Abstract
Acetic acid bacteria (AAB) are widely used in acetic acid fermentation due to their remarkable ability to oxidize ethanol and high tolerance against acetic acid. In Acetobacter pasteurianus, nucleotide excision repair protein UvrA was up-regulated 2.1 times by acetic acid when compared with that without acetic acid. To study the effects of UvrA on A. pasteurianus acetic acid tolerance, uvrA knockout strain AC2005-ΔuvrA, uvrA overexpression strain AC2005 (pMV24-uvrA), and the control strain AC2005 (pMV24), were constructed. One percent initial acetic acid was almost lethal to AC2005-ΔuvrA. However, the biomass of the UvrA overexpression strain was higher than that of the control under acetic acid concentrations. After 6% acetic acid shock for 20 and 40 min, the survival ratios of AC2005 (pMV24-uvrA) were 2 and 0.12%, respectively; however, they were 1.5 and 0.06% for the control strain AC2005 (pMV24). UvrA overexpression enhanced the acetification rate by 21.7% when compared with the control. The enzymes involved in ethanol oxidation and acetic acid tolerance were up-regulated during acetic acid fermentation due to the overexpression of UvrA. Therefore, in A. pasteurianus, UvrA could be induced by acetic acid and is related with the acetic acid tolerance by protecting the genome against acetic acid to ensure the protein expression and metabolism.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acetic acid is a highly important organic acid that is widely used in chemistry, medicine, and food industry. Acetic acid has an inhibitory effect on some microorganism growth and metabolism when its concentration reaches 5 g/L. Acetic acid bacteria (AAB) are gram-negative, aerobic bacteria belonging to the Acetobacteraceae family. The remarkable ability of AAB to oxidize ethanol and high tolerance to acetic acid makes it widely used in the acetic acid fermentation industry (Hattori et al. 2011; Sengun and Karabiyikli 2011). In addition to acetic acid, AAB are important functional microorganisms in the production of different vinegars, including traditional Chinese cereal vinegars that are generally produced through solid-state fermentation, traditional European fruit vinegars produced through liquid-surface fermentation, and the vinegar produced in liquid-submerged fermentation with pure strains of AAB (Solieri and Giudici 2009; Nie et al. 2015).
A high acetic acid tolerance of AAB is crucial for industrial acetic acid and vinegar production. According to previous reports, the acetic acid tolerance mechanism of AAB is mainly related to (i) ethanol oxidation by membrane-bound alcohol dehydrogenase (ADH) (Trcek et al. 2007; Trcek et al. 2006)—the high ADH activity in the Gluconacetobacter cells and high acetic acid stability of the enzyme enable these species to grow and stay metabolically active at 10% acetic acid (Trcek et al. 2006); (ii) tricarboxylic acid (TCA) cycle involving citrate synthase (CS) and aconitase (Fukaya et al. 1990); (iii) the acetic acid assimilation by transferase of acetyl coenzyme AarC (Mullins et al. 2008); (iv) the ATP-binding cassette (ABC) transporter playing a role in pumping the acetic acid out of the AAB (Nakano and Fukaya 2008), (v) press tolerance proteins induced by acetic acid, such as molecular chaperones DnaK and GroEL, to ensure proper protein folding in adverse environments—co-overexpression of GrpE with DnaK/J in Acetobacter pasteurianus resulted in an improved growth under different pressure conditions (Ishikawa et al. 2010; Okamoto-Kainuma et al. 2004); (vi) changes in cell morphology and membrane composition and the pellicle (capsular polysaccharides) formation (Trcek et al. 2007); and (vii) the metabolism of some amino acids (Akiko et al. 2002; Ishikawa et al. 2010; Okamoto-Kainuma et al. 2004).
The weak electrolytes and lipophilic properties of acetic acid can cause the reduction of intracellular pH, and the low intracellular pH will result in the release of DNA purines and pyrimidines and causes damage to the genome (Hahn et al. 1999; Van de Guchte et al., 2002). Saccharomyces cerevisiae could lead to chromosomal DNA breakdown into fragments when treated with acetic acid (Ribeiro et al. 2006). For microorganisms, DNA repair is a highly complex phenomenon, and one of the processes is nucleotide excision repair (NER) (Kuper and Kisker 2012; Van Houten et al. 2005). Several proteins, including excinuclease UvrA, B, C, D, and RecA, have been proven to be important for strain tolerance against acidic conditions (Grinholc et al. 2015; Sancar and Rupp 1983). Among them, UvrA, which belongs to the NER pathway, is involved in DNA repair of prokaryotic microorganisms and is the first induced protein of the NER mechanism in bacteria (Doolittle et al. 1986; Kuper and Kisker 2012; Van Houten et al. 2005; Verhoeven et al. 2002). In Lactobacillus helveticus UvrA contributed to acid and oxidative tolerance (Cappa et al. 2005).
