Abstract
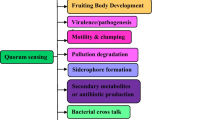
The social behaviour of bacteria for the fulfilment of different physiological activities is defined as Quorum Sensing (QS). This ranges from conjugation, symbiosis, virulence, antibiotic production, sporulation and biofilm formation. Streptococcus pyogenes which is also named as group A streptococcus (GAS) is a Gram-positive bacteria, is reported to cause diseases strictly in human. The different QS mechanisms in GAS (group A streptococcus) reported till date include Rgg-SHP quorum sensing pathway, SilC (streptococcal invasion locus) quorum-sensing pathway, Lantibiotic regulatory systems, LuxS and AI-2. The proteins of Rgg family are conserved transcription factors, which is modulated by short peptides, thus involve in the biofilm formation and virulence of bacteria. The SilC mechanism involved in the invasive tissue disease and also in the biofilm formation, Lantibiotic regulatory systems aids bacteria in adopting different immune evasion strategies and thus allow them to persist in the harsh hostile environment. Lastly, LuxS and AI-2 are the common mechanisms in all the different bacterial species including streptococcus for the virulence, motility and bio-film formation. The current chapter focuses on the detail mechanism of all the four different pathways along with the role of Quorum Sensing for the establishment of disease in the host, the immune evasion strategies of bacteria using Quorum sensing (QS) and future clinical perspective with possible applications. This may help to increase our vision towards putative vaccine targets by exploiting the mechanisms involved in Quorum Sensing.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Group A Streptococcus pyogenes(GAS) are gram positive, non motile bacteria. It resides mainly in oropharynx [1]. GAS is clinically important bacteria. GAS is responsible for various human infections within the range from benign to life threatening. It causes infections like pyoderma (skin infections), tonsillitis, pharyngitis (strep throat), impetigo, streptococcal toxic shock syndrome (STSS), scarlet fever, endocarditis and necrotizing fasciitis [2]. GAS is a very clever micro-organism, it has the potential of host immune modulation and evasion [3]. There has been various post immune sequelae has been reported. The GAS classified as “nephrotogenic” is responsible for the cause of Acute poststreptococcal glomerulonephritis (APSGN). It is described as disorder arises due to deposition of immune complexes, which affects the kidney. The average death cases reported in a year is approximately 5000 and 47,000 APSGN patient has been estimated [4]. The another class of post infection upshot is the repeated episodes of GAS causes post-inflectional sequel coined as Rheumatic heart disease (RHD). The anticipated global burden of disease caused by GAS is 18 million cases per year and near about 517,000 deaths [5]. Perhaps, the bacteria are very sensitive to penicillin [6]. In many cases, the diagnosis of disease becomes too late which results in severe pathological conditions. These squeal of disease is not handled by simple penicillin thus, resultant of infections may not be manageable in few cases. Thus, understanding the mechanism involved in bacterial infections is of great importance.
Box 1: Clinical Symptoms and Signs of Various Diseases Caused by Group A Streptococcus (GAS)
S.No. | Disease | Type | Clinical signs and symptoms | Associated M types | References |
|---|---|---|---|---|---|
1. | Impetigo | Superficial | Skin pustules that mature into honey colored scabs | 33,41,42,52,53,70 | [7] |
2. | Scarlet fever | Superficial | Deep red rashes on skin, strawberry tongue, Pharyngitis | [8] | |
3. | Pharyngitis | Superficial | Sore throat and fever | 1,3,5,6,12,14,17,19,24 | |
4. | Acute rheumatic fever | Sequelae | Carditis, polyarthritis, syndehman chorea (jerky movements), subcutaneous nodules | 1,3,5,6,11,12,14,17,18,19,24,27,29,30,32,41 | |
5. | Rheumatic heart disease | Sequelae | Mitral valve and aortic valve get affected, regurtation and stenosis occur. Difficulty in breathing | 1,3,5,6,11,12,14,17,18,19,24,27,29,30,32,41 | |
6. | Acute poststreptococcal glomerulonephritis | Sequelae | Complement deficiency, immune complex deposition, urinary sedimentation, hypertension and edema | 1,4,12,49,55,57,60 | |
7. | Bacteremia | Invasive | High fever, vomiting and nausea | [20] | |
8. | Puerperal sepsis | Invasive | Abdominal pain in pregnancy, fever and chills | 28 | [21] |
9. | Cellulitis | Invasive | Tender and swollen part of skin | [22] | |
