Abstract
The direct fermentative production of l-serine by Corynebacterium glutamicum from sugars is attractive. However, superfluous by-product accumulation and low l-serine productivity limit its industrial production on large scale. This study aimed to investigate metabolic and bioprocess engineering strategies towards eliminating by-products as well as increasing l-serine productivity. Deletion of alaT and avtA encoding the transaminases and introduction of an attenuated mutant of acetohydroxyacid synthase (AHAS) increased both l-serine production level (26.23 g/L) and its productivity (0.27 g/L/h). Compared to the parent strain, the by-products l-alanine and l-valine accumulation in the resulting strain were reduced by 87 % (from 9.80 to 1.23 g/L) and 60 % (from 6.54 to 2.63 g/L), respectively. The modification decreased the metabolic flow towards the branched-chain amino acids (BCAAs) and induced to shift it towards l-serine production. Meanwhile, it was found that corn steep liquor (CSL) could stimulate cell growth and increase sucrose consumption rate as well as l-serine productivity. With addition of 2 g/L CSL, the resulting strain showed a significant improvement in the sucrose consumption rate (72 %) and the l-serine productivity (67 %). In fed-batch fermentation, 42.62 g/L of l-serine accumulation was achieved with a productivity of 0.44 g/L/h and yield of 0.21 g/g sucrose, which was the highest production of l-serine from sugars to date. The results demonstrated that combined metabolic and bioprocess engineering strategies could minimize by-product accumulation and improve l-serine productivity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
l-Serine is a nonessential amino acid, but it is of significant commercial value in cosmetic and pharmaceutical industries (Ikeda and Takeno 2013); it is known to be an ingredient of skin lotions to ensure a proper hydration status and selected infusion solutions (Stolz et al. 2007). In addition, l-serine is used as a precursor for the synthesis of other amino acids including cysteine and tryptophan, phospholipid, and one-carbon units in vivo (Netzer et al. 2004). Currently, the industrial production of l-serine relies mainly on extraction from protein hydrolysates and enzymatic or cellular conversion (Hsiao and Wei 1986; Izumi et al. 1993). However, these processes are limited for further large-scale production of l-serine due to low yield and high cost (Leuchtenberger et al. 2005). Thus, the direct fermentative production of l-serine from sugars has attracted increasing attention in recent years (Eggeling 2007; Lai et al. 2012; Li et al. 2012).
Compared with the amounts of the other amino acids, accumulation of l-serine in microorganisms is more difficult due to the role of l-serine as a central intermediate for a number of cellular reactions. Recently, microbial fermentation technology combined with system metabolic engineering has constantly improved titer of l-serine overproducers, mainly engineered Escherichia coli and Corynebacterium glutamicum strains. It is worth noting that the attempts to obtain l-serine-producing strains by random mutagenesis had not been successful before (Peters-Wendisch et al. 2005). Gu et al. (2014) reported production of 8.34 g/L l-serine by an engineered E. coli strain in which sdaA, iclR, arcA, and aceB genes were knocked out, and the serA FR, serB, and serC genes were overexpressed. Meanwhile, recent studies have demonstrated that C. glutamicum might be more advantageous as an l-serine overproducer. Peters-Wendisch et al. (2005) reported that by overexpression the genes encoding l-serine synthesis enzymes, releasing the feedback inhibition of 3-phosphoglycerate dehydrogenase (PGDH), and disruption or weakening of l-serine degradation pathways, the resulting engineered C. glutamicum could produce 9.03 g/L l-serine directly from glucose. Subsequently, by reducing folate supply to decreasing serine hydroxymethyltransferase (SHMT) activity and medium optimization, Stolz et al. (2007) further improved l-serine production to 36.25 g/L from a mixture of glucose and fructose in fed-batch fermentation, which was the highest production of l-serine as reported.
