Abstract
Quorum sensing (QS) is an important protective mechanism that allows bacteria to adapt to its environment. A limited number of signal molecules play the key role of transmitting information in this mechanism. Signals are transmitted between individual bacterium through QS systems, resulting in the expression of specific genes. QS plays an important role in a variety of bacterial processes, including drug resistance, biofilm formation, motility, adherence, and virulence. Most Gram-positive and Gram-negative bacteria possess QS systems, mainly the LuxS/AI-2-mediated QS system. Evidence has been brought that LuxS/AI-2 system controls major virulence determinants in Streptococcus suis and, as such, the ability of this bacterial species to cause infections in humans and pigs. Understanding the S. suis LuxS/AI-2 system may open up novel avenues for decreasing the drug resistance and infectivity of S. suis. This article focuses on the progress made to date on the S. suis LuxS/AI-2-mediated QS system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Quorum sensing (QS) is a cell-to-cell communication mechanism that mediates coordinated adaptation of bacteria by regulating the expression of numerous genes. QS has two distinct characteristics. The first relates to its complexity, which is reflected by the wide variety of signaling molecules exhibiting various functions and by the different types of communication between different QS systems. The second is the diversity of QS systems in terms of distribution, signaling molecules, and mechanisms as well as induction and transport of signaling molecules (Grandclement et al. 2016; Hawver et al. 2016; Ma et al. 2017a). QS systems are currently divided into different classes: the LuxR-LuxI systems of Gram-negative bacteria, the auto-inducing peptide (AIP) of Gram-positive bacteria, and the LuxS/AI-2 system found in both Gram-positive and Gram-negative bacteria. Many studies have shown that QS systems are responsible for group behavior, cell luminescence, antibiotic resistance, plasmid transfer, virulence factor gene expression, and biofilm formation (Asif and Acharya 2012; Kalia et al. 2015; Miller et al. 2015; Singh et al. 2016). Streptococcus suis is a zoonotic pathogen that is mainly responsible for septicemia, meningitis, pneumonia, arthritis, and even sudden death in humans and pigs worldwide (Segura et al. 2016). S. suis can be divided into 33 serotypes, of which serotype 2 (S. suis 2) is the most pathogenic and the most harmful to the swine industry, especially in East and Southeast Asian countries such as Thailand, Vietnam, and China (Hatrongjit et al. 2015; Wertheim et al. 2009; Yu et al. 2006). To date, S. suis has spread to more than 30 countries and regions around the world (Feng et al. 2014). According to reports, there were more than 1600 cases of human-infected S. suis in the world, and the vast majority of the patients were from Southeast Asia (Chatzopoulou et al. 2015). Consequently, S. suis is a constant threat to the health of humans and pigs. The LuxS/AI-2 QS system is believed to be involved in the virulence and drug resistance of S. suis.
QS mechanism
Bacteria possessing a QS system are capable of producing and releasing signaling molecules known as autoinducers (AIs), which increase from concentration as a function of cell density (Rémy et al. 2016; Tan et al. 2017). Bacteria can monitor variations in the concentrations of autoinducers to track changes in cell numbers and to collectively alter the global pattern of gene expression. QS signaling molecules include acyl-homoserine lactones (AHLs), AIP, autoinducer-2 (AI-2), diffusible signal factors (DSFs), fatty acids, and partial ester compounds. Many Gram-negative bacteria rely on the secretion of AHLs to communicate and coordinate group behavior, such as the production of extracellular enzymes and toxins (Williams 2007), biofilm formation (Alagely et al. 2011; Kim et al. 2014; Parsek and Greenberg 2005; Shih and Huang 2002), antibiotic resistance (Bainton et al. 1992), and bacterial motility (Eberl et al. 1996). The structure of AIP in Gram-positive bacteria differs according to the species (Malone et al. 2007; Novick and Muir 1999). It cannot be transported on its own across the cell wall and generally requires an ATP-binding-cassette (ABC) transport system or other membrane channel proteins for translocation outside the cell. The binary signal system of bacteria can regulate the transcriptional expression of target genes through a complex signal transduction process (Zollmann et al. 2015). The LuxS/AI-2-mediated QS system mediates interspecies and intraspecies information exchanges between Gram-positive and Gram-negative bacteria (He et al. 2015; Thompson et al. 2015).
LuxS
The luxS gene is highly conserved in bacteria. For instance, a comparative sequence analysis of luxS in Streptococcus mutans and Streptococcus pyogenes showed that the two genes share 84% identity and 92% similarity (Merritt et al. 2003). High identity and similarity of the above luxS genes were also found with those from Streptococcus pneumoniae, Lactococcus lactis, Clostridium perfringens, Neisseria meningitidis, Escherichia coli, and Haemophilus influenza (Merritt et al. 2003).
The LuxS protein is a homodimeric metallo-enzyme that contains two identical tetrahedral metal-binding sites. The core of each metal binding site contains a divalent zinc ion with two histidines, a cysteine and a water molecule, which structure is similar to active sites identified in some amidases and peptidases (Hilgers and Ludwig 2001). The LuxS system is involved in the synthesis of autoinducer AI-2, which is a furanosyl borate diester (Sperandio et al. 2003).