In our previous research, the proteome of A. pasteurianus under the conditions of acetic acid being present and absent was analyzed (Zheng et al. 2017). Especially, we wish to find a probable extra mechanism for acetic acid tolerance of AAB besides the mechanisms mentioned above. Fortunately, the expression of excision repair protein UvrA was found improved by acetic acid. And then, the uvrA gene (APA01_RS07300) was knocked out and overexpressed in A. pasteurianus to study the effect on acetic acid tolerance and acetic acid fermentation collaboratively with other proteins responsible for acetic acid tolerance. Therefore, a rational strategy could be proposed to improve acetic acid fermentation.
Materials and methods
Strains and plasmids
A. pasteurianus AC2005 was registered in the Chinese General Microbiological Culture Collection Center (numbered CGMCC 3089). Escherichia coli JM109 was used for construction of recombinant vectors.
The plasmid pMD18-T (Takara) was used for cloning and sequencing analysis. Plasmid pMV24 (Apr, lacZ), given as a gift by the Mizkan Group Corporation, Japan, was used for overexpression of uvrA in A. pasteurianus (Fukaya et al. 1989). A suicide plasmid pSUP202 was used to construct the replacement vector pSUP202-uvrA::Km. E. coli HB101 containing plasmid pRK2013 was used as assistance to knockout the gene of A. pasteurianus AC2005 (Wei et al. 2014).
Media and culture conditions
Strains of E. coli were grown at 37 °C on Luria–Bertani (LB) medium. A. pasteurianus AC2005 was cultured in YPG medium (1% yeast extract, 1% peptone, and 3% glucose) at 30 °C. GYEA medium (3% glucose, 1.5% yeast extract, 3.5% ethanol, 2% CaCO3, 1.7% agar) was used for DNA manipulation of A. pasteurianus AC2005.
GY medium (3% glucose, 1.5% yeast extract) and GYA medium (3% glucose, 1.5% yeast extract, and acetic acid were added to proper concentrations) were used for proteome assay and for analyzing acetic acid inhibition on cell growth. Acetic acid shock experiments were performed in GYA medium containing 4 or 6% acetic acid conditions to analyze the survival of A. pasteurianus under high acetic acid concentration stress.
The acetic acid fermentation was performed with GPAE medium (2% glucose, 2% peptone, 1% acetic acid, and 8% ethanol). The A. pasteurianus AC2005 seed medium was GYE medium (3% glucose, 1.5% yeast extract, and 3.5% ethanol). The acetic acid fermentation was implemented in a 5-L self-inspiriting fermenter (Nanjing Huike Bioengineering Equipment Corporation, Nanjing, China). The strains were grown for 24 h at 30 °C in the GYE medium and then were transferred into the GPAE medium with 10% inoculum and grown at 30 °C and 0.15 vvm.
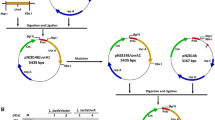
uvrA disruption and overexpression in A. pasteurianus
The deletions of uvrA from the chromosome of A. pasteurianus AC2005 were performed using the reported strategy with minor modifications (Wei et al. 2014; Zhu et al. 2011). Two fragments for the homologous recombination and the kanamycin resistance gene were obtained by polymerase chain reaction (PCR) using the primers uvrA-1/2 and kan-1/2. The PCR products were digested and ligated to the suicide plasmid pSUP202, yielding the gene replacement vector pSUP202-uvrA::Km. It was then transferred into A. pasteurianus AC2005 by triparental mating using E. coli JM109 bearing the respective vector as the donor and E. coli HB101 bearing the plasmid pRK2013 as the helper strain. The positive strains can grow on YGEA medium containing kanamycin and produce a transparent zone by producing acetic acid. The gene disruption was confirmed by PCR using the primers of uvrA-1/2 and kan-3/4.
The gene uvrA with the promoter sequence was amplified using A. pasteurianus AC2005 genomic DNA as a template, and the primers uvrA-3/4 were used for PCR. The pMV24 plasmid was used for constructing the uvrA overexpression vector pMV24-uvrA. The control strain A. pasteurianus AC2005 (pMV24) and the overexpression strain A. pasteurianus AC2005 (pMV24-uvrA) were constructed by electroporation with the plasmid pMV24 and plasmid pMV24-uvrA, respectively. Primers used in this study are listed in Table 1. DNA manipulation was performed according to standard protocols (Sambrook and Russell 2016).