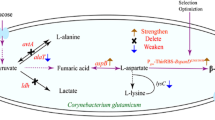
In our previous study, a wild-type strain C. glutamicum SYPS-062, which could directly produce l-serine from sugar, was isolated from soil (Xu et al. 2014; Zhang et al. 2014a). Subsequently, in order to obtain strains with higher l-serine production, random mutagenesis with diethylsulfate (DES) and directed selection with d-serine as the analog were employed in wild-type C. glutamicum SYPS-062. The mutant C. glutamicum SYPS-062-33a was obtained, which could accumulate 11.40 g/L l-serine from sucrose (unpublished data). After that, the engineered C. glutamicum 33a ∆SS was constructed by deletion of the 591 bp of the C-terminal domain of serA encoding 3-phosphoglycerate dehydrogenase and deletion of sdaA encoding l-serine dehydratase (Fig. 1). The resulting strain could produce 21.27 g/L l-serine with a productivity of 0.22 g/L/h directly from sucrose (unpublished data). However, there still existed large amounts of by-products, especially l-alanine and l-valine, which will bring difficulty to l-serine industrial recovery processes due to similar isoelectric point. Moreover, the resulting strain showed weak growth and low l-serine productivity, which limited l-serine production on large scale.
Biosynthesis pathways of l-serine, l-valine, and l-alanine in C. glutamicum. PGDH 3-phosphoglycerate dehydrogenase, PAST phosphoserine aminotransferase, PSP phosphoserine phosphatase, l -SerDH l-serine dehydroxylase, SHMT serine hydroxymethyltransferase, AlaT alanine aminotransferase, AvtA valine-pyruvate aminotransferase, AHAS acetohydroxyacid synthase, PDHC pyruvate dehydrogenase complex, PYC pyruvate carboxylase, TCA tricarboxylic acid, THF tetrahydrofolate, PEP phosphoenolpyruvate, α-KG α-ketoglutaric acid, Glu l-glutamate, O-Val 2-oxo-isovalerate. Dotted arrows represent pathways consisting of more than one reaction
In this study, metabolic and bioprocess engineering was used to minimize by-products accumulation and improve l-serine productivity. Deletion of alaT and avtA combined with introduction of an attenuated mutant of acetohydroxyacid synthase (AHAS) (∆C-T ilvN) was carried out to reduce by-products l-alanine and l-valine. Subsequently, medium optimization was performed to increase the cell growth and l-serine productivity. Finally, an industrially feasible production process was established to overproduce l-serine.
Materials and methods
Strains and plasmids
The bacterial strains and plasmids used in this study are listed in Table 1. C. glutamicum SYPS-062-33a, derived from C. glutamicum SYPS-062 (Zhang et al. 2014a) by random mutagenesis, was deposited in CGMCC with the No. 8667. The engineered C. glutamicum 33a ∆SS with deletion of the 591 bp of the C-terminal domain of serA and deletion of sdaA from C. glutamicum SYPS-062-33a was stored in our laboratory. In this study, this strain was used as the parent strain for generating the mutant strains and E. coli JM109 was used for plasmid construction.
Medium and culture conditions
For strain construction, E. coli was cultivated in Luria-Bertain (LB) medium at 37 °C on a rotary shaker at 120 rpm, and C. glutamicum was cultivated in Brain Heart Infusion (BHI; Difco) medium at 30 °C on a rotary shaker at 120 rpm. When appropriate, 50 mg/L kanamycin was added into the medium. For l-serine fermentations in shake flasks, the seed medium consisted of (per liter) 20 g glucose, 37 g yeast extract, 10 g corn steep liquor, 10 g (NH4)2SO4, 0.2 g K2HPO4, 0.3 g NaH2PO4, and 0.5 g MgSO4·7H2O. The fermentation medium consisted of (per liter) 100 g sucrose, 30 g (NH4)2SO4, 3 g KH2PO4, 0.5 g MgSO4·7H2O, 30 mg protocatechuic acid, 20 mg FeSO4·7H2O, 20 mg MnSO4·H2O, 50 μg biotin, 450 μg thiamine, and 60 g CaCO3. The initial pH of the media was adjusted to 7.2. The seed medium, with an overnight optical density at 562 nm (OD562) of approximately 25, was inoculated into 25 mL fermentation medium with a final OD562 of 1 in a 250-mL flask at 30 °C for 120 h. Samples were withdrawn at regular intervals to measure biomass, sugar, and amino acid concentrations.