Structure and activity of LuxS in S. suis
Analysis of the three-dimensional crystal structure of S. suis LuxS revealed the presence of four LuxS monomers in each asymmetric unit (PDB ID 4XCH) (Fig. 1). The LuxS monomers consist of four antiparallel β-sheets and four antiparallel alpha helices in the order H1-S1-S2-H2-S3-S4-H3-H4 (Wang et al. 2015). Moreover, ion coupled plasma mass spectrometry has shown that Zn2+ is the main component of the active center of S. suis LuxS. This is consistent with the study by Ruzheinikov et al. (2001), who investigated the Bacillus subtilis LuxS. In a previous study, Zhu et al. (2003) determined the activity of LuxS by measuring the release of homocysteine using Ellman’s reagent. They found that the ligand environment of LuxS metalloproteinase is similar to the envelopellase of polypeptides; LuxS in the form of Fe2+ rapidly deactivates under aerobic conditions. However, the LuxS protein composed of Zn2+ or Co2+ is very stable, and the catalytic ability of Co-LuxS was the same as that of Fe-LuxS and 10 times stronger than that of Zn-LuxS. Rajan et al. (2005) also reported the presence of Fe2+ in the LuxS of Bacillus subtilis. These results suggest that different bacterial LuxS proteins may have different metal ions and that this may impact their catalytic efficiency. A bioinformatic analysis has revealed that there are possible evolutionary mutations at amino acid positions 80 and 87, which are located near the substrate binding site (Wang et al. 2015). These two amino acid mutations have a marked effect on the binding of the substrate, the catalytic activity of the enzyme, the production of AI-2 by Streptococcus spp., and the ability to form biofilms. In vitro and in vivo tests have shown that the absence of mutations in these two amino acids can inhibit the production of AI-2 and the formation of biofilm (Wang et al. 2011).
Metabolism
LuxS is not only involved in the production of the AI-2 signaling molecule but also plays an important role in central bacterial metabolism and is part of the activated methyl cycle (Trappetti et al. 2017). LuxS is mainly responsible for the hydrolysis of S-adenosine homocysteine to S-adenosylmethionine (SAMe). SAMe is a ubiquitous biomolecule that serves primarily as a methyl donor. It is the main route by which bacteria recycle methyl groups and is key to polyamine formation and vitamin synthesis by bacteria (Bonhoure et al. 2015). A mutation or deletion of luxS results in the loss of SAMe function and the inhibition of AI-2 synthesis, indicating that QS activity will likely be affected if the luxS mutation causes a change in the relevant phenotype. The induction of luxS can also lead to changes in the extracellular concentrations of other metabolites. A recombinant LuxS assay showed that the extracellular concentration of S-ribosyl homocysteine (SAMe with a LuxS function) is significantly higher in the culture supernatants of LuxS-deficient strains than in the wild-type strain (Challan Belval et al. 2006). Based on the above results, it is possible that numerous SAMe pathway intermediates inside and outside LuxS mutants are modified.
LuxS/AI-2 system
LuxS plays a fundamental role in LuxS/AI-2-mediated QS system, while AI-2 is a by-product of bacterial methyl metabolism and balances the metabolism in activated methyl cycles (AMC) (Yang and Defoirdt 2015). In this process, the methyl group is removed from SAMe and is converted into thioglucoside homocysteine (SAH), which represents a toxic metabolite. SAH is subsequently converted by a 5′-methylthioadenosine/S-adenosyl homocysteine nucleosidase (Pfs) into adenine and thioglycoside-type homocysteine (SRH). SRH is then converted into 4,5-dihydroxy-2,3-pentanedione (DPD) and homocysteine acid (HCY) by LuxS (Malladi et al. 2011; Tavender et al. 2008). Bassler et al. studied luminescence in Vibrio harveyi and found that S-glycosylated homocystease is encoded by luxS (Bassler et al. 2010). LuxS catalyzes the cleavage of the thioether linkage of SRH to produce HCY and DPD. DPD is formed by self-cyclization, yielding AI-2 (Fig. 2) (Han and Lu 2009c; Ma et al. 2015; Miller et al. 2004; Winzer et al. 2003). As the bacterial density increases, AI-2-mediated QS is activated, LuxO phosphorylation ceases, and LuxO and LuxR act together to transcribe the luxCDABE operon, causing the bacteria to emit light. There are currently more than 55 known luxS homologs, but only a few of the molecular structures of AI-2 have been clearly determined. Han et al. and Cao et al. analyzed the S. suis genome and showed that luxS can produce AI-2 signaling molecules (Cao et al. 2011; Han and Lu 2009a).
