Abstract
The uvrA gene of Acetobacter pasteurianus AC2005 coding for subunit A of the excinuclease ABC complex involved in the nucleotide excision repair mechanism was identified. Gene uvrA was amplified using A. pasteurianus AC2005 genomic DNA as a template. Then the pMV24 plasmid, an expression vector of Acetobacter, was used for constructing the recombinant plasmid pMV24-uvrA. UvrA was expressed in Escherichia coli JM109, and its molecular weight was about 91.1 kDa. With 0.5 % acetic acid shock for 20 and 40 min, the survival rates of recombinant strain E. coli JM109/pMV24-uvrA were 0.48 and 0.056 %, which increased by 17.5 and 10.2 times, respectively, compared with those of E. coli JM109/pMV24. All these demonstrate that the expression of repair excinuclease UvrA could increase the acetic acid tolerance of the strain.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Acetate ion in the microorganism is toxic mainly to itself, and acetobacter can improve its resistance to a high concentration of acetic acid with a unique mechanism. Studies suggest that there are two main mechanisms that confer high acetic acid concentration to the bacteria. One is to maintain the intracellular pH relatively constant, like the proton pump, overoxidation of acetic acid, changes in fatty acid composition in membrane, etc.; all these could affect the permeability of the proton [1, 2]. Another is to repair the damage of cellular components caused by high concentrations of acetic acid, like improvement of the stability of enzyme under high concentrations of acetic acid, correct folding of the protein involved by molecular chaperone, and so on [3–5].
Nucleotide excision repair (NER) is a mechanism commonly used to maintain the integrity of DNA and the proteins involved in this mechanism are mainly UvrA, UvrB, and UvrC [6]. UvrA is the initial induced protein in bacteria that test various structurally unrelated DNA lesions and excise and repair them. In prokaryotic microorganism, repair excinuclease UvrA involves in the excision and repair of DNA, and this repair mechanism is applicable to repair of many DNA damages [7]. According to the literature, in Thermus thermophilus, UvrA was overexpressed induced by IPTG and it makes the strain resist high temperature at neutral pH and resist low pH at room temperature. In Lactobacillus helveticus, UvrA was activated by exposure to UV radiation and oxidative stress, and the expression of uvrA was inducible by pH; UvrA contributes to acid and oxidative tolerance in L. helveticus [8].
Acetobacter pasteurianus is an important acid-producing bacterium during solid-state fermentation of vinegar, and is one of the bacteria commonly used in pure liquid fermentation worldwide. As its important application value, the whole-genome of A. pasteurianus has been sequenced completely [9, 10]. This research is focused on the repair excinuclease UvrA from A. pasteurianus AC2005. Using genetic engineering technology, we study the effects of UvrA on the acetic acid tolerance of Escherichia coli, which would lay the foundation for further clarifying the function of repair excinuclease.
2 Materials and Methods
2.1 Bacterial Strains, Plasmids, and Growth Conditions
Acetobacter pasteurianus AC2005, stored in the laboratory, was cultured in GYE media (2 % glucose, 1.5 % yeast extract, 3.5 % ethanol) at 30 °C. E. coli JM109 were used as hosts for the cloning experiments and E. coli was grown at 37 °C on Luria–Bertani (LB) broth supplemented with or without 100 μg/mL ampicillin. The pMD19-T simple vector (TaKaRa) was used for both cloning and sequencing analysis. The pMV24 plasmid (Apr, lacZ), gifted by Mizkan Group Corporation, Japan, was used for expression of the gene in E. coli.
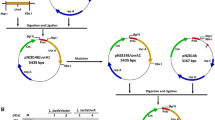
2.2 Construction of the Recombinant Plasmid and Expression of the Target Protein
We used expression vector pMV24 (Apr, 3,854 bp) and selected two restriction sites (EcoRI and XbaI) to construct recombinant plasmid. As pMV24 had a lactose promoter, the target protein was expressed induced by IPTG.
