister EB, Gao C, Versalovic J. Compositional and functional features of the gastrointestinal microbiome and their effects on human health. Gastroenterology. 2014;146:1449–58.
Sabatino A, Regolisti G, Brusasco I, Cabassi A, Morabito S, Fiaccadori E. Alterations of intestinal barrier and microbiota in chronic kidney disease. Nephrol Dial Transplant. 2015;30:924–33. This review is relevant because it discusses in depth the cross-talk between the kidney and the intestine in CKD and ESRD.
•• Vaziri ND, Zhao YY, Pahl MV. Altered intestinal microbial flora and impaired epithelial barrier structure and function in CKD: the nature, mechanisms, consequences and potential treatment. Nephrol Dial Transplant. 2016;31:737–46. This review gives a thorough description on the mechanisms involved in the derangement of intestinal barrier in CKD and ESRD.
Baumgart DC, Dignass U. Intestinal barrier function. Curr Opin Clin Nutr Metab Care. 2002;5:685–94.
Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415–45.
Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A. 2005;102:11070–5.
Turnbaugh PJ, Bäckhed F, Fulton L, Gordon JI. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe. 2008;3:213–23.
Bäckhed F, Manchester JK, Semenkovich CF, Gordon JI. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc Natl Acad Sci. 2007;104:979–84.
Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, Kau L, et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice gut microbiota from twins metabolism in mice. Science. 2013;341:1241214.
Karlsson FH, Tremaroli V, Nookaew I, Bergström G, Behre CJ, Fagerberg B, et al. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature. 2013;498:99–103.
Ley R, Turnbaugh P, Klein S, Gordon J. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–3.
Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–31.
Gao Z, Yin J, Zhang J, Ward RE, Martin RJ, Lefevre M, et al. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes. 2009;58:1509–17.
Lin HV, Frassetto A, Kowalik EJ, Nawrocki AR, Lu MM, Kosinski JR, et al. Butyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms. PLoS One. 2012;7:e35240.
Wichmann A, Allahyar A, Greiner TU, Plovier H, Lundén GO, Larsson T, et al. Microbial modulation of energy availability in the colon regulates intestinal transit. Cell Host Microbe. 2013;14:582–90.
Vallon V, Docherty NG. Intestinal regulation of urinary sodium excretion and the pathophysiology of diabetic kidney disease: a focus on glucagon-like peptide 1 and dipeptidyl peptidase 4 in diabetic kidney disease. Exp Physiol. 2014;99:1140–5.
Allin KH, Nielsen T, Pedersen O. Mechanisms in endocrinology: gut microbiota in patients with type 2 diabetes mellitus. Eur J Endocrinol. 2015;172:167–77.
Ramezani A, Massy ZA, Meijers B, Evenepoel P, Vanholder R, Raj DS. Role of the gut microbiome in uremia: a potential therapeutic target. Am J Kidney Dis. 2016;67:483–98.
Vaziri ND, Wong J, Pahl M, Piceno YM, Yuan J, DeSantis TZ, et al. Chronic kidney disease alters intestinal microbial flora. Kidney Int. 2012;83:308–15.
De Angelis M, Montemurno E, Piccolo M, Vannini L, Lauriero G, Maranzano V, et al. Microbiota and metabolome associated with immunoglobulin a nephropathy (IgAN). PLoS One. 2014;9:e99006.
Piccolo M, De Angelis M, Lauriero G, Montemurno E, Di Cagno R, Gesualdo L, et al. Salivary microbiota associated with immunoglobulin A nephropathy. Microb Ecol. 2015;70:557–65.
David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–63.
Krishnamurthy VMR, Wei G, Baird BC, Murtaugh M, Chonchol MB, Raphael KL, et al. High dietary fiber intake is associated with decreased inflammation and all-cause mortality in patients with chronic kidney disease. Kidney Int. 2012;81:300–6.
Bammens B, Verbeke K, Vanrenterghem Y, Evenepoel P. Evidence for impaired assimilation of protein in chronic renal failure. Kidney Int. 2003;64:2196–203.