Detection of gene transcription
For quantitative real-time PCR (RT-PCR) experiments, strains were cultured at different acetic acid concentrations (0, 1, and 2%), and the cells were collected when the OD610 nm (optical density under 610 nm) reached about 0.6.
Total RNA was isolated using RNAplus Kit (TaKaRa Biotechnology, Dalian, China) by following the manufacturer’s procedure. Total RNA was treated with DNase I for 30 min at 37 °C to remove residual DNA. RNA samples were reverse-transcribed with RevertAid™ First Strand cDNA Synthesis Kit (Takara Biotechnology, Dalian, China) according to the manufacturer’s instructions. Subsequently, the quantitative gene analysis was performed on an ABI StepOnePlus Real-Time PCR System (StepOnePlus, Applied Biosystems, USA) using the oligonucleotides listed in Table 1. The 16S rRNA gene was used as the internal standard. The 2Ct method was applied to analyze the data.
Acetic acid tolerance analysis
The acetic acid tolerance of strains AC2005 (pMV24), AC2005-ΔuvrA, and AC2005 (pMV24-uvrA) that expressed UvrA in different levels was analyzed by comparing their OD at 610 nm after 48-h cultivation under different initial acetic acid concentrations with 10% inoculum.
To test the tolerance of the strains toward higher acetic acid stresses, shock experiments were performed. Strains were grown overnight at 30 °C in GY medium and then were diluted with fresh GY medium at a ratio of 1:100 to grow to a mid-exponential phase (reaching an OD610 mm of 0.6). Acetic acid was added to final concentrations of 2, 4, and 6% (v/v). After 20 and 40 min of incubation at 30 °C, cells were added into an Oxford cup and grew on GYEA medium, and the survival of cells was observed. Viable bacteria were determined by spread plating serial dilutions onto the GYEA medium.
Genome integrality analysis
For genome damage analysis, overnight cultures of AC2005 (pMV24), AC2005-ΔuvrA, and AC2005 (pMV24-uvrA) were diluted 10-fold into fresh YPG medium and grown to mid-exponential phase (when the OD610 nm reached about 0.6). Then, cells were harvested and resuspended into 10 mL of GY medium and GYA media with acetic acid concentrations (v/v) of 2, 4, and 6% to incubate for 40 min at 30 °C. Furthermore, genomic DNA was isolated with a TIANamp Bacteria DNA Kit (TIANGEN, Beijing, China). DNA strand breaks were also demonstrated at the single-cell level by terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) assay with the In Situ Cell Death Detection Kit, fluorescein, from Roche Molecular Biochemicals (Mannheim, Germany) (Gavrieli et al. 1992; Trcek et al. 2006). The integrality of the genomic DNA was analyzed by 1.0% agarose gel electrophoresis (40 V, 60 min) by comparing the trailing (Gavrieli et al. 1992; Poorbagher et al. 2016).
Effect of UvrA expression on acetic acid fermentation
To test the effect of UvrA on acetic acid fermentation, acetic acid fermentations were performed in a 5-L fermenter as mentioned above. The growth and the acetic acid were monitored, and the relative transcription of uvrA, adh, cs, and dnaK was mainly detected.
Analytical methods
The cell growth was monitored based on the OD value by a spectrophotometer (UVmini-1240, Shimadzu, Kyoto, Japan) at 610 nm. The acidity of the broth was titrated by 0.1 M NaOH with phenolphthalein as an indicator. All experiments were performed in triplicate. The results were expressed as mean values with standard error. Analysis of the differences between the categories was calculated with a confidence interval of 95% with SPSS (least significant differences) analysis.
Results
Effect of acetic acid on cell growth and protein expression
The growths of A. pasteurianus AC2005 under different concentrations of acetic acid added at initial time were compared at 48 h. As shown in Fig. 1a, the cell growth reduced with the increase of acetic acid. When the initial acetic acid concentration was above 3%, the time for doubling of initial OD was more than 80 h (data not shown).