10. | Necrotizing fasciitis | Invasive | Tissue destruction, vomiting, skin lesions, fever | 1,3,28 | [22] |
11. | Streptococcal toxic shock syndrome | Invasive | High fever and multi-organ failure | 1,3 | [23] |
Epidemology, Mechanism and Mode of Infection
The GAS isolates has been distinguished on the basis of emm protein. The emm typing is done from the 5′ region of the gene encoding the emm protein. This region is variable among all isolates, so far there are 200 types of strains has been identified [24, 25]. The GAS colonize in the throat on the epithelial surface, but sometimes also resides in the vaginal and anal linings. It has been reported that GAS resides on the epithelial surface but also able to breach the lining and propagate insides the organs [26, 27]. The GAS is spread through skin to skin infection and saliva, it become prevalent in overcrowding areas and has been observed more in school children and aboriginal population [2]. GAS has the capability to perform antigenic mimicry which help them to sustain its growth and maintain its virulence against the immune system of host. Various strategies has been reported in case of GAS immune evasion. It has been reported that cytoslin streptolysin (SLO) which is defined as the pore forming bacterial protein inhibit the fusion of lyosome during the phagocytosis event and thus escape from the harsh acidic mileu [28]. The SLO protein has also induces the death the cell death while residing inside the macrophages and neutrophils [29].
Biofilms Formation and Quorum Sensing
Apart from targeting the host immune cells, this bacteria also modulate its own gene expression inorder to survive in the harsh hostile environment and prevail the infections in the host. Biofilms formation has been reported as one of the pathogen modulatory exercise in Streptococcus pyogenes (GAS) [30]. Biofilms are the enveloped structures consist of sessile bacteria enclosed in a matrix of polymeric substance. Biofilms are formed by bacteria using the well defined phenomena which are stated as Quorum sensing (QS) [31, 32]. QS Is used by bacteria for fulfilling their various activities which includes the collective traits such as virulence, biofilm formation, swarming, conjugation. QS is reported in gram positive and gram negative bacteria both, for the persistent of infection and evasion of immune responses of host. Socialization among bacteria includes biosynthesis, secretion and detection of ligand named as auto inducers. Production of these chemical substances in enormous amount triggers the cascade of QS, results in the altered gene expression. The auto-inducers in gram positive and gram negative bacteria behaves differently. In gram-negative bacteria N-acyl homoserine lactones derivatives act as auto inducers which diffuse freely to and fro from the cells and directly interact with intracellular regulatory proteins. In gram positive bacteria, the pro-peptides are formed which processed and form ligand which binds with the receptors like as the membrane bound sensor which have the histidine protein kinase activity (refer to Box no. 2) [33]. Quorum sensing in Streptococcus pyogenes has been classified under four system.
-
1.
Rgg-SHP quorum sensing pathway: Rgg are the class of regulatory proteins which are poorly studied in case of gram positive bacteria. After the name of Regulator gene of glucosyl-transferase in Streptococcus gornodii, the RGG termed was coined. In Streptococcus pyogenes paralogs of Rgg has been identified, such as RopB (Rgg) (spy49_1691), Rgg2 (spy49_0415), Rgg3 (spy49_0449c) and ComR (Rgg4) (spy49_0032). Rgg2 and Rgg3 are located in opposite directions to each other in the close proximity of SHPs genes (Short hydrophobic genes). Study was done in the Rgg2 and Rgg3 mutant strains, and its role in the quorum sensing has been demonstrated. It has been shown that the Rgg2 with Rgg3 are involved in the biofilm formation and also in the positive feedback mechanism by expressing the SHPs genes. [34]
RopB: RopB is a dimeric protein primarily engaged in the positive and negative regulation of most of the genes. SpeB i.e. Streptococcal pyrogenic exotoxin are the proteins involved in extracellular matrix formation, role of SpeB in the virulence has been reported [35]. RopB belongs to the member of Rgg family and curbs the expression of n numbers of genes, out of them SpeB has been reported as the most studied one. Studies reveal that RopB dependent SpeB expression is regulated with the density of GAS and the peptides releases by the bacteria regulates the virulence activity.