2.3 Shock Experiments
To test the tolerance of the strains toward extremely high acetate stresses, shock experiments were performed with much higher concentrations of acetic acid. Strains were grown overnight at 37 °C in LB medium containing 100 mg/mL ampicillin, then diluted at a ratio of 1:100 into fresh LB medium containing 100 mg/mL ampicillin, and grown at 37 °C to logarithmic phase reaching an OD600 value of 0.6, then added 1 mM IPTG and inoculated for 4–5 h to induce the expression of UvrA. After that acetic acid was added to final concentrations of 0.5 % (v/v). With 40 min incubation at 37 °C, samples were removed and the number of viable bacteria was determined by spread plating serial dilutions onto LB agar containing 100 mg/mL ampicillin. The plates were incubated at 37 °C for about 16 h before enumeration of the colonies. At the same time, the cultures shocked for 20 and 40 min were serially diluted, plated onto LB/agar plates with the Oxford Cup, and incubated at 37 °C for 16 h and then photographed.
3 Results
3.1 Sequence Analysis of the uvrA in A. pasteurianus AC2005
The uvrA gene of A. pasteurianus AC2005 was cloned and its nucleotide sequence was determined. Analysis of the sequence revealed a gene of 2,514 nt that encoded a protein with 837 amino acids (aa) and a predicted molecular mass of 91.1 kDa. In this study, UvrA was expressed in E. coli JM109 induced by 1 mM IPTG. By SDS-PAGE, as shown in Fig. 18.1, a significant band of the protein induced appeared at about 91.1 kDa, and its molecular weight was in line with expectation.
The result of comparison indicates that the protein has high homology with the genus Acetobacter and Gluconobacter. The protein sequence contains a conserved region of NER enzymes.
The sequence obtained was compared with the uvrA gene sequence (GenBank: 8435212) of A. pasteurianus IFO3283-01 in GenBank. The result showed that the coding sequence from A. pasteurianus AC2005 has a similarity of 93.2 % with the corresponding region from A. pasteurianus IFO3283-01. Compared with the protein UvrA from A. pasteurianus AC2005 speculated by the coding region with UvrA from Acetobacter IFO3283-01, the amino acid sequence has a similarity of 98.6 %. Therefore, we can determine the amplified bands for uvrA gene of A. pasteurianus.
3.2 Effect of uvrA Expression on the Growth of E. coli Under Acidic Conditions
To determine the effect on cell growth caused by the expression of UvrA, the experiment studies the growth curves of the strains with the same initial amount of bacteria cells in LB medium. As acetic acid treatment could significantly reduce the final biomass of different strains, E. coli JM109/pMV24 and E. coli JM109/pMV24-uvrA were grown in LB broth supplemented with or without 0.05 % (vol/vol) acetic acid and their growth curves were determined. As shown in Fig. 18.2, in the presence of acetic acid, the growth of control strain E. coli JM109/pMV24 in early logarithmic phase and steady growth was suppressed obviously. However, the growth of recombinant strain E. coli JM109/pMV24-uvrA in the presence or absence of acetic acid is similar. It showed that the expression of UvrA increased the acetic acid tolerance of recombinant strain, and it initially proved that UvrA was related to the acetic acid tolerance.
As we all know acetic acid is highly toxic to E. coli cells, and the concentration of acetic acid for use in E. coli is often under 0.1 %. Then acetic acid shock experiments were subsequently performed to test the tolerance of uvrA expression strain toward acetic acid at higher concentration. The final concentrations of acid required to adjust cultures of E. coli to 0.5 % acetic acid were pH 3. After shocking with 0.5 % acetic acid for different times, the survival curves for the control strain E. coli JM109/pMV24 and the recombinant strain E. coli JM109/pMV24-uvrA are as shown in Fig. 18.3. The changeable survival rate after the acetic acid treatment reflected that the expression of UvrA affects acetic acid tolerance of E. coli. With increasing shock time, the recombinant strain showed a clearly increased tolerance to acetic acid as compared to the control strain exposed to 0.5 % acetic acid. With 0.5 % acetic acid shock for 20 and 40 min, the survival rates of recombinant strain E. coli JM109/pMV24-uvrA were 0.48 and 0.056 %, which increased by 17.5 and 10.2 times, respectively, compared with those of control strain E. coli JM109/pMV24. And with 0.5 % acetic acid shock for 40 min, the plaques of two strains on solid plate were observed. Although the number of colonies of control strain under normal culture condition was slightly higher than the recombinant strain, the number was 10 times higher than control strain after acetic acid shock. All these demonstrate that the expression of repair excinuclease UvrA could increase the acetic acid tolerance of the strain.