The synthetic pathway of AI-2 molecules in bacteria. S-adenosylmethionine (SAM), the major methyl donor in metabolic processes, is responsible for the production of AI-2. SAM transfers methyl groups to methyl acceptors with the aid of methyltransferases to produce S-adenosylhomocysteine (SAH). SAH is hydrolyzed to S-ribose homocysteine (SRH) with the involvement of the nucleoside Pfs with a concomitant release of adenine. The thioether bond of SRH is cleaved under the action of the LuxS protein and produces homocysteine and 4,5-dihydroxy-2,3-pentanedione (DPD). It is reported that AI-2 is the result of DPD self-cycling reaction, involving least two reactions. SAM can also form the main triamine spermidine by decarboxylation with diamine and putrescine. The reaction is also accompanied by the release of the toxic by-product 5′-thiomethyladenosine (MTA), which is then modified by Pfs, producing adenine and 5′-thiomethylribose (MTR). The exact export mechanism of AI-2 is unknown
Gene expression levels of LuxS/AI-2 system in S. suis
The absence of luxS leads to changes in the gene expression and phenotype of bacteria, and the LuxS protein directly regulates the gene expression of bacteria and the functions of various proteins, including enzymes involved in biofilm formation, bacteriocin synthesis, cell competence, and acid resistance. Hasona et al. (2007) used gene chips to analyze the decreased expression of LuxS protein in Streptococcus mutans, and found that the expression of 81 genes related to cell competence, including chaperones, proteases, cell envelope synthetases, DNA repair, and replication enzymes, was upregulated. On the other hand, the downregulation of 35 genes involved in ribosomal proteins, and biosynthetic enzymes of amino acids and proteins was also observed. Sztajer et al. (2008), through a global transcriptome analysis of a luxS null mutant of S. mutans, found that the expressions of 585 genes were still affected after adding chemically pure DPD to the medium. It suggested that the LuxS enzyme plays an important role in the methyl transfer metabolism.
The Pfs enzyme converts S-adenosylhomocysteine into S-ribosylhomocysteine and adenine and plays an important role in bacterial metabolism and synthesis of AI-2 (Cao et al. 2011; Han and Lu 2009a). AI-2 activity and the transcription of pfs reach their highest levels in the late logarithmic growth phase of S. suis 2 while the transcription of luxS is higher during the stationary phase. On the other hand, the transcription of pfs and the production of AI-2 remain the same while the transcription of luxS and the production of AI-2 do not (Han and Lu 2009b). The differences in transcription between the S. suis wild-type and the luxS deletion mutant (ΔluxS) strains were determined using an Agilent microarray. The results showed that 312 genes are differentially expressed in wild-type cells, of which 144 were upregulated and 168 were downregulated. By introducing DPD into the ΔluxS strain, 79 genes were differentially expressed. Of these, 29 were related to the production of S. suis virulence factors and the uptake of iron were regulated by the LuxS/AI-2-mediated QS system (Cao et al. 2011). We also constructed a S. suis luxS+ overexpressing strain and showed by real-time PCR that the expression of luxS increases throughout all the growth phases, while the level of pfs expression remains unchanged. Overexpressing luxS did not increase the level of pfs expression or AI-2 production. The overexpressing strain formed more biofilms, which increased with the incubation time. However, the bacteria grew more than the wild-type strain, indicating that the production of AI-2 is not correlated with the transcription of luxS. Although the expression of luxS is constitutive, the transcription of pfs is possibly correlated with AI-2 production in S. suis (Wang et al. 2013). These results suggest that the production of AI-2 is controlled by the interaction between luxS and pfs and increases only when LuxS does not increase the production of AI-2.
Characteristics of LuxS/AI-2 system in S. suis
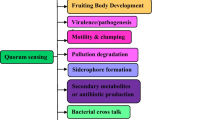
The LuxS/AI-2 system impacts on various biological properties of bacteria. It is considered as one of the most important global regulatory networks in bacteria; the regulation of the expression of the corresponding genes is modulated as the bacterial concentrations change. In general, regulating gene expression often causes a variety of phenotypic changes. Many physiological functions of bacteria have been found to be regulated by the LuxS/AI-2 system, including bacterial luminescence, antibiotic susceptibility, plasmid transfer, virulence, gene expression, and biofilm formation (Ma et al. 2015; Wang et al. 2017). In S. suis, current research is mainly focused on the impacts of the LuxS/AI-2-mediated QS system on the regulation of the biological characteristics described below.
Bacterial growth
By observing the growth curves and the morphological changes of bacterial mutants and wild-type cells, physiological changes displayed by the mutants can be more intuitively understood. The growth rate of a ΔluxS S. suis mutant was found to be lower than that of the wild-type strain. The logarithmic growth phase of the ΔluxS S. suis mutant lagged behind that of the wild-type strain, and the growth rate of the mutant was lower than that of the wild-type strain. The wild-type strain reached the quiescent period 90 min ahead of the ΔluxS strain. Light microscopy showed that cells of the ΔluxS strain aggregate and that the length of the bacterial chains was shorter than that of the wild-type strain. Electron microscopy revealed that the thickness of the capsule of the ΔluxS mutant was thinner than that of the wild-type strain (Cao et al. 2011).
Cell adhesion
The first steps in the infection of a host cell by a pathogen are usually adhesion and then colonization. By using site-specific integration, isogenic mutations were generated in luxS, and the resulting Lactobacillus acidophilus mutants resulted in a 58% reduction in adhesion to Caco-2 cells (Buck et al. 2009). The same phenomena also appear in Actinobacillus pleuropneumoniae (Li et al. 2011) and Lactobacillus plantarum (Jia et al. 2018).