Rgg2 (spy49_0415) and Rgg3 (spy49_0449c): Biofilm formation and lysozyme resistance are the main functions of these proteins. These are cytoplasmic in nature and formed for the pro-peptides forms such as SHPs. The exportation of SHPs is done by a transporter named as PptAB. The ABC transporter PptAB is conserved among all firmicutes but their cognate substrate and functions among different species is remain uncleared. Recently in 2016, study was done by creating PptAB mutant GAS, and it has shown that this specific ABC transporter is important for the SHPs protein translocation [36]. Biofilm synthesis by Rgg 2/3 sensing pathway in GAS has been reported.
To understand the mechanism involve in biofilm formation, processed pheromones from bacterial supernatant were isolated, bioluminescent reporters were tagged and mass spectrometry were used to detect and characterize it. SHPs of different length were detected and there synthetic peptides has been used to validate the biofilm growth assay. Rgg SHP interactions are validated by using fluorescence polarization assay [37]. Thus, Rgg2/3 serve as a cytoplasmic receptor for SHPs and PptAB act as transporter for such proteins which involve in biofilm growth and virulence in Streptococcus pyogenes (GAS) [36].
ComR (Rgg4) (spy49_0032): ComR is the another class of Rgg member involved in the horizontal gene transfer. Competence is the state where the bare DNA is uptake from the outer environment. The uptake of the foreign sequence is regulated by the series of genes and the expression of these genes is master regulated by the cascade of molecules. In streptococcus species the master regulator defined as SigX has been identified. But not all class of streptococcus is naturally competent. In GAS, the type II ComRS quorum sensing has been identified and the novel pheromone which regulates this pathway has been identified. The stability of SigX has been identified and the another class of protein called cytoplasm protease (ClpP) regulates its stability. Figure 1 explains the proposed model for competence gene regulation in GAS. It was hypothesized on the basis of previous findings in S.mutans that the expression of sigX is regulated by ComRS. The secretion of ComS is done by bacterial cell, its further processing and maturation is done by an unknown process which forms a sigX-inducing peptide (XIP).The released XIP in the extracellular environment get imported with the Opp transporter. Inside the cytosol XIP binds with ComR, thus results in the dimer. This dimer binds with the P1 promoter of which is upstream of comS and sigX, thus activates there expression. The accumulation of SigX is depend upon the ClpP protease. SigX and RNA polymerase together bind with promoter region named as CIN box which activates the transcription of late competence genes. While this study explains the presence of competence genes in GAS, and these genes express on the density depended manner. It gives us the clear report of ComR rgg regulator in Streptococcus pyogenes but still the in vitro transfer of DNA in GAS is not possible. Using radio labelled DNA it has been demonstrated that the transformation is blocked at particular stage. [38]
-
2.
SilC ( s treptococcal i nvasion l ocus) quorum-sensing pathway:
The Sil system is the first Quorum sensing network explained in Streptococcus pyogenes. The Sil is a locus which gets activated by the pheromones defined as SilCR. The overall cascade is run through the two- component system termed as SilA-SilB. In vivo virulence genes were identified in the GAS with the help of polymorphic-tag-lengths transposon-mutagenesis (PTTM).The transposon is inserted in the locus termed as Sil locus, which results in low virulence in mice model. The movement of the strain from skin to spleen is also get attenuated. The Sil consist of five genes named as Sil A-E. The two component system is encoded by silA and silB, while the ABC transporters are encoded by silD and silE. There is an ORF next to combox promoter which is called as silC [39]. The DNA promoter region required for the activation of SilA pathway has been characterized. It is consist of two direct repeats of 10 bp with 11 bp of spacer. With wide array of bioinformatic analysis, 13 bacteriocin genes were identified that are under the regulation of SilA. Using the GFP accumulation, the SilCR signalling has become more clear. Using a little amount of synthetic SilCR, the autoinduction in GAS demonstrated the ability of naturally producing SilCR [40]. The overall study suggest the role of Sil in colonization and virulence.