Mafra D, Barros AF, Fouque D. Dietary protein metabolism by gut microbiota and its consequences for chronic kidney disease patients. Futur Microbiol. 2013;8:1317–23.
Nyangale EP, Mottram DS, Gibson GR. Gut microbial activity, implications for health and disease: the potential role of metabolite analysis. J Proteome Res. 2012;11:5573–85.
Bammens B, Evenepoel P, Verbeke K, Vanrenterghem Y. Impairment of small intestinal protein assimilation in patients with end-stage renal disease: extending the malnutrition–inflammation–atherosclerosis concept. Am J Clin Nutr. 2004;80:1536–43.
Montemurno E, Cosola C, Dalfino G, Daidone G, De Angelis M, Gobbetti M, et al. What would you like to eat, Mr CKD Microbiota? A Mediterranean Diet, please! Kidney Blood Press Res. 2014;39:114–23.
Barrios C, Beaumont M, Pallister T, Villar J, Goodrich JK, Clark A, et al. Gut–microbiota–metabolite axis in early renal function decline. PLoS One. 2015;10:e0134311.
Wong J, Piceno YM, DeSantis TZ, Pahl M, Andersen GL, Vaziri ND. Expansion of urease- and uricase-containing, indole- and p-cresol-forming and contraction of short-chain fatty acid-producing intestinal microbiota in ESRD. Am J Nephrol. 2014;39:230–7.
Kang JY. The gastrointestinal tract in uremia. Dig Dis Sci. 1993;38:257–68.
Vaziri N, Freel W, Hatch M. Effect of chronic experimental renal insufficiency on urate metabolism. J Am Soc Nephrol. 1995;6:1313–7.
Hatch M, Vaziri ND. Enhanced enteric excretion of urate in rats with chronic renal failure. Clin Sci (Lond). 1994;86:511–6.
Hatch M, Freel RW, Vaziri ND. Intestinal excretion of oxalate in chronic renal failure. J Am Soc Nephrol. 1994;5:1339–43.
Neirynck N, Vanholder R, Schepers E, Eloot S, Pletinck A, Glorieux G. An update on uremic toxins. Int Urol Nephrol. 2013;45:139–50.
Sirich TL, Funk BA, Plummer NS, Hostetter TH, Meyer TW. Prominent accumulation in hemodialysis patients of solutes normally cleared by tubular secretion. J Am Soc Nephrol. 2014;25:615–22.
Vanholder R, Meert N, Schepers E, Glorieux G. Uremic toxins: do we know enough to explain uremia? Blood Purif. 2008;26:77–81.
Vanholder R, Baurmeister U, Brunet P, Cohen G, Glorieux G, Jankowski J, et al. A bench to bedside view of uremic toxins. J Am Soc Nephrol. 2008;19:863–70.
Viaene L, Meijers BKI, Bammens B, Vanrenterghem Y, Evenepoel P. Serum concentrations of p-cresyl sulfate and indoxyl sulfate, but not inflammatory markers, increase in incident peritoneal dialysis patients in parallel with loss of residual renal function. Perit Dial Int. 2014;34:71–8.
Dobre M, Meyer TW, Hostetter TH. Searching for uremic toxins. Clin J Am Soc Nephrol. 2013;8:322–7.
Meijers BKI, Claes K, Bammens B, De Loor H, Viaene L, Verbeke K, et al. p-Cresol and cardiovascular risk in mild-to-moderate kidney disease. Clin J Am Soc Nephrol. 2010;5:1182–9.
Liabeuf S, Barreto DV, Barreto FC, Meert N, Glorieux G, Schepers E, et al. Free p-cresylsulphate is a predictor of mortality in patients at different stages of chronic kidney disease. Nephrol Dial Transplant. 2010;25:1183–91.
Jourde-Chiche N, Dou L, Cerini C, Dignat-George F, Brunet P. Vascular incompetence in dialysis patients—protein-bound uremic toxins and endothelial dysfunction. Semin Dial. 2011;24:327–37.