Comparative analysis of adhesion of the S. suis wild-type strain and the ΔluxS mutant may provide insight regarding regulation of adherence by the LuxS/AI-2 system. The ability of the luxS mutant to adhere to human epithelial laryngeal carcinoma cells and human umbilical vein endothelial cells was found to be lower than that of the wild-type strain. Moreover, low concentrations of exogenous AI-2 added to the S. suis wild-type strain and luxS mutant increased adhesion to the host cells, while high concentrations of AI-2 decreased adhesion, with 4 and 6 μM AI-2 being the most efficient at improving adhesion (Yang et al. 2014).
Biofilm formation
Biofilms are produced by bacteria and embed the entire bacterial population. Biofilms have a significant effect on bacterial virulence and drug resistance. In recent years, studies have found that QS systems and two-component regulatory systems play an important regulatory role in the formation and development of bacterial biofilms (Christiaen et al. 2014; O'Loughlin et al. 2013). Among them, the LuxS/AI-2-mediated QS system has attracted more and more attention due to its widespread existence in Gram-positive and Gram-negative bacteria.
The ability of S. suis to form biofilm was significantly increased when low amount of AI-2 was added to the growth medium, while the addition of high concentrations of AI-2 suppressed the ability to form biofilm. The addition of 2 μM AI-2 significantly increased biofilm formation at 24 h but had no effect at 48 h (Yang et al. 2014). These results showed that the ability of S. suis to form biofilm is enhanced by the overexpression of AI-2 and the incubation time (Wang et al. 2013).
Hemolytic activity
Hemolysis can cause anemia, sepsis, and other symptoms. As such, bacterial hemolytic activity is an important indicator of pathogenicity. Pecharki et al. (2008) found that the hemolytic activity of a Streptococcus intermedius luxS mutant was five times lower than that of the wild-type strain. Supplementing the culture medium with the AI-2 precursor 4,5-dihydroxy-2,3-pentanedione restored the hemolytic activity of the mutant. In our study, it was found that the maximum dilution ratio of culture supernatant required to lyse 50% of erythrocytes is 1:16, 1:2, and 1:16 for the wild-type, ΔluxS, and luxS complement strains of S. suis 2 HA9801, respectively. This suggests that luxS can modulate the hemolytic ability of bacteria (Wang et al. 2011).
Bacterial virulence
Virulence factor gene expression by bacterial pathogens is required to cause infections. Virulence gene expression must be precisely regulated if pathogens are to infect a host and cause a disease. LuxS has been reported to play a critical role in regulating both bacterial virulence and interspecies QS in a broad variety of bacteria (Jones et al. 2010; Li et al. 2011; Ma et al. 2017b). The expression of the virulence genes gdh, cps, mrp, gapdh, sly, fbps, and ef was found to be 0.66-, 0.61-, 0.45-, 0.48-, 0.29-, 0.57-, and 0.38-fold lower, respectively, in the ΔluxS mutant strain than in the wild-type strain of S. suis. Zebrafish infection experiments have shown that the virulence of ΔluxS strain is 10 times lower than that of the wild-type strain and that complemented strains showed a partially restored level of virulence (Wang et al. 2011). Experiments in a pig model have also shown that the number of ΔluxS bacteria infecting the pig lung, brain, and joints is significantly lower than that of the wild-type strain (Cao et al. 2011).
Stress responses
Bacterial stress responses, referring to changes in the physiology and biochemistry of bacteria, are caused by variations in their normal living environment (Pham et al. 2017); it may also cause morphologic changes of the bacteria (Oh et al. 2015). The stress response is a spontaneous adaptation of the bacteria to the external environment. A previous study showed that inactivation of the luxS gene results in a wide range of phenotypic changes, including an increase in tolerance to H2O2 (Cao et al. 2011). Similar results were also observed in our laboratory (unpublished data). Under oxidative stress conditions, the wild-type strain of S. suis treated with H2O2 exhibited sensitivity to H2O2, whereas the ΔluxS was more resistant to H2O2. On the other hand, the acid resistance assay showed that the inactivation of the luxS gene results in a rapid decrease in the number of S. suis cells. Similarly, in the presence of an iron chelator, the growth of ΔluxS was significantly decreased (unpublished data).