-
3.
Lantibiotic regulatory systems: During the establishment of infection, colonization and niche formation, bacteria meet to high nutrition demand and competition. To minimize this competition, bacteria have evolved with various strategies which help them to successfully establish the infection without the interference of host immune system. Thus, the Lantibiotic system helps bacteria to adopt various immune strategies and successfully survive in harsh hostile environment. The bacteria synthesize bacteriocins, which is defined antimicrobial peptides (AMP) which kill the bacteria of same or different species. These AMP kills its neighbouring bacteria with different modes such as pore formation, cell wall synthesis inhibitors. Lantibiotics are the class I bacteriocin identified in Streptococcus pyogenes and also in some other class such as staphylococcus. Lantiobiotics are synthesized in unprocessed form define as with the leader sequence located in the N terminal while the C terminal is involved in post translational modifications. The enzymatic chain of reactions i.e. the dehydration of serine/threonine in the C terminal region leads to the formation of 2,3-dehydroalanine (Dha) and 2,3-dehydrobutyrine (Dhb). The further addition of thiol group from the nearby cysteine group results in the formation lanthionine and methyllanthionine respectively. These specialized stable thioether rings are important for biological activities. The recognition of substrate is done by leader peptide [41]. The another class of bacteriocin define as classIIb has been identified in M18 S. pyogenes strain, the promoter region upstream to class II b has been identified using recombinase-based in vivo expression technology (RIVET) system, the activity of the promoter has been studied in mouse. It has been shown that the peptides named as SpbM and SpbN, both are essential for antimicrobial activity. It has been also shown that the S.pyogenes immunity genes are encoded downstream of spbN [42]. It has been reported that S.pyogenes has evade immune response by hiding inside the macrophages, SalY which is homologous to lac-operon of lantibiotic has been identified in S.pyogenes and its mutant study has revealed that the SalY is crucial for bacteria survival inside macrophages [43]. While lantibiotic act as a AMP for their neighbouring micro-organisms, it also has proven for adopting immune evasion strategies such as dwelling inside the macrophages (as discussed above). The lantibiotic as described is the crucial system for niche establishment is also the type of quorum sensing mechanism, where the production of lantibiotics occurred through density depended manner. The auto-regulator are the lantibiotics with the promoter region responsible for the synthesis of peptides [44]. The overall setup is arranged in the operon. The production of lantibiotic occurs in propeptide form which further processed and transported out of the cell. The mature lantibiotic is also sensed by the two regulatory system (TCS), thus the production of lantibiotic is sensed and regulated. In one of the study in S.pyogenes SF370, role of TCS in case of quorum sensing has been established. Since SF370 is a strain which lacks bacteriocin synthesis was co-cultured with nisin A and demonstrated SrtRK TCS (of SF370 strain) is susceptible to ex-bacteriocin, thus regulating the ABC transporter SrtFEG. It has been also demonstrated that TCS is also crucial for the S.pyogenes when cultured with nisin A-forming Lactococcus lactis [45]. Streptin and streptococin are the other two classes of lantibiotics studied in case of GAS [46].
-
4.