Effect of acetic acid on cell growth and uvrA transcription. a Cell growth of the wild strain of A. pasteurianus AC2005. Cells were cultured in GY (0% acetic acid) and GYA medium (1, 2, 3, and 4% acetic acid), respectively, for 48 h. b Transcription levels of uvrA induced by acetic acid. The cells were harvested when OD610 nm reached about 0.6
To further study the effect of acetic acid on cells, the differential protein expressions of A. pasteurianus AC2005 under 0 and 1% acetic acid concentrations were analyzed by using the proteome assay (Zheng et al. 2017). The major up-regulated proteins were related with energy production and conversion, amino acid transport and metabolism, carbohydrate transport and metabolism, and ribosomal structure (Zheng et al. 2017). That differential protein expression is consistent with previous reports (Wang et al. 2015; Xia et al. 2016). Interestingly, three of the proteins, excinuclease ABC subunit A (UvrA) (GI:258541116), NAD-dependent DNA ligase (NAD-DNA ligase) (GI:918718363), and DNA recombination/repair protein (RecA) (GI:256632226), involved in DNA recombination and repair were up-regulated when compared with those without acetic acid. RecA and NAD-DNA ligase were up-regulated 1.8 times and 1.5 times, respectively. Especially, UvrA was up-regulated 2.1 times. However, its relation with acetic acid tolerance of AAB was rarely studied. Thus, we analyzed the relative transcription of uvrA under acetic acid stress by RT-PCR. As shown in Fig. 1b, the relative transcriptions of uvrA increased with the increase of acetic acid concentrations. Furthermore, the analysis of genome integrality by agarose gel electrophoresis showed that the diffusion increased with the increase of acetic acid (the same as the Fig. 3b). Those results indicated that acetic acid could affect the genome integrality, and some proteins related to DNA repair, such as UvrA, were up-regulated under acetic acid conditions, together with other proteins related to acetic acid tolerance.
Effect of UvrA on acetic acid tolerance and genomic DNA integrality
To analyze the effects of UvrA on A. pasteurianus acetic acid tolerance, three strains, uvrA knockout strain AC2005-ΔuvrA, uvrA overexpression strain AC2005 (pMV24-uvrA), and the control strain AC2005 (pMV24), were constructed according to the method described above. Strains were grown in GYA media supplemented with different acetic acid concentrations to compare the effect of UvrA on cell growth. As shown in Fig. 2a, all strains grew similarly at 0% (P < 0.05) without acetic acid press; however, the knockout strains hardly grew when the concentration of acetic acid was more than 1%. Whereas, the biomass of AC2005 (pMV24-uvrA) was 20.6 and 31.7% higher than that of the control under 1 and 2% acetic acid, respectively. The growth of the control and AC2005-ΔuvrA was almost suppressed by 3% acetic acid concentration even after 72 h of cultivation, but the biomass of AC2005 (pMV24-uvrA) increased by 66.1% (data not shown). Then, uvrA transcriptions were compared under acetic acid concentration of 0, 1, and 2%, since three stains hardly grow when the initial acetic acid concentration was more than 2%. As shown in Fig. 2b, uvrA transcription was positively correlated with the growth of strains under acetic acid conditions. Thus, strain AC2005 (pMV24-uvrA) showed a better cell growth under acetic acid stress than the control due to the overexpression of UvrA.
Effect of UvrA expressions on cell growth. a Cell growth. Cells were cultured in GY and GYA media for 48 h. b Transcription of uvrA. The cells were harvested when OD610 nm reached about 0.6. Cells of AC2005-ΔuvrA under 1 and 2% acetic acid conditions were collected after 24 h of cultivation since it hardly grew
Furthermore, acetic acid shock experiments were performed to determine the effect of UvrA overexpression on the acetic acid tolerance. As shown in Fig. 3a, the viable cells decreased with the increase of acetic acid concentration and treatment period. The knockout strain hardly grew after the 40-min acetic acid shock and showed a clearly decreased tolerance to acetic acid than those of the control. The viable cells of UvrA overexpression strain are more than those of the control strain exposed in the same conditions. The decrease of survival ratio with the acetic acid concentration and treatment period reflected the toxicity of acetic acid to cells (shown in Fig. 3b). With 6% acetic acid shock for 20 and 40 min, the survival ratio of overexpression strain AC2005 (pMV24-uvrA) was 2 and 0.12%, respectively. However, it was 1.5 and 0.06% for the control strain AC2005 (pMV24).