Future prospects
Given the ongoing increase in the antibiotic resistance of bacterial pathogens and the important role played by the LuxS/AI-2 system in intercellular communication, metabolism, and virulence, a novel antibacterial strategy based on interference with the LuxS/AI-2-mediated QS system can be proposed. A better knowledge of the mechanisms of bacterial regulation and the regulation of AI-2 uptake is essential in order to be able to control bacteria through inhibition of pathogen signaling. More specifically, in-depth studies are required to identify the genes involved in the uptake of AI-2 by S. suis, the genes associated with AI-2, the target proteins of S. suis that are regulated by AI-2, the downstream target proteins regulated following AI-2 binding to the receptor protein, and the regulation of the QS signaling pathway. These issues need to be urgently addressed. Gene chip technology is a widely used DNA analytical approach for detecting gene transcription levels through nucleic acid hybridization. Many studies have used this approach to identify a large number of genes affected by LuxS and AI-2 in S. suis. Investigations by our group to identify the S. suis AI-2 receptor are ongoing. It is planned to construct a random S. suis mutant library using the transposon Tn917 and find the gene-mediated downstream regulatory network of AI-2 and its receptor through gene chip analysis and proteomics. With the deepening research on QS systems, researchers have developed a variety of quorum sensing inhibitors (QSIs), including both natural and synthetic agents. These QSIs can be divided into three categories: (1) non-peptide small-molecule substances such as garlic extract, thiolactones, and AI-2 analogues; (2) peptides such as leader peptide for oligopeptide signal molecules (AgrD); and (3) proteins such as QS degrading enzymes. These inhibitors can block QS by inhibiting signal production, blocking signal receptors or disrupting QS signals, and also providing an alternative way of controlling microbial pathogenesis (Pan and Ren 2009). In addition, the advantage of using QSI is not only to prevent bacterial growth but also to avoid the production of bacterial resistant strains (Saurav et al. 2017). Consequently, QSIs inhibitors appear to have good prospects for the prevention and control of pathogens.
References
Alagely A, Krediet CJ, Ritchie KB, Teplitski M (2011) Signaling-mediated cross-talk modulates swarming and biofilm formation in a coral pathogen Serratia marcescens. ISME J 5(10):1609–1620. https://doi.org/10.1038/ismej.2011.45
Asif M, Acharya M (2012) Quorum sensing: a Nobel target for antibacterial agents. Avicenna J Med 2(4):97–99. https://doi.org/10.4103/2231-0770.110743
Bainton NJ, Bycroft BW, Chhabra SR, Stead P, Gledhill L, Hill PJ, Rees CE, Winson MK, Salmond GP, Stewart GS (1992) A general role for the lux autoinducer in bacterial cell Signalling: control of antibiotic biosynthesis in Erwinia. Gene 116(1):87–91. https://doi.org/10.1016/0378-1119(92)90633-Z
Bassler BL, Wright M, Showalter RE, Silverman MR (2010) Intercellular Signalling in Vibrio Harveyi: sequence and function of genes regulating expression of luminescence. Mol Microbiol 9(4):773–786
Bonhoure N, Byrnes A, Moir RD, Hodroj W, Preitner F, Praz V, Marcelin G, Jr CS, Martinezlopez N, Singh R (2015) Loss of the Rna polymerase iii repressor Maf1 confers obesity resistance. Genes Dev 29(9):934–947. https://doi.org/10.1101/gad.258350.115
Buck BL, Azcarateperil MA, Klaenhammer TR (2009) Role of Autoinducer-2 on the adhesion ability of Lactobacillus Acidophilus. J Appl Microbiol 107(1):269–279
Cao M, Feng Y, Wang C, Zheng F, Li M, Liao H, Mao Y, Pan X, Wang J, Hu D (2011) Functional definition of Luxs, an Autoinducer-2 (Ai-2) synthase and its role in full virulence of Streptococcus Suis Serotype 2. J Microbiol 49(6):1000–1011
Challan Belval S, Gal L, Margiewes S, Garmyn D, Piveteau P, Guzzo J (2006) Assessment of the roles of Luxs, S-Ribosyl homocysteine, and autoinducer 2 in cell attachment during biofilm formation by Listeria Monocytogenes Egd-E. Appl Environ Microbiol 72(4):2644–2650. https://doi.org/10.1128/AEM.72.4.2644-2650.2006
Chatzopoulou M, Voulgaridou I, Papalas D, Vasiliou P, Tsiakalou M (2015) Third case of Streptococcus Suis infection in Greece. Case Rep Infect Dis 2015:505834. https://doi.org/10.1155/2015/505834