LuxS and AI-2:- The autoinducer -2 (AI-2) was first reported in gram negative bacteria called Vibrio harveyi and later it has been identified its involvement in QS depended mechanism for the production of luciferase activity. LuxS is an enzyme identified for the production of AI-2. The LuxS enzyme has been identified in both gram negative and gram positive bacteria. As LuxS is the key component of this system which diverts the interest for the identification of lux/AI in gram positive bacteria. The mechanism involved in this type of QS is widely differing as explained in the former types of QS. This type of communication skill of bacteria is not peptide depended. The AI-2 synthesis pathway is adjunct with a metabolic pathway coined as activated methyl cycle (AMC). This AMC pathway main focus is to utilize the S-adenosylmethionine (SAM) and decompose its toxic by-products. SAM is an important provider of methyl groups required for the processing of building blocks such as DNA, RNA, protein and other biological activities of an organism. During the course of such events the toxic in between product like S-adenosylhomocysteine (SAH) is formed, which further degraded by the nucleosides to form S-rybosylhomocysteine (SRH) and adenine. The break-down of SRH to homocysteine and 4,5-dihydroxy 2,3-pentanedione (DPD) is carried out by LuxS. Pro-AI-2 molecules are formed by the spontaneous cyclization of DPD which further reacted with borate leads to the signal identified by Vibrios [47]. LuxS and AI-2 role in causing virulence, biofilm formation has been studied in various bacteria. To examine the role of LuxS in S.pyogenes biological activities, a LuxS mutant was designed of an M3 serotype. Functional characterization of the mutant explained its internalization by HEp-2 cells with greater efficiency as compare to the wild type strain. Expression of genes such as speB (streptococcal pyrogenic exotoxin B), hasA (hyaluronic acid synthesis) which are known to involve in the internalization of SP268 strain were checked in case of wild type and mutant strain. There was increase in mutation of emm3 and reduced level of expression in case of speB in mutant strain. Previously, it was considered that SpeB and M3 proteins are involve in internalization by epithelial and endothelial cells. Later, it was cleared that only M3 helps in internalization while the SpeB protein impede the GAS uptake. Thus, the work which was performed in LuxS mutant strain gives the more light on the LuxS/AI-2 pathway and its importance on GAS internalization mechanism [48]. In another study conducted on M1 and M19 strain of Streptococcus pyogenes explains the involvement of the luxS/AI-2 pathway in the metabolism and adaptation of the bacteria in tough host environment. It has been reported that the expression of LuxS and AI-2 get reduced at low pH and thus explains its adaptation capability under stress condition. In order to mimicking the host environment S.pyogenes were grown in RPMI with 10% serum and decreased level of both the genes were observed. It has been also observed that LuxS mutant S.pyogenes strain can be successfully enters and survive inside epithelial cells and macrophages [40]. Thus, suggesting that this QS type helps bacteria to communicate in such a way which results the organism to sustain its life within its targets. Its aid in internalizing, releasing proteins for adopting different immune evasion strategies.
Conclusion and Perspective
The Streptococcus pyogenes is responsible to cause many types of disease in human. Understanding the pattern of bacterial survival and retention inside the host is essential. Like other bacterial species, it has been seen that the S.pyogenes is also a social micro-organism which sustain its life inside biofilms and colonize in its host [1]. The wide clear picture of QS in S.pyogenes is portraited by Sil system which explains the role of QS in virulence and colonization. There are other type of QS circuits that has been explained in S.pyogenes called as type II ComRS quorum sensing which involved in control of DNA transfer. Lantibiotic, a class of bacteriocin has also been identified in this species which shows the antimicrobial activities and form the AMP such as Streptin and streptococin. Its role for insulting the host immune system attack was explained. LuxS/AI-2 pathway an another class of QS and its importance during the course of internalization and establishment of the infection has been clearly explained. Though, the work related to QS system in S.pyogenes has been established, the information in this area is very little for clinical applications. The clear pathogenesis of this bacteria and its QS system is needed to be explored more. From the past research evidences, it has been clear that QS involves in the establishment of infection such as biofilm formation, colonization and adhesion. It has also been clear that this bacteria adopt various immune evasion strategies such as hiding inside macrophages by exploiting the QS system [40, 43].It is apparent that this species as similar to other bacteria perform their various biological activities by using QS. To understand the molecular pathways and there component may help to target and block there growth. The term which is well defined this phenomena is “Quorum quenching”. Blocking of QS can be done by using enzymatic degradation of pathways components, using of inhibitors against signal molecules [49]. Thus, Quorum quenching would be the next possible therapeutic clinical step for clearing out the infection. In desire of finding out the therapeutic targets, the QS system in this species is need to be explored with greater extent as it may be the future promising treatment directions.
References
Brouwer, S., Barnett, T. C., Rivera-hernandez, T., Rohde, M., & Walker, M. J. (2016). Streptococcus pyogenes adhesion and colonization. FEBS Letters, 590, 3739–3757. https://doi.org/10.1002/1873-3468.12254.
Cunningham, M. W. (2000). Pathogenesis of group A streptococcal infections. Clinical Microbiology Reviews, 13(3), 470–511.