Ito S, Yoshida M. Protein-bound uremic toxins: new culprits of cardiovascular events in chronic kidney disease patients. Toxins (Basel). 2014;6:665–78.
Dou L, Jourde-Chiche N, Faure V, Cerini C, Berland Y, Dignat-George F, et al. The uremic solute indoxyl sulfate induces oxidative stress in endothelial cells. J Thromb Haemost. 2007;5:1302–8.
Yu M, Kim YJ, Kang D-H. Indoxyl sulfate-induced endothelial dysfunction in patients with chronic kidney disease via an induction of oxidative stress. Clin J Am Soc Nephrol. 2011;6:30–9.
Barreto FC, Barreto DV, Liabeuf S, Meert N, Glorieux G, Temmar M, et al. Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin J Am Soc Nephrol. 2009;4:1551–8.
Rossi M, Campbell KL, Johnson DW, Stanton T, Vesey DA, Coombes JS, et al. Protein-bound uremic toxins, inflammation and oxidative stress: a cross-sectional study in stage 3–4 chronic kidney disease. Arch Med Res. 2014;45:309–17.
Chow J. Probiotics and prebiotics: a brief overview. J Ren Nutr. 2002;12:76–86.
de Vrese M, Schrezenmeir J. Probiotics, prebiotics and synbiotics. Adv Biochem Eng Biotechnol. 2008;111:1–66.
Griffiths EA, Duffy LC, Schanbacher FL, Qiao H, Dryja D, Leavens A, et al. In vivo effects of bifidobacteria and lactoferrin on gut endotoxin concentration and mucosal immunity in Balb/c mice. Dig Dis Sci. 2004;49:579–89.
Lovshin JA, Drucker DJ. Incretin-based therapies for type 2 diabetes mellitus. Nat Rev Endocrinol. 2009;5:262–9.
Veldhorst MAB, Westerterp KR, Westerterp-Plantenga MS. Gluconeogenesis and protein-induced satiety. Br J Nutr. 2012;107:595–600.
Monolayers C-C, Peng L, Li Z, Green RS, Holzman IR, Lin J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase. J Nutr. 2009;139:1619–25.
Vrieze A, Van Nood E, Holleman F, Salojarvi J, Kootte RS, Bartelsman JF, et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143:913–916.e7.
Yadav H, Lee JH, Lloyd J, Walter P, Rane SG. Beneficial metabolic effects of a probiotic via butyrate-induced GLP-1 hormone secretion. J Biol Chem. 2013;288:25088–97.
Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U S A. 2013;110:9066–71.
Jakobsdottir G, Xu J, Molin G, Ahrne S, Nyman M. High-fat diet reduces the formation of butyrate, but increases succinate, inflammation, liver fat and cholesterol in rats, while dietary fibre counteracts these effects. PLoS One. 2013;8:e80476.
Cani PD, Neyrinck AM, Fava F, Knauf C, Burcelin RG, Tuohy KM, et al. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia. 2007;50:2374–83.
Robertson MD, Bickerton AS, Dennis AL, Vidal H, Frayn KN. Insulin-sensitizing effects of dietary resistant starch and effects on skeletal muscle and adipose tissue metabolism. Am J Clin Nutr. 2005;82:559–67.
Salonen A, Lahti L, Salojarvi J, Holtrop G, Korpela K, Duncan SH, et al. Impact of diet and individual variation on intestinal microbiota composition and fermentation products in obese men. ISME J. 2014;8:2218–30.
Kovatcheva-Datchary P, Nilsson A, Akrami R, Lee YS, De Vadder F, Arora T, et al. Dietary fiber-induced improvement in glucose metabolism Is associated with increased abundance of Prevotella. Cell Metab. 2015;22:971–82.
Takayama F, Taki K, Niwa T. Bifidobacterium in gastro-resistant seamless capsule reduces serum levels of indoxyl sulfate in patients on hemodialysis. Am J Kidney Dis. 2003;41:S142–5.