Christiaen SE, Matthijs N, Zhang XH, Nelis HJ, Bossier P, Coenye T (2014) Bacteria that inhibit quorum sensing decrease biofilm formation and virulence in Pseudomonas Aeruginosa Pao1. Pathog Dis 70(3):271–279. https://doi.org/10.1111/2049-632X.12124
Eberl L, Winson MK, Sternberg C, Stewart GS, Christiansen G, Chhabra SR, Bycroft B, Williams P, Molin S, Givskov M (1996) Involvement of N-acyl-L-Hormoserine lactone autoinducers in controlling the multicellular behaviour of Serratia Liquefaciens. Mol Microbiol 20(1):127–136. https://doi.org/10.1111/j.1365-2958.1996.tb02495.x
Feng Y, Zhang H, Wu Z, Wang S, Cao M, Hu D, Wang C (2014) Streptococcus Suis infection: an emerging/reemerging challenge of bacterial infectious diseases? Virulence 5(4):477–497. https://doi.org/10.4161/viru.28595
Grandclement C, Tannieres M, Morera S, Dessaux Y, Faure D (2016) Quorum quenching: role in nature and applied developments. FEMS Microbiol Rev 40(1):86–116. https://doi.org/10.1093/femsre/fuv038
Han XG, Lu CP (2009a) Biological activity and identification of a peptide inhibitor of Luxs from Streptococcus Suis Serotype 2. FEMS Microbiol Lett 294(1):16–23. https://doi.org/10.1111/j.1574-6968.2009.01534.x
Han XG, Lu CP (2009b) Detection of Autoinducer-2 and analysis of the profile of Luxs and Pfs transcription in Streptococcus Suis Serotype 2. Curr Microbiol 58(2):146–152. https://doi.org/10.1007/s00284-008-9291-9
Han XG, Lu CP (2009c) In vitro biosynthesis of autoinducer 2 of Steptococcus Suis Serotype 2 using recombinant Luxs and Pfs. Enzym Microb Technol 44(1):40–45. https://doi.org/10.1016/j.enzmictec.2008.09.009
Hasona A, Zuobihasona K, Crowley PJ, Abranches J, Ruelf MA, Bleiweis AS, Brady LJ (2007) Membrane composition changes and physiological adaptation by Streptococcus Mutans signal recognition particle pathway mutants. J Bacteriol 189(4):1219–1230
Hatrongjit R, Kerdsin A, Gottschalk M, Dan T, Hamada S, Oishi K, Akeda Y (2015) First human case report of Sepsis due to infection with Streptococcus Suis Serotype 31 in Thailand. BMC Infect Dis 15(1):392. https://doi.org/10.1186/s12879-015-1136-0
Hawver LA, Jung SA, Ng WL (2016) Specificity and complexity in bacterial quorum-sensing systems. FEMS Microbiol Rev 40(5):738–752. https://doi.org/10.1093/femsre/fuw014
He Z, Liang J, Tang Z, Ma R, Peng H, Huang Z (2015) Role of the Luxs gene in initial biofilm formation by Streptococcus Mutans. J Mol Microbiol Biotechnol 25(1):60–68. https://doi.org/10.1159/000371816
Hilgers MT, Ludwig ML (2001) Crystal structure of the quorum-sensing protein Luxs reveals a catalytic metal site. Proc Natl Acad Sci U S A 98(20):11169. https://doi.org/10.1073/pnas.191223098
Jia FF, Zheng HQ, Sun SR, Pang XH, Liang Y, Shang JC, Zhu ZT, Meng XC (2018) Role of Luxs in stress tolerance and adhesion ability in Lactobacillus Plantarum Klds1.0391. Biomed Res Int 2018:4506829. https://doi.org/10.1155/2018/4506829
Jones MB, Peterson SN, Benn R, Braisted JC, Jarrahi B, Shatzkes K, Ren D, Wood TK, Blaser MJ (2010) Role of Luxs in Bacillus Anthracis growth and virulence factor expression. Virulence 1(2):72–83
Kalia M, Yadav VK, Singh PK, Sharma D, Pandey H, Narvi SS, Agarwal V (2015) Effect of cinnamon oil on quorum sensing-controlled virulence factors and biofilm formation in Pseudomonas Aeruginosa. PLoS One 10(8):e0135495. https://doi.org/10.1371/journal.pone.0135495
Kim AL, Park SY, Lee CH, Lee CH, Lee JK (2014) Quorum quenching Bacteria isolated from the sludge of a wastewater treatment plant and their application for controlling biofilm formation. J Microbiol Biotechnol 24(11):1574–1582
Li L, Xu Z, Zhou Y, Li T, Sun L, Chen H, Zhou R (2011) Analysis on Actinobacillus Pleuropneumoniae Luxs regulated genes reveals pleiotropic roles of Luxs/Ai-2 on biofilm formation, adhesion ability and Iron metabolism. Microb Pathog 50(6):293–302
Ma YP, Ke H, Hao L (2015) Luxs/Ai-2 quorum sensing is involved in antimicrobial susceptibility in Streptococcus Agalactiae. Fish Pathol 50(1):8–15. https://doi.org/10.3147/jsfp.50.8
Ma H, Wang X, Zhang Y, Hu H, Ren H, Geng J, Ding L (2017a) The diversity, distribution and function of N-acyl-Homoserine lactone (Ahl) in industrial anaerobic granular sludge. Bioresour Technol 247:116–124. https://doi.org/10.1016/j.biortech.2017.09.043
Ma Y, Hao L, Ke H, Liang Z, Ma J, Liu Z, Li Y (2017b) Luxs/Ai-2 in Streptococcus Agalactiae reveals a key role in acid tolerance and virulence. Res Vet Sci 115:501–507. https://doi.org/10.1016/j.rvsc.2017.07.032