Effect of UvrA expressions on acetic acid tolerance. a The bacterial colony after acetic acid shock of 2, 4, and 6%, respectively. The cells were treated with GYA media containing 2, 4, and 6% acetic acid, respectively, for 40 min. The dilution ratio from left to right is 1:10 to 1:10000. b The survival of strains after acetic acid shock of 2, 4, and 6%, respectively. The cells were treated with GYA media containing 2, 4, and 6% acetic acid, respectively, for 40 min. The survival ratio was calculated as the ratio of the viable cell count after and before treatment
The DNA could be damaged by acids to result in the breakage of a DNA strand and the decrease of the genome length. DNA damage analysis is an important method in screening chemicals and other factors for potential genotoxic and cytotoxic effects, which could be detected by agarose gel electrophoresis (Drouin et al. 1996). In this study, the TUNEL apoptosis detection kit (FITC) and the agarose gel electrophoresis were used to analyze the qualitative DNA damage caused by acetic acid (Ribeiro et al. 2006). As shown in Fig. 4a, b, the number of FITC-positive cells that indicated the DNA damage caused by acetic acid and the diffusion of DNA samples increased with the increase of acetic acid concentrations. When the concentration of acetic acid is 6%, FITC-positive cells of AC2005 (pMV24), AC2005-ΔuvrA, and AC2005 (pMV24-uvrA) were 7.00-, 9.23-, and 4.30-folds higher than those without acetic acid, respectively. Clearly, UvrA overexpression would help to protect the integrality of the genome, and knockout of uvrA resulted in serious damage. Especially, strain AC2005 (pMV24-uvrA) showed an improved acetic acid tolerance and genome integrality because of the overexpression of UvrA with its own promoter, which was induced by acetic acid.
Effect of UvrA expressions on genome integrality under acetic acid conditions. a TUNEL assay of FITC-positive cells. The cells were treated with GY medium and GYA media containing 2, 4, and 6% acetic acid, respectively, for 40 min. Letters present significantly different LSD tests at P < 0.05. b Agarose gel electrophoresis assay. 1, AC2005 (pMV24); 2, AC2005-ΔuvrA; 3, AC2005 (pMV24-uvrA). The cells were treated with GY medium and GYA media containing 2, 4, and 6% acetic acid, respectively, for 40 min
All these results demonstrated that the overexpression of UvrA could increase the acetic acid tolerance of A. pasteurianus and knockout of uvrA will result in sensitivity to acetic acid. Briefly, acetic acid can cause DNA damage to inhibit A. pasteurianus growth, and UvrA that relates to the nucleotide excision repair process is responsible for acetic acid tolerance by protecting the genome from acetic acid damage.
Effect of UvrA on acetic acid fermentation
Acetic acid fermentations were performed with three strains. The time curves are shown in Fig. 5a. The cell growth and acetic acid production of knockout strain were almost inhibited due to the toxicity of 1% initial acetic acid concentration, while the growth and the acetic acid production of overexpression strain were significantly higher than those of the control strain (P < 0.05) after 9 h. For AC2005 (pMV24-uvrA), the highest acidity (8.5 g/100 mL) was obtained at 47 h of fermentation that was shorter than the control strain by 7 h. The average acetification rate of AC2005 (pMV24-uvrA) was 1.57 g/(L h), which was 21.7% high when compared with the control (1.29 g/(L h)).
To confirm the reason for improved acetic acid fermentation, relative transcriptions of genes adh, uvrA, cs, and dnaK encoding the proteins ADH, UvrA, CS, and DnaK, respectively, which have been proved responsible for ethanol oxidation and acetic acid tolerance in AAB, were analyzed. As shown in Fig. 5b, the relative transcription of uvrA, adh, cs, and dnaK in AC2005 (pMV24-uvrA) were 9-, 3.7-, 3.6-, and 1.5-folds higher, respectively, than those of the control strain due to the overexpression of UvrA. Thus, UvrA not only improved the acetic acid tolerance of A. pasteurianus by reducing the genome damage caused by acetic acid, but also enhanced the expression of proteins related to ethanol oxidation and acetic acid tolerance to improve acetic acid fermentation.
Discussion
The destruction to bacteria by acetic acid is caused by intensifying the intracellular acidic environment and causing an uncoupling effect acting as a lipotrope (Yin et al. 2017). The remarkable acetic acid tolerance is an essential character to AAB, especially for acetic acid fermentation. The mechanism of this important property has been widely studied. However, few reports analyzed the acetic acid tolerance mechanism of AAB, considering the nucleotide repair. In general, the genome of all organisms is stable to ensure the metabolism and reproduction. Physical and chemical mutagens induce DNA lesions and reduce its molecular weight, besides stress conditions such as heat and peroxide (Brennan et al. 2000; Drouin et al. 1996; Greer and Zamenhof 1962). An intracellular acidic environment would cause the loss of more DNA purines and pyrimidines than the relative neutral environment (Cotter and Hill 2003). To resist these damages, some mechanisms are generated in the organism. The NER is one of the most common mechanisms to maintain the completeness of DNA. This repair system essentially repairs all DNA lesions and plays a backup role for other repair systems (De Laat et al. 1999; Sancar and Tang 1993). It is related to acid, heat, oxidation tolerances, etc. (Cappa et al. 2005; Yamamoto et al. 1996; Zheng et al. 2015). UvrA is the initial protein and is mainly involved in the original DNA damage detection and identification (Kuper and Kisker 2012; Van Houten et al. 2005). Furthermore, the overexpression of UvrA from A. pasteurianus could increase the tolerance of E. coli to acetic acid, heat, and peroxide (Zheng et al. 2015).