Martin, W. J., Steer, A. C., Smeesters, P. R., et al. (2015). Post-infectious group A streptococcal autoimmune syndromes and the heart. Autoimmunity Reviews, 14(8), 710–725. https://doi.org/10.1016/j.autrev.2015.04.005.
Carapetis, J. R., Steer, A. C., Mulholland, E. K., & Weber, M. (2005, November). The global burden of group A streptococcal diseases. The Lancet Infectious Diseases, 5, 685–694.
Sika-Paotonu, D., Beaton, A., Raghu, A., et al. (2017). Acute rheumatic fever and rheumatic heart disease. In J. J. Ferretti, D. L. Stevens, & V. A. Fischetti (Eds.), Streptococcus pyogenes: Basic biology to clinical manifestations. https://www.ncbi.nlm.nih.gov/books/NBK425394/.
Šmitran, A., Vuković, D., Gajić, I., Marinković, J., Ranin, L. (2015, August). Effects of penicillin and erythromycin on adherence of invasive and noninvasive isolates of Streptococcus pyogenes to laminin. Memórias do Instituto Oswaldo Cruz, 110, 684–686. https://doi.org/10.1590/0074-02760150092.
Bowen, A. C., Tong, S. Y., Chatfield, M. D., & Carapetis, J. R. (2014). The microbiology of impetigo in indigenous children: Associations between Streptococcus pyogenes, Staphylococcus aureus, scabies, and nasal carriage. BMC Infectious Diseases, 14(1), 727.
Perea-mejı, L. M., Inzunza-montiel, A. E., & Cravioto, A. (2002). Molecular characterization of group A streptococcus strains isolated during a scarlet fever outbreak. Journal of Clinical Microbiology, 40(1), 278–280. https://doi.org/10.1128/JCM.40.1.278.
Shea, P. R., Ewbank, A. L., Gonzalez-Lugo, J. H., Martagon-Rosado, A. J., Martinez-Gutierrez, J. C., Rehman, H. A., et al. (2011). Group a Streptococcus emm gene types in pharyngeal isolates, Ontario, Canada, 2002–2010. Emerging Infectious Diseases, 17(11), 2010.
Fam, A. (2009). Diagnosis and treatment of streptococcal pharyngitis. 383–390.
Bright, P. D., Mayosi, B. M., & Martin, W. J. (2016.;(table 1)). An immunological perspective on rheumatic heart disease pathogenesis: More questions than answers. Heart, 1527–1532. https://doi.org/10.1136/heartjnl-2015-309188.
Raynes, J. M., Frost, H. R., Williamson, D. A., Young, P. G., Baker, E. N., Steemson, J. D., et al. (2016). Serological evidence of immune priming by group a streptococci in patients with acute rheumatic fever. Frontiers in Microbiology, 7, 1119.
Maurice, J. (2013). Rheumatic heart disease back in the limelight rheumatic heart disease is drawing renewed attention from the health community and from. Lancet, 382(9898), 1085–1086. https://doi.org/10.1016/S0140-6736(13)61972-8.
Kalil, J. (2006, March). Molecular mimicry in autoimmune pathogenesis of rheumatic heart disease. Autoimmunity. https://doi.org/10.1080/08916930500484674.
Tandon, R. (2012). Rheumatic fever pathogenesis: Approach in research needs change. Annals of Pediatric Cardiology. https://doi.org/10.4103/0974-2069.99621.
Cunningham, M. W. (2012). Streptococcus and rheumatic fever. Current Opinion in Rheumatology, 24(4), 408–416. https://doi.org/10.1097/BOR.0b013e32835461d3.
Beaton, A., & Carapetis, J. (2015). The 2015 revision of the Jones criteria for the diagnosis of acute rheumatic fever: Implications for practice in low-income and middle-income countries. Heart Asia, 7, 7–11. https://doi.org/10.1136/heartasia-2015-010648.
Jackson, S. J., Steer, A. C., & Campbell, H. (2011). Systematic review: Estimation of global burden of non- suppurative sequelae of upper respiratory tract infection: Rheumatic fever and post-streptococcal glomerulonephritis. Tropical Medicine & International Health, 16(1), 2–11. https://doi.org/10.1111/j.1365-3156.2010.02670.x.