Construction of deletion mutant
Chromosomal DNA isolated from C. glutamicum 33a ∆SS was used as the template for PCR. The isolation of plasmids from E. coli was performed using plasmid mini-preps kits according to the protocol from Sangon (Shanghai). The preparation of competent cells and electroporation of C. glutamicum were performed according to the published methods (Van der Rest et al. 1999). Disruption of the gene was performed using the nonreplicable integration vector pK18mobsacB, which allows for marker-free deletion of the target gene (Schäfer et al. 1994). PCR primers for this study are listed in Table 1. For construction of pK18mobsacB ∆alaT, DNA fragments located upstream and downstream of the alaT gene were amplified by PCR using the primer pairs: alaT-1/alaT-2 for the upstream fragment and alaT-3/alaT-4 for the downstream fragment. Two PCR fragments used as the template were subjected to crossover PCR using the primer pair of alaT-1 and alaT-4. The resulting product with truncated alaT gene was digested with SalI and HindIII and then inserted into vector pK18mobsacB to construct pK18mobsacB ∆alaT. The truncated alaT gene from pK18mobsacB ∆alaT was verified by DNA sequencing. Similar approach was applied to construct pK18mobsacB ∆avtA and pK18mobsacB ∆C-T ilvN. The recombinant plasmids were electroporated into C. glutamicum to disrupt the gene using the method described by Schäfer et al. (1994).
Enzyme assays
The AHAS activity was determined according to the published method (Leyval et al. 2003). The C. glutamicum cells were harvested by centrifugation, washed three times by cold 2 % KCl, and then resuspended in the buffer consisting of 100 mM potassium phosphate buffer (pH 7.3), 0.5 mM dithiothreitol (DTT), and 20 % glycerol. The crude enzyme was prepared by sonication, and the homogenate was centrifuged at 12,000 rpm for 30 min at 4 °C. The reaction mixture contained 100 mM potassium phosphate buffer (pH 7.3), 50 mM sodium pyruvate, 10 mM MgCl2, and 100 μM flavin adenine dinucleotide (FAD). The reaction was started by adding 100 μL of crude cell extract to 900 μL reaction mixture in 37 °C for 20 min and terminated by adding 100 μL of 50 % H2SO4. Then, the reaction mixture was incubated at 37 °C for 30 min to transform α-acetolactate into acetoin. The concentration of acetoin was determined by gas chromatography by the method of Zhang et al. (2014b). One unit of the enzyme activity was defined as formation of 1 nmol α-acetolactate per milligram protein per minute. Protein concentrations were determined by the method of Bradford (1976).
Fed-batch fermentations in 5-L stirred-tank bioreactor
In fed-batch fermentation, 5-L stirred-tank bioreactor (Baoxing, Shanghai) was used for l-serine production with an effective working volume of 2.5 L. The medium for fed-batch fermentations consisted of (per liter) 100 g sucrose, 30 g (NH4)2SO4, 2 g corn steep liquor, 3 g KH2PO4, 0.5 g MgSO4·7H2O, 30 mg protocatechuic acid, 20 mg FeSO4·7H2O, 20 mg MnSO4·H2O, 50 μg biotin, and 450 μg thiamine. The feeding medium contained 800 g/L sucrose, and the feed was started when the residual sugar concentration was below 15 g/L. The temperature was maintained at 30 °C, and the pH was adjusted to 7.0 with addition of 25 % (v/v) NH4OH. The dissolved oxygen concentration was maintained around 10 % of air saturation by supplying air at 1 vvm (air volume/working reactor volume/min) and by automatically controlling the agitation speed.
Analytical procedures
The cell density was measured with optical density at 562 nm (UNICO UV-2000, America). The concentration of sugars (sucrose, glucose, and fructose) was determined using an Agilent 1100 Series HPLC (Agilent Technologies, Santa Clara, CA, USA), equipped with a Cosmosil Sugar-D (4.6 × 250 mm) and a refractive index detector (RID). The mobile phase consisted of acetonitrile (75:25, v/v), and the flow rate was adjusted to 1 mL/min. For amino acid analysis, phenylisothiocyanate (PITC) was used as a precolumn derivatization agent. The levels of l-serine and other amino acids were analyzed by HPLC according to the published method (Chen et al. 2011). All assays were performed by triplicate cultures.