In this research, we demonstrated that acetic acid destroyed bacteria by affecting the genome integrality, and A. pasteurianus could reduce this negative effect on genomes by its DNA repair mechanism including nucleotide repair excinuclease UvrA. Moreover, the genome damage of A. pasteurianus increases with the increase of acetic acid accompanied with growth delay. A. pasteurianus could repair genome damage caused by the up-regulated expression of UvrA. In A. pasteurianus, UvrA was induced by acetic acid and the relative transcription level of uvrA increased with the increase of acetic acid to protect the genomic DNA by acetic acid. Overexpression of UvrA in A. pasteurianus protected the genomic DNA to a certain extent, while uvrA knockout resulted in the exacerbation of genome damage. A concentration of 1% acetic acid was almost lethal to uvrA knockout strain AC2005-ΔuvrA. As a result of the increase of genome integrality due to the overexpression of UvrA, some enzymes involved in ethanol oxidation, TCA cycle, and molecular chaperone were up-regulated to improve acetic acid fermentation (shown in Fig. 6).
References
Akiko OK, Wang Y, Sachiko K, Kenji T, Yukimichi K, Fujiharu Y (2002) Cloning and characterization of groESL operon in Acetobacter aceti. J Biosci Bioeng 94:140–147. https://doi.org/10.1016/S1389-1723(02)80134-7
Brennan L, Morris G, Wasson G, Hannigan B, Barnett Y (2000) The effect of vitamin C or vitamin E supplementation on basal and H2O2-induced DNA damage in human lymphocytes. Brit J Nutr 84(2):195–202. https://doi.org/10.1017/S0007114500001422
Cappa F, Cattivelli D, Cocconcelli P (2005) The uvrA gene is involved in oxidative and acid stress responses in Lactobacillus helveticus CNBL1156. Res Microbiol 156(10):1039–1047. https://doi.org/10.1016/j.resmic.2005.06.003
Cotter P, Hill C (2003) Surviving the acid test: responses of gram-positive bacteria to low pH. Microbiol Mol Biol R 67(3):429–453. https://doi.org/10.1128/mmbr.67.3.429-453.2003
De Laat W, Jaspers N, Hoeijmakers J (1999) Molecular mechanism of nucleotide excision repair. Genes Dev 13:768–785. https://doi.org/10.1101/gad.13.7.768
Doolittle R, Johnson M, Husain I, Houten B, Thomas D, Sancar A (1986) Domainal evolution of a prokaryotic DNA repair protein and its relationship to active-transport proteins. Nature 323:451–453. https://doi.org/10.1038/323451a0
Drouin R, Gao S, Holmquist G (1996) Agarose gel electrophoresis for DNA damage analysis. Technologies for Detection of DNA Damage & Mutations. Springer, Boston, pp 37–43. https://doi.org/10.1007/978-1-4899-0301-3_337-43
Fukaya M, Takemura H, Okumura H, Kawamura Y, Horinouchi S, Beppu T (1990) Cloning of genes responsible for acetic acid resistance in Acetobacter aceti. J Bacteriol 172:2096–2104 http://jb.asm.org/content/172/4/2096
Fukaya M, Tayama K, Tamaki T, Tagami H, Okumura H, Kawamura Y (1989) Cloning of the membrane-bound aldehyde dehydrogenase gene of Acetobacter polyoxogenes and improvement of acetic acid production by use of the cloned gene. Appl Environ Microbiol 55:171–176 http://aem.asm.org/content/55/1/171
Gavrieli Y, Sherman Y, Ben-Sasson S (1992) Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. Cell Bio 119:493–501. https://doi.org/10.1083/jcb.119.3.493
Greer S, Zamenhof S (1962) Studies on depurination of DNA by heat. J Mol Biol 4(3):123–141. https://doi.org/10.1016/S0022-2836(62)80046-1
Grinholc M, Rodziewicz A, Forys K, Rapackazdonczyk A, Kawiak A, Domachowska A, Golunski G, Wolz C, Mesak L, Becker K (2015) Fine-tuning recA expression in Staphylococcus aureus for antimicrobial photoinactivation: importance of photo-induced DNA damage in the photoinactivation mechanism. Appl Microbiol Biotechnol 99(21):9161–9176. https://doi.org/10.1007/s00253-015-6863-z