Speers, D. J., Levy, A., Gichamo, A., Eastwood, A., & Leung, M. J. (2017). M protein gene (emm type) analysis of group a Streptococcus isolates recovered during an acute glomerulonephritis outbreak in northern western Australia. Pathology, 49(7), 765–769.
Sriskandan, S., & Altmann, D. M. (2008). The immunology of sepsis. The Journal of Pathology, 214, 211–223. https://doi.org/10.1002/path.
Zakour, N. L. B., Venturini, C., Beatson, S. A., & Walker, M. J. (2012). Analysis of a streptococcus pyogenes puerperal sepsis cluster by use of whole-genome sequencing. Journal of Clinical Microbiology, 50(7), 2224–2228. https://doi.org/10.1128/JCM.00675-12.
Martin, J.M., Green, M. (2006). Group A streptococcus. 140–148. https://doi.org/10.1053/j.spid.2006.07.001.
Low, D. E. (2013). Toxic Shock syndrome major advances in pathogenesis, but not treatment. Critical Care Clinics, 29(3), 651–675. https://doi.org/10.1016/j.ccc.2013.03.012.
Vähäkuopus, S., Vuento, R., Siljander, T., & Syrjänen, J. (2012). Distribution of emm types in invasive and non-invasive group A and G streptococci. European Journal of Clinical Microbiology, 1251–1256. https://doi.org/10.1007/s10096-011-1436-2.
Whatmore, A. M., & Kumar, M. P. (2018). EMM types of streptococcus pyogenes in Chennai. Indian Journal of Medical Microbiology, 19(3), 161–163.
Rudolph, K., Bruce, M. G., Bruden, D., et al. (2016). Epidemiology of invasive group A streptococcal disease in Alaska, 2001 to 2013. Journal of Clinical Microbiology, 54(1), 134–141. https://doi.org/10.1128/JCM.02122-15 Editor.
Cole, J. N., Barnett, T. C., Nizet, V., & Walker, M. J. (2011). Molecular insight into invasive group A streptococcal disease. Nature Publishing Group, 9(10), 724–736. https://doi.org/10.1038/nrmicro2648.
Bentley, C.C., Shakhnovic, E.A., Wessels, M.R. (2005). Cytolysin-dependent evasion of lysosomal killing.
Timmer, A. M., Timmer, J. C., Pence, M. A., et al. (2009). Streptolysin O promotes group A streptococcus immune evasion by accelerated macrophage apoptosis*. Journal of Biological Chemistry, 284(2), 862–871. https://doi.org/10.1074/jbc.M804632200.
Fiedler, T., Köller, T., & Kreikemeyer, B. (2015). Streptococcus pyogenes biofilms—Formation, biology, and clinical relevance. Frontiers in Cellular and Infection Microbiology, 5, 15.
Marks, L. R., Mashburn-Warren, L., Federle, M. J., & Hakansson, A. P. (2014). Streptococcus pyogenes biofilm growth in vitro and in vivo and its role in colonization, virulence, and genetic exchange. The Journal of Infectious Diseases, 210(1), 25–34.
Young, C., Holder, R.C., Dubois, L., Sean, D. (2016). Streptococcus pyogenes biofilm introduction to biofilms. 1–34.
Miller, M. B., & Bassler, B. L. (2001). Quorum sensing in bacteria. Annual Review of Microbiology, 55, 165–199.
Chang, J. C., LaSarre, B., Jimenez, J. C., Aggarwal, C., & Federle, M. J. (2011). Two group a streptococcal peptide pheromones act through opposing rgg regulators to control biofilm development. PLoS Pathogens, 7(8), e1002190. https://doi.org/10.1371/journal.ppat.1002190.
Makthal, N., Gavagan, M., Do, H., Olsen, R. J., Musser, J. M., & Kumaraswami, M. (2016). Structural and functional analysis of RopB: A major virulence regulator in Streptococcus pyogenes. Molecular Microbiology, 99(6), 1119–1133. https://doi.org/10.1111/mmi.13294.
Chang, J. C., & Federle, M. J. (2016). PptAB exports Rgg quorum-sensing peptides in streptococcus. PLoS One, 11(12), 1–12. https://doi.org/10.1371/journal.pone.0168461.