Results
Deletion of alaT and avtA reduced l-alanine synthesis and improved l-serine production
In C. glutamicum, the transaminases AlaT and AvtA are known to be responsible for l-alanine formation from pyruvate (Marienhagen et al. 2005). Here, in order to reduce the accumulation of by-product l-alanine, deletion of alaT or/and avtA genes were carried out in C. glutamicum 33a ∆SS (Fig. 1). Firstly, single deletion for alaT or avtA in C. glutamicum 33a ∆SS was explored. As shown in Table 2, in shake-flask fermentations, when alaT was deleted alone, l-alanine was reduced by 28 % (from 9.80 to 7.06 g/L) at 96 h. With avtA deletion, the concentration of l-alanine could reduce by 63 % (from 9.80 to 3.62 g/L). The results indicated that AvtA is the principal l-alanine supplying enzyme in C. glutamicum 33a ∆SS. With regard to the l-serine titer, 22.54 g/L by C. glutamicum 33a ∆SS ∆alaT and 24.46 g/L by C. glutamicum 33a ∆SS ∆avtA of l-serine were obtained in the flask culture, which showed 6 and 15 % increase in l-serine production compared to the parent strain (21.27 g/L).
Subsequently, when alaT and avtA were deleted simultaneously, the resulting strain C. glutamicum 33a ∆SS ∆alaT ∆avtA accumulated l-serine to 25.37 g/L, whereas l-alanine was further decreased to 1.52 g/L (Table 2). The results indicated that deletion of alaT and avtA simultaneously resulted in a cumulative reduction of l-alanine formation and therefore increased l-serine production. However, with alaT and avtA deletion, another by-product l-valine was increased by 10 %.
Introduction of an attenuated mutant of AHAS reduced l-valine synthesis and improved l-serine production
In C. glutamicum 33a ∆SS ∆alaT ∆avtA, l-valine was still the main by-product in the fermentation medium. Previous study suggested that deletion of C-terminal domain in the regulatory subunit ilvN led to a decreased AHAS activity with twofold-lower K m for the substrate pyruvate and fourfold-lower V max (Blombach et al. 2009). Moreover, the AHAS is the key enzyme for the formation of l-valine in C. glutamicum (Bartek et al. 2010; Elišáková et al. 2005). Here, in order to reduce l-valine production, the recombinant strain C. glutamicum 33a ∆SS ∆alaT ∆avtA ∆C-T ilvN was constructed by deletion of ∆C-T ilvN. The results of cell growth, l-serine, and by-product accumulation were shown in Fig. 2 and Table 2. When compared to the C. glutamicum 33a ∆SS ∆alaT ∆avtA, the resulting strain showed similar growth rate and reached to a maximal OD562 of 48 after 96 h. Meanwhile, this strain could produce 26.23 g/L l-serine, 1.23 g/L l-alanine, and 2.63 g/L l-valine in shake-flask fermentations. The by-products l-valine and l-alanine were reduced by 64 and 19 %, respectively. The specific activity of modified AHAS (∆C-T ilvN) in the resulting strain was 30.2 U/mg, which was decreased by 68 % when compared to the parental AHAS (95.6 U/mg). The results indicated that decreased AHAS activity could reduce carbon flux towards the branched-chain amino acids (BCAAs) and lead to a drastically decreased l-valine production, which therefore redirected carbon flux towards l-serine synthesis.
Growth, sugar consumption, and l-serine, l-valine, and l-alanine accumulation during representative shake-flask batch fermentations of C. glutamicum 33a ∆SS ∆alaT ∆avtA ∆C-T ilvN in the fermentation medium. Black squares indicate growth OD562; open squares indicate residual sugar; black circles indicate l-serine; black triangles indicate l-alanine; open triangles indicate l-valine
Optimization of medium to improve l-serine productivity with C. glutamicum 33a ∆SS ∆alaT ∆avtA ∆C-T ilvN
In an industrial fermentation, fermentation medium is of critical importance because medium composition can significantly affect product yield, productivity, and overall process economics (Kennedy and Krouse 1999). Here, the influences of corn steep liquor, yeast extract, beef extract and tryptone on cell growth, l-serine production, and by-product accumulation were investigated. As shown in Table 3, corn steep liquor (CSL) and beef extract significantly stimulated cell growth and increased sucrose consumption rate as well as l-serine productivity. Compared to beef extract, CSL showed more advantageous for l-serine production. With addition of 2 g/L CSL, l-serine productivity was enhanced to 0.45 g/L/h, an increase of 67 % compared to the control. Within 56 h of the fermentation, 98.2 % of the sugars were consumed for l-serine production and the fermentation time was shortened by 40 h (from 96 to 56 h). However, as the CSL concentration was increased, l-serine yield and productivity decreased remarkably, and by-products l-alanine and l-valine accumulation were improved correspondingly. The results showed that addition of 2 g/L CSL was the most suitable for l-serine production, and high level of CSL might lead to the degradation of l-serine, which therefore increased by-products l-alanine and l-valine accumulation (Netzer et al. 2004).