Malladi VL, Sobczak AJ, Meyer TM, Pei D, Wnuk SF (2011) Inhibition of Luxs by S-Ribosylhomocysteine analogues containing a [4-Aza]ribose ring. Bioorg Med Chem 19(18):5507–5519
Malone CL, Boles BR, Horswill AR (2007) Biosynthesis of Staphylococcus Aureus autoinducing peptides by using the Synechocystis Dnab mini-Intein. Appl Environ Microbiol 73(19):6036–6044. https://doi.org/10.1128/AEM.00912-07
Merritt J, Qi F, Goodman SD, Anderson MH, Shi W (2003) Mutation of Luxs affects biofilm formation in Streptococcus Mutans. Infect Immun 71(4):1972–1979
Miller ST, Xavier KB, Campagna SR, Taga ME, Semmelhack MF, Bassler BL, Hughson FM (2004) Salmonella Typhimurium recognizes a chemically distinct form of the bacterial quorum-sensing signal Ai-2. Mol Cell 15(5):677–687. https://doi.org/10.1016/j.molcel.2004.07.020
Miller KP, Wang L, Chen YP, Pellechia PJ, Benicewicz BC, Decho AW (2015) Engineering nanoparticles to silence bacterial communication. Front Microbiol 6(Article 189):189. https://doi.org/10.3389/fmicb.2015.00189
Novick RP, Muir TW (1999) Virulence gene regulation by peptides in Staphylococci and other gram-positive Bacteria. Curr Opin Microbiol 2(1):40–45. https://doi.org/10.1016/S1369-5274(99)80007-1
Oh E, Mcmullen L, Jeon B (2015) Impact of oxidative stress defense on bacterial survival and morphological change in Campylobacter Jejuni under aerobic conditions. Front Microbiol 6:295
O'Loughlin CT, Miller LC, Siryaporn A, Drescher K, Semmelhack MF, Bassler BL (2013) A quorum-sensing inhibitor blocks Pseudomonas Aeruginosa virulence and biofilm formation. Proc Natl Acad Sci U S A 110(44):17981–17986. https://doi.org/10.1073/pnas.1316981110
Pan J, Ren D (2009) Quorum sensing inhibitors: a patent overview. Expert Opin Ther Pat 19(11):1581–1601. https://doi.org/10.1517/13543770903222293
Parsek MR, Greenberg EP (2005) Sociomicrobiology: the connections between quorum sensing and biofilms. Trends Microbiol 13(1):27–33. https://doi.org/10.1016/j.tim.2004.11.007
Pecharki D, Petersen FC, Scheie AA (2008) Luxs and expression of virulence factors in Streptococcus Intermedius. Oral Microbiol Immunol 23(1):79–83. https://doi.org/10.1111/j.1399-302X.2007.00395.x
Pham HN, Michalet S, Bodillis J, Nguyen TD, Nguyen TKO, Le TPQ, Haddad M, Nazaret S, Dijoux-Franca MG (2017) Impact of metal stress on the production of secondary metabolites in Pteris Vittata L. and associated rhizosphere bacterial communities. Environ Sci Pollut Res Int 24(20):16735–16750. https://doi.org/10.1007/s11356-017-9167-2
Rajan R, Zhu J, Hu X, Pei D, Bell CE (2005) Crystal structure of S-Ribosylhomocysteinase (Luxs) in complex with a catalytic 2-ketone intermediate. Biochemistry 44(10):3745–3753. https://doi.org/10.1021/bi0477384
Rémy B, Plener L, Elias M, Daudé D, Chabrière E (2016) Des Enzymes Pour Bloquer La Communication Bactérienne, Une Alternative Aux Antibiotiques ? Ann Pharm Fr 74(6):413–420. https://doi.org/10.1016/j.pharma.2016.06.005
Ruzheinikov SN, Das SK, Sedelnikova SE, Hartley A, Foster SJ, Horsburgh MJ, Cox AG, McCleod CW, Mekhalfia A, Blackburn GM, Rice DW, Baker PJ (2001) The 1.2 a structure of a novel quorum-sensing protein, Bacillus Subtilis Luxs. J Mol Biol 313(1):111–122. https://doi.org/10.1006/jmbi.2001.5027
Saurav K, Costantino V, Venturi V, Steindler L (2017) Quorum sensing inhibitors from the sea discovered using bacterial N-acyl-Homoserine lactone-based biosensors. Mar Drugs 15(3):53. https://doi.org/10.3390/md15030053
Segura M, Calzas C, Grenier D, Gottschalk M (2016) Initial steps of the pathogenesis of the infection caused by Streptococcus Suis: fighting against non-specific defenses. FEBS Lett 590(21):3772–3799. https://doi.org/10.1002/1873-3468.12364
Shih PC, Huang CT (2002) Effects of quorum-sensing deficiency on Pseudomonas Aeruginosa biofilm formation and antibiotic resistance. J Antimicrob Chemother 49(2):309–314
Singh RP, Desouky SE, Nakayama J (2016) Quorum quenching strategy targeting gram-positive pathogenic Bacteria. Adv Exp Med Biol 901:109–130. https://doi.org/10.1007/5584_2016_1
Sperandio V, Torres AG, Jarvis B, Nataro JP, Kaper JB (2003) Bacteria-host communication: the language of hormones. Proc Natl Acad Sci U S A 100(15):8951–8956. https://doi.org/10.1073/pnas.1537100100