Ranganathan N, Friedman EA, Tam P, Rao V, Ranganathan P, Dheer R. Probiotic dietary supplementation in patients with stage 3 and 4 chronic kidney disease: a 6-month pilot scale trial in Canada. Curr Med Res Opin. 2009;25:1919–30.
Ranganathan N, Ranganathan P, Friedman EA, Joseph A, Delano B, Goldfarb DS, et al. Pilot study of probiotic dietary supplementation for promoting healthy kidney function in patients with chronic kidney disease. Adv Ther. 2010;27:634–47.
Miranda Alatriste PV, Urbina Arronte R, Gomez Espinosa CO, de los Espinosa Cuevas M. Effect of probiotics on human blood urea levels in patients with chronic renal failure. Nutr Hosp. 2014;29:582–90.
Bliss DZ, Stein TP, Schleifer CR, Settle RG. Supplementation with gum arabic fiber increases fecal nitrogen excretion and lowers serum urea nitrogen concentration in chronic renal failure patients consuming a low-protein diet. Am J Clin Nutr. 1996;63:392–8.
Meijers BKI, De Preter V, Verbeke K, Vanrenterghem Y, Evenepoel P. p-Cresyl sulfate serum concentrations in haemodialysis patients are reduced by the prebiotic oligofructose-enriched inulin. Nephrol Dial Transplant. 2010;25:219–24.
Salmean YA, Segal MS, Langkamp-Henken B, Canales MT, Zello GA, Dahl WJ. Foods with added fiber lower serum creatinine levels in patients with chronic kidney disease. J Ren Nutr. 2013;23:e29–32.
Nakabayashi I, Nakamura M, Kawakami K, Ohta T, Kato I, Uchida K, et al. Effects of synbiotic treatment on serum level of p-cresol in haemodialysis patients: a preliminary study. Nephrol Dial Transplant. 2011;26:1094–8.
Cruz-Mora J, Martínez-Hernández NE, Martín del Campo-López F, Viramontes-Hörner D, Vizmanos-Lamotte B, Muñoz-Valle JF, et al. Effects of a symbiotic on gut microbiota in Mexican patients with end-stage renal disease. J Ren Nutr. 2014;24:330–5.
Rossi M, Johnson DW, Morrison M, Pascoe EM, Coombes JS, Forbes JM, et al. Synbiotics easing renal failure by improving gut microbiology (SYNERGY): a randomized trial. Clin J Am Soc Nephrol. 2016;11:223–31.
De Angelis M, Montemurno E, Vannini L, Cosola C, Cavallo N, Gozzi G, et al. Effect of whole-grain barley on the human fecal microbiota and metabolome. Appl Environ Microbiol. 2015;81:7945–56.
Cosola C, De Angelis M, Rocchetti MT, Montemurno E, Maranzano V, Dalfino G, et al. Beta-glucans supplementation associates with reduction in p-cresyl sulfate levels and improved endothelial vascular reactivity in healthy individuals. PLoS One. 2017;12(1):e0169635. doi:10.1371/journal.pone.0169635.
Hida M, Aiba Y, Sawamura S, Suzuki N, Satoh T, Koga Y. Inhibition of the accumulation of uremic toxins in the blood and their precursors in the feces after oral administration of Lebenin, a lactic acid bacteria preparation, to uremic patients undergoing hemodialysis. Nephron. 1996;74:349–55.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
A.S., G.R., C.C., L.G., and E.F. declare that they have no competing interests.
Human and Animal Rights and Informed Consent
This article contains studies with human subjects performed by one of the authors, L.G. In this case, all procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the Helsinki declaration. This article does not contain any studies with animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Microvascular Complications—Nephropathy
Rights and permissions
About this article
Cite this article
Sabatino, A., Regolisti, G., Cosola, C. et al. Intestinal Microbiota in Type 2 Diabetes and Chronic Kidney Disease. Curr Diab Rep 17, 16 (2017). https://doi.org/10.1007/s11892-017-0841-z
Published:
DOI: https://doi.org/10.1007/s11892-017-0841-z