High level production of l-serine by fed-batch fermentations with C. glutamicum 33a ∆SS ∆alaT ∆avtA ∆C-T ilvN
In order to scale up for l-serine production from sucrose, 5-L fed-batch fermentations were carried out to evaluate the suitability of C. glutamicum 33a ∆SS ∆alaT ∆avtA ∆C-T ilvN. The cell growth, l-serine production, by-product accumulation, and sugar consumption were shown in Fig. 3. Within 96 h of the fermentation, this strain exhibited a fast growth and reached its maximum OD562 of 86.5. The l-serine production continuously increased, and the maximum titer achieved was 42.62 g/L with a productivity of 0.44 g/L/h and yield of 0.21 g/g sucrose. Meanwhile, there still existed little by-products l-alanine and l-valine. However, when compared to the titer of l-serine, the accumulation of by-products l-alanine and l-valine were relatively lower. Notably, owing to the resulting engineered strain without exogenous plasmid, we did not observe a stability problem in fed-batch fermentation. Thus, the results obtained in this study demonstrated the possibility of efficiently producing l-serine by engineered C. glutamicum 33a ∆SS ∆alaT ∆avtA ∆C-T ilvN on large scale.
Growth, sugar consumption, and l-serine, l-valine, and l-alanine accumulation during a representative fed-batch fermentation of C. glutamicum 33a ∆SS ∆alaT ∆avtA ∆C-T ilvN in the optimized fermentation medium. Black squares indicate growth OD562; open squares indicate residual sugar; black circles indicate l-serine; black triangles indicate l-alanine; open triangles indicate l-valine
Discussion
Currently, the pathway of l-alanine and l-valine biosyntheses in C. glutamicum has been demonstrated. The transaminases AlaT and AvtA are the l-alanine supplying enzymes and have substrate specificity for l-alanine formation. The AlaT converts pyruvate to l-alanine in a glutamate-dependent reaction, whereas AvtA uses specifically l-valine as amino donors for l-alanine formation (Marienhagen et al. 2005; Leyval et al. 2003). Compared with AvtA, AlaT is the principal l-alanine supplying enzyme and has higher flux efficiency towards l-alanine synthesis (Marienhagen and Eggeling 2008; Hou et al. 2012). However, in this work, AvtA plays the major role in l-alanine synthesis in C. glutamicum 33a ∆SS, which is not consistent with the reports before. This could attribute to substrate specificity of AlaT and AvtA. The l-glutamate as amino donor for AlaT was almost undetectable in C. glutamicum 33a ∆SS, limiting the conversion of pyruvate into l-alanine. Meanwhile, l-valine, as the precursor for synthesis of l-alanine catalyzed by AvtA, was the main by-product. The excessive accumulation of l-valine will promote the l-alanine formation by AvtA, which makes AvtA have the major role in l-alanine synthesis. The results implicated that the presence of AlaT and AvtA together confers the flexibility of l-alanine synthesis in C. glutamicum. When alaT and avtA are both deleted, the concentration of l-alanine could reduce by 84 % and l-serine titer was improved by 20 % compared to the parent strain, indicating that carbon flow towards l-alanine synthesis was redirected to the formation of l-serine. Unexpectedly, the resulting strain was not auxotrophic for l-alanine and showed similar growth rate compared to the parent strain. Moreover, there is still little l-alanine existing in the fermentation medium. Wieschalka et al. (2012) showed that deletion of the transaminase genes alaT and avtA led to drastically reduced formation of l-alanine as a by-product of pyruvate production. The resulting engineered C. glutamicum still showed growth in the minimal medium without addition of l-alanine. The results showed that other transaminase might exist, which could catalyze the formation of l-alanine under these conditions.