Sztajer H, Lemme A, Vilchez R, Schulz S, Geffers R, Yip CYY, Levesque CM, Cvitkovitch DG, Wagnerdöbler I (2008) Autoinducer-2-regulated genes in Streptococcus Mutans Ua159 and global metabolic effect of the Luxs mutation. J Bacteriol 190(1):401–415
Tan KH, How KY, Tan JY, Yin WF, Chan KG (2017) Cloning and characterization of the autoinducer synthase gene from lipid-degrading Bacteriumcedecea Neteri. Front Microbiol 8(e48053):72. https://doi.org/10.3389/fmicb.2017.00072
Tavender TJ, Halliday NM, Hardie KR, Winzer K (2008) Luxs-independent formation of Ai-2 from Ribulose-5-phosphate. BMC Microbiol 8(1):98
Thompson JA, Oliveira RA, Djukovic A, Ubeda C, Xavier KB (2015) Manipulation of the quorum sensing signal Ai-2 affects the antibiotic-treated gut microbiota. Cell Rep 10(11):1861–1871. https://doi.org/10.1016/j.celrep.2015.02.049
Trappetti C, Mcallister LJ, Chen A, Hui W, Paton AW, Oggioni MR, Mcdevitt CA, Paton JC (2017) Autoinducer 2 signaling via the phosphotransferase Frua drives galactose utilization by Streptococcus Pneumoniae, resulting in Hypervirulence. Mbio 8(1):e02269–e02216. https://doi.org/10.1128/mBio.02269-16
Wang Y, Zhang W, Wu Z, Zhu X, Lu C (2011) Functional analysis of Luxs in Streptococcus Suis reveals a key role in biofilm formation and virulence. Vet Microbiol 152(1):151–160
Wang Y, Li Y, Zhang Z, Fan H, Cheng X, Lu C (2013) Overexpression of Luxs cannot increase Autoinducer-2 production, only affect the growth and biofilm formation in Streptococcus Suis. Sci World J 2013(2):924276–924276. https://doi.org/10.1155/2013/924276
Wang Y, Yi L, Wang S, Fan H, Ding C, Mao X, Lu C (2015) Crystal structure and identification of two key amino acids involved in Ai-2 production and biofilm formation in Streptococcus Suis Luxs. PLoS One 10(10):e0138826. https://doi.org/10.1371/journal.pone.0138826
Wang X, Li X, Ling J (2017) Streptococcus Gordonii Luxs/Autoinducer-2 quorum-sensing system modulates the dual-species biofilm formation with Streptococcus Mutans. J Basic Microbiol 57(7):605–616. https://doi.org/10.1002/jobm.201700010
Wertheim HF, Nguyen HN, Taylor W, Lien TT, Ngo HT, Nguyen TQ, Nguyen BN, Nguyen HH, Nguyen HM, Nguyen CT (2009) Streptococcus Suis, an important cause of adult bacterial meningitis in northern Vietnam. PLoS One 4(6):e5973. https://doi.org/10.1371/journal.pone.0005973
Williams P (2007) Quorum sensing, communication and cross-kingdom Signalling in the bacterial world. Microbiology 153(Pt 12):3923–3938. https://doi.org/10.1099/mic.0.2007/012856-0
Winzer K, Hardie KR, Williams P (2003) Luxs and Autoinducer-2: their contribution to quorum sensing and metabolism in Bacteria. Adv Appl Microbiol 53:291–396
Yang Q, Defoirdt T (2015) Quorum sensing positively regulates flagellar motility in pathogenic Vibrio Harveyi. Environ Microbiol 17(4):960–968. https://doi.org/10.1111/1462-2920.12420
Yang W, Li Y, Zhang Z, Fan H, Cheng X, Lu C (2014) Biofilm formation, host-cell adherence, and virulence genes regulation of Streptococcus Suis in response to Autoinducer-2 signaling. Curr Microbiol 68(5):575–580. https://doi.org/10.1007/s00284-013-0509-0
Yu H, Jing H, Chen Z, Zheng H, Zhu X, Wang H, Wang S, Liu L, Zu R, Luo L (2006) Human Streptococcus Suis outbreak, Sichuan, China. Emerg Infect Dis 12(6):914–920. https://doi.org/10.3201/eid1206.051194
Zhu J, Dizin E, Hu X, Wavreille AS, Junguk Park A, Pei D (2003) S-ribosylhomocysteinase (Luxs) is a mononuclear iron protein†. Biochemistry 42(16):4717–4726
Zollmann T, Moiset G, Tumulka F, Tampe R, Poolman B, Abele R (2015) Single liposome analysis of peptide translocation by the Abc transporter Tapl. Proc Natl Acad Sci U S A 112(7):2046–2051. https://doi.org/10.1073/pnas.1418100112
Acknowledgements
This study was funded by the National Key Research and Development Program of China (No. 2018YFD0500100), the National Natural Science Foundation of China (31772761, 31540095), the Science and Technology Development Project of Henan Province (182300410047, 162300410067), and the China Scholarship Council (CSC).
Author information
Authors and Affiliations
Contributions
YW and LY designed the concept of the review article. YXW, YW, and LYS contributed to writing the manuscript. DG critically read and corrected the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Wang, Y., Wang, Y., Sun, L. et al. The LuxS/AI-2 system of Streptococcus suis. Appl Microbiol Biotechnol 102, 7231–7238 (2018). https://doi.org/10.1007/s00253-018-9170-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-018-9170-7