Aggarwal, C., Jimenez, J. C., Nanavati, D., & Federle, M. J. (2014). Multiple length peptide-pheromone variants produced by streptococcus pyogenes directly bind Rgg proteins to confer transcriptional regulation. The Journal of Biological Chemistry, 289(32), 22427–22436. https://doi.org/10.1074/jbc.M114.583989.
Mashburn-Warren, L., Morrison, D. A., & Federle, M. J. (2012). The cryptic competence pathway in streptococcus pyogenes is controlled by a peptide pheromone. Journal of Bacteriology, 194(17), 4589–4600. https://doi.org/10.1128/JB.00830-12.
Hidalgo-grass, C., Ravins, M., Dan-goor, M., Jaffe, J., Moses, A. E., & Hanski, E. (2002). A locus of group A streptococcus involved in invasive disease and DNA transfer. Molecular Microbiology, 46, 87–99.
Belotserkovsky, L., Baruch, M., Peer, A., et al. (2009). Functional analysis of the quorum-sensing streptococcal invasion locus (sil). PLoS Pathogens, 5(11), e1000651. https://doi.org/10.1371/journal.ppat.1000651.
Dischinger, J., Wiedemann, I., Bierbaum, G. (n.d.). H-GS. Handbook of biologically active peptides (2nd Edition). https://www.sciencedirect.com/science/article/pii/B9780123850959000191.
Armstrong, B. D., Herfst, C. A., Tonial, N. C., Wakabayashi, A. T., Zeppa, J. J., & McCormick, J. K. (2016). Identification of a two-component class IIb bacteriocin in Streptococcus pyogenes by recombinase-based in vivo expression technology. Scientific Reports, 6, 36233.
Phelps, H. A., & Neely, M. N. (2007). SalY of the streptococcus pyogenes lantibiotic locus is required for full virulence and intracellular survival in macrophages. Infection and Immunity, 75(9), 4541–4551. https://doi.org/10.1128/IAI.00518-07.
Kleerebezem, M. (2004). Quorum sensing control of lantibiotic production; nisin and subtilin autoregulate their own biosynthesis. 25. https://doi.org/10.1016/j.peptides.2003.10.021.
Kawada-matsuo, M., Tatsuno, I., Arii, K., et al. (2016). Two-component systems involved in susceptibility to nisin A in streptococcus pyogenes. Applied and Environmental Microbiology, 82(19), 5930–5939. https://doi.org/10.1128/AEM.01897-16.Editor.
Wescombe, P. A., & Tagg, J. R. (2003). Purification and characterization of streptin, a type A1 lantibiotic produced by streptococcus pyogenes. Applied and Environmental Microbiology, 69(5), 2737–2747. https://doi.org/10.1128/AEM.69.5.2737.
Schauder, S. Shokat, K. Surette, M. G. Bassler, B. L. (2001). The LuxS family of bacterial autoinducers: Biosynthesis of a novel quorum-sensing signal molecule. https://doi.org/10.1046/j.1365-2958.2001.02532.x.
Marouni, M. J., & Sela, S. (2003). The luxS gene of streptococcus pyogenes regulates expression of genes that affect internalization by epithelial cells. Infection and Immunity, 71(10), 5633–5639. https://doi.org/10.1128/IAI.71.10.5633.
Basavaraju, M., Sisnity, V. S., & Palaparthy, R. (2016). ScienceDirect Quorum quenching: Signal jamming in dental plaque biofilms. Journal of Dental Sciences, 11(4), 349–352. https://doi.org/10.1016/j.jds.2016.02.002.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Sahu, P., Pallaval Veera Bramhachari (2018). Quorum Sensing in Streptococcus pyogenes and Their Role in Establishment of Disease. In: Pallaval Veera Bramhachari (eds) Implication of Quorum Sensing System in Biofilm Formation and Virulence. Springer, Singapore. https://doi.org/10.1007/978-981-13-2429-1_23
Download citation
DOI: https://doi.org/10.1007/978-981-13-2429-1_23
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-2428-4
Online ISBN: 978-981-13-2429-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)