Hahn K, Faustoferri R, Quivey J (1999) Induction of an AP endonuclease activity in Streptococcus mutans during growth at low pH. Mol Microbiol 31:1489–1498. https://doi.org/10.1046/j.1365-2958.1999.01292.x
Hattori R, Yamada K, Kikuchi M, Hirano S, Yoshida N (2011) Intramolecular carbon isotope distribution of acetic acid in vinegar. J Agric Food Chem 59:9049–9053. https://doi.org/10.1021/jf200227e
Ishikawa M, Okamoto-Kainuma A, Jochi T, Suzuki I, Matsui K, Kaga T, Koizumi Y (2010) Cloning and characterization of grpE in Acetobacter pasteurianus NBRC 3283. J Biosci Bioeng 109(1):25–31. https://doi.org/10.1016/j.jbiosc.2009.07.008
Kuper J, Kisker C (2012) Damage recognition in nucleotide excision DNA repair. Curr Opin Struct Biol 22(1):88–93. https://doi.org/10.1016/j.sbi.2011.12.002
Mullins E, Francois J, Kappock T (2008) A specialized citric acid cycle requiring succinyl-coenzyme A (CoA):acetate CoA-transferase (AarC) confers acetic acid resistance on the acidophile Acetobacter aceti. J Bacteriol 190(14):4933–4940. https://doi.org/10.1128/JB.00405-08
Nakano S, Fukaya M (2008) Analysis of proteins responsive to acetic acid in Acetobacter: molecular mechanisms conferring acetic acid resistance in acetic acid bacteria. Int J Food Microbiol 125(1):54–59. https://doi.org/10.1016/j.ijfoodmicro.2007.05.015
Nie Z, Zheng Y, Du H, Xie S, Wang M (2015) Dynamics and diversity of microbial community succession in traditional fermentation of Shanxi aged vinegar. Food Microbiol 47:62–68. https://doi.org/10.1016/j.fm.2014.11.006
Okamoto-Kainuma A, Yan W, Fukaya M, Tukamoto Y, Ishikawa M, Koizumi Y (2004) Cloning and characterization of the dnaKJ operon in Acetobacter aceti. J Biosci Bioeng 97:339–342. https://doi.org/10.1016/S1389-1723(04)70216-9
Poorbagher H, Moghaddam M, Eagderi S, Farahmand H (2016) Estimating the DNA strand breakage using a fuzzy inference system and agarose gel electrophoresis, a case study with toothed carp Aphanius sophiae exposed to cypermethrin. Ecotoxicology 25(5):1040–1046. https://doi.org/10.1007/s10646-016-1647-5
Ribeiro G, Corte-Real M, Johansson B (2006) Characterization of DNA damage in yeast apoptosis induced by hydrogen peroxide, acetic acid, and hyperosmotic shock. Mol Biol Cell 17(10):4584–4591. https://doi.org/10.1091/mbc.E06-05-0475
Sambrook J, Russell D (2016) Molecular cloning: a laboratory manual (third edition). Cold Spring Harbor Laboratory 49:895–909 http://www.openisbn.org/download/0879695765
Sancar A, Rupp W (1983) A novel repair enzyme: UVRABC excision nuclease of Escherichia coli cuts a DNA strand on both sides of the damaged region. Cell 33:249–260. https://doi.org/10.1016/0092-8674(83)90354-9
Sancar A, Tang M (1993) Nucleotide excision repair. Photochem Photobiol 57(5):905–921. https://doi.org/10.1111/j.1751-1097.1993.tb09233.x
Sengun I, Karabiyikli S (2011) Importance of acetic acid bacteria in food industry. Food Control 22(5):647–656. https://doi.org/10.1016/j.foodcont.2010.11.008
Solieri L, Giudici P (2009) Vinegars of the world. Springer, Milan, pp 1–16. https://doi.org/10.1007/978-88-470-0866-3_1
Trcek J, Jernejc K, Matsushita K (2007) The highly tolerant acetic acid bacterium Gluconacetobacter europaeus adapts to the presence of acetic acid by changes in lipid composition, morphological properties and PQQ-dependent ADH expression. Extremophiles 11(4):627–635. https://doi.org/10.1007/s00792-007-0077-y
Trcek J, Toyama H, Czuba J, Misiewicz A, Matsushita K (2006) Correlation between acetic acid resistance and characteristics of PQQ-dependent ADH in acetic acid bacteria. Appl Microbiol Biotechnol 70(3):366–373. https://doi.org/10.1007/s00253-005-0073-z