Expectably, with the deletion of alaT and avtA, another by-product l-valine was increased correspondingly. Recently, Blombach et al. (2009) reported that introduction of an attenuated mutant of AHAS (∆C-T ilvN) in l-lysine-producing strain of C. glutamicum could decrease the carbon flow towards BCAAs and improve l-lysine production. The modified variant of AHAS also led to drastically increased pyruvate production due to the reduced carbon flow from pyruvate towards l-valine (Wieschalka et al. 2012). However, in C. glutamicum 33a ∆SS ∆alaT ∆avtA ∆C-T ilvN, pyruvate was not been detected in the fermentation medium (data not shown). The l-alanine and l-valine syntheses in C. glutamicum were mainly derived from the glycolysis pathway (Jojima et al. 2010). Reduction of by-products l-alanine and l-valine accumulation by introduction of an attenuated mutant of AHAS could redirect the carbon flow towards l-serine synthesis. In particular, this modification did not result in the generation of an auxotrophy for BCAAs and was suitable for l-serine production. The combined results indicated that deletion of alaT and avtA and introduction of an attenuated mutant of acetohydroxyacid synthase (AHAS) could increase both l-serine production level and its productivity. Through these strategies, the by-products l-alanine and l-valine were significantly decreased, which therefore will facilitate purification process and decrease the cost of production of l-serine.
Industrial production of l-serine demands for high yield, titer, and productivity. In this study, C. glutamicum 33a ∆SS ∆alaT ∆avtA ∆C-T ilvN still showed weak cell growth rate and low l-serine productivity in the fermentation medium. In the presence of 2 g/L CSL, cell growth, sucrose consumption rate, and l-serine productivity were significantly enhanced. However, compared to the control, addition of CSL did not increase the yield of l-serine. The results indicated that the major role of CSL was to promote cell growth and accelerate the consumption of sucrose, which therefore improved l-serine productivity. With an increase of CSL concentration, the final biomass was enhanced. However, l-serine production was decreased and by-products l-alanine and l-valine accumulation were increased. This is probably due to the increase of folate from the CSL (Stolz et al. 2007), which stimulated the conversion of l-serine to glycine plus C1 units and increased growth (Fig. 1). Stolz et al. (2007) reported that in C. glutamicum, reducing the folate supply to decrease SHMT activity could enhance l-serine production. However, a phenomenon cannot be ignored is that the increased l-serine production by decreasing levels of folate supply is always at the expense of cell growth (Stolz et al. 2007; Zhang et al. 2014a). Our previous study also found that in C. glutamicum SYPS-062, folate metabolic pathway was limited due to the low activity of aminodeoxychorismate synthase (ADC), which was probably the main reason resulting in the poor growth and accumulation of l-serine (Zhang et al. 2014a). Meanwhile, high level of CSL might lead to the degradation of l-serine to pyruvate-derived metabolites such as l-alanine even if sdaA was deleted (Netzer et al. 2004). Thus, the proper concentration of CSL is a key for cell growth and l-serine production.
In fed-batch fermentations, the production of l-serine in C. glutamicum 33a ∆SS ∆alaT ∆avtA ∆C-T ilvN is relatively high. However, we observed a reduced Y P/S in fed-batch fermentations compared to cultivations in shake flasks. The result demonstrated the importance of optimizing process conditions, which may further improve l-serine production. Therefore, optimization process parameters for pilot-scale cultivation of the resulting strain will be carried out later.
References
Bartek T, Zönnchen E, Klein B, Gerstmeir R, Makus P, Lang S, Oldiges M (2010) Analysing overexpression of l-valine biosynthesis genes in pyruvate-dehydrogenase-deficient Corynebacterium glutamicum. J Ind Microbiol Biotechnol 37:263–270
Blombach B, Hans S, Bathe B, Eikmanns BJ (2009) Acetohydroxyacid synthase, a novel target for improvement of L-lysine production by Corynebacterium glutamicum. Appl Environ Microbiol 75:419–427
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Chen Q, Wang Q, Wei G, Liang Q, Qi Q (2011) Production in Escherichia coli of poly (3-hydroxybutyrate-co-3-hydroxyvalerate) with differing monomer compositions from unrelated carbon sources. Appl Environ Microbiol 77:4886–4893
Eggeling L (2007) L-Serine and glycine