4 Discussion
We have constructed a recombinant strain of E. coli with the help of the pMV24 plasmid which is an expression vector of Acetobacter. UvrA was expressed in E. coli JM109, and its molecular weight was about 91.1 kDa. Homology analysis of the A. pasteurianus uvrA gene product revealed high level homology to the Acetobacter and Gluconobacter UvrA proteins. The protein sequence contained a conserved region of NER enzymes was found. Comparison of the A. pasteurianus AC2005 and A. pasteurianus IFO3283-01 UvrA protein demonstrated 98.3 % identity and 98.6 % similarity.
With 0.5 % acetic acid shock for 20 and 40 min, the survival rates of recombinant strain E. coli JM109/pMV24-uvrA increased by 17.5 and 10.2 times, respectively, compared with those of E. coli JM109/pMV24. These demonstrate that the expression of repair excinuclease UvrA could increase the acetic acid tolerance of the strain.
References
Hanna MN, Ferguson RJ, Li YH et al (2001) uvrA is an acid-inducible gene involved in the adaptive response to low pH in Streptococcus mutans. J Bacteriol 183(20):5964–5973
Mullins EA, Francois JA, Kappock TJ (2008) A specialized citric acid cycle requiring succinyl-coenzyme A (CoA): acetate CoA-transferase (AarC) confers acetic acid resistance on the acidophile Acetobacter aceti. J Bacteriol 190(14):4933–4940
Matsushita K, Inoue T, Adachi O, Toyama H (2005) Acetobacter aceti possesses a proton motive force-dependent efflux system for acetic acid. J Bacteriol 187(13):4346–4352
Nakano S, Fukaya M, Horinouchi S (2006) Putative ABC transporter responsible for acetic acid resistance in Acetobacter aceti. Appl Environ Microbiol 72(1):497–505
Trcek J, Toyama H, Czuba, Misiewicz A, Matsushita K (2006) Correlation between acetic acid resistance and characteristics of PQQ-dependent ADH in acetic acid bacteria. Appl Microbiol Biotechnol 70(3):366–373
Van Houten B, Croteau DL, DellaVecchia MJ, Wang H, Kisker C (2005) ‘Close-fitting sleeves’: DNA damage recognition by the UvrABC nuclease system. Mutat Res 577:92–117
Kuper J, Kisker C (2012) Damage recognition in nucleotide excision DNA repair. Sciverse Sci Direct 22:88–93
Cappa F, Cattivelli D (2005) Pier Sandro Cocconcelli. The uvrA gene is involved in oxidative and acid stress responses in Lactobacillus helveticus CNBL1156. Res Microbiol 156:1039–1047
Azuma Y, Hosoyama A, Matsutani M et al (2009) Whole-genome analyses reveal genetic instability of Acetobacter pasteurianus. Nucleic Acids Res 37(17):5768–5783
Matsutani M, Hirakawa H, Saichana N et al (2012) Genome-wide phylogenetic analysis of differences in thermotolerance among closely related Acetobacter pasteurianus strains. Microbiology 158(1):229–239
Acknowledgments
This work was supported by the National High Technology Research and Development Program of China (2012AA022108, 2013AA102106), the National Natural Science Foundation of China (31201406), the Natural Science Foundation of Tianjin, China, (13JCQNJC10000).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer-Verlag Berlin Heidelberg
About this paper
Cite this paper
Zheng, Y., Chen, X., Wang, J., Yin, H., Wang, L., Wang, M. (2015). Expression of Gene uvrA from Acetobacter pasteurianus and Its Tolerance to Acetic Acid in Escherichia coli . In: Zhang, TC., Nakajima, M. (eds) Advances in Applied Biotechnology. Lecture Notes in Electrical Engineering, vol 333. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-662-46318-5_18
Download citation
DOI: https://doi.org/10.1007/978-3-662-46318-5_18
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-662-46317-8
Online ISBN: 978-3-662-46318-5
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)