Van de Guchte M, , Serror P, Chervaux C, Smokvina T, Ehrlich S, Maguin E (2002) Stress responses in lactic acid bacteria. Antonie Van Leeuwenhoek 82(1–4):187 doi:https://doi.org/10.1023/A:1020631532202, 216
Van Houten B, Croteau D, DellaVecchia M, Wang H, Kisker C (2005) ‘Close-fitting sleeves’: DNA damage recognition by the UvrABC nuclease system. Mutat Res 577(1–2):92–117. https://doi.org/10.1016/j.mrfmmm.2005.03.013
Verhoeven E, Wyman C, Moolenaar G, Goosen N (2002) The presence of two UvrB subunits in the UvrAB complex ensures damage detection in both DNA strands. EMBO J 21:4196–4205. https://doi.org/10.1093/emboj/cdf396
Wang B, Shao Y, Chen T, Chen W, Chen F (2015) Global insights into acetic acid resistance mechanisms and genetic stability of Acetobacter pasteurianus strains by comparative genomics. Sci Rep 5:18330. https://doi.org/10.1038/srep18330
Wei L, Zhu D, Zhou J, Zhang J, Zhu K, Du L, Hua Q (2014) Revealing in vivo glucose utilization of Gluconobacter oxydans 621H Δmgdh strain by mutagenesis. Microbiol Res 169(5–6):469–475. https://doi.org/10.1016/j.micres.2013.08.002
Xia K, Zang N, Zhang J, Zhang H, Li Y, Liu Y, Feng W, Liang X (2016) New insights into the mechanisms of acetic acid resistance in Acetobacter pasteurianus using iTRAQ-dependent quantitative proteomic analysis. Int J Food Microbiol 238:241–251. https://doi.org/10.1016/j.ijfoodmicro.2016.09.016
Yamamoto N, Kato R, Kuramitsu S (1996) Cloning, sequencing and expression of the uvrA gene from an extremely thermophilic bacterium, Thermus thermophilus HB8. Gene 171:103–106. https://doi.org/10.1016/0378-1119(96)00052-2
Yin H, Zhang R, Xia M, Bai X, Mou J, Zheng Y, Wang M (2017) Effect of aspartic acid and glutamate on metabolism and acid stress resistance of Acetobacter pasteurianus. Microb Cell Factories 16:109–115. https://doi.org/10.1186/s12934-017-0717-6
Zheng Y, Chen X, Wang J, Yin H, Wang L, Wang M (2015) Expression of gene uvrA from Acetobacter pasteurianus and its tolerance to acetic acid in Escherichia coli. In: Zhang TC, Nakajima M (eds) Advances in Applied Biotechnology. Lecture Notes in Electrical Engineering, vol 333. Springer, Berlin, Heidelberg, pp 163–169. https://doi.org/10.1007/978-3-662-46318-5_18
Zheng Y, Zhang R, Yin H, Bai X, Chang Y, Xia M, Wang M (2017) Acetobacter pasteurianus metabolic change induced by initial acetic acid to adapt to acetic acid fermentation conditions. Appl Microbiol Biotechnol 101:7007–7016. https://doi.org/10.1007/s00253-017-8453-8
Zhu K, Lu L, Wei L, Wei D, Imanaka T, Hua Q (2011) Modification and evolution of Gluconobacter oxydans for enhanced growth and biotransformation capabilities at low glucose concentration. Mol Biotechnol 49(1):56–64. https://doi.org/10.1007/s12033-011-9378-6
Acknowledgements
The authors would like to acknowledge the Mizkan Group Corporation, Japan, for their plasmid pMV24 and Dr. Wei Liujing (East China University of Science and Technology, China) for gifting the plasmids pSUP202 and pRK2013.
Funding
This work was supported by the National Natural Science Foundation of China (31201406, 31671851), Tianjin Municipal Science and Technology Commission (16YFZCNC00650, 17PTGCCX00190), Rural Affairs Committee of Tianjin (201701180), Program for Changjiang Scholars and the Innovative University Research Team (IRT15R49), and the Innovative Research Team of Tianjin Municipal Education Commission (TD13-5013).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Zheng, Y., Wang, J., Bai, X. et al. Improving the acetic acid tolerance and fermentation of Acetobacter pasteurianus by nucleotide excision repair protein UvrA. Appl Microbiol Biotechnol 102, 6493–6502 (2018). https://doi.org/10.1007/s00253-018-9066-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-018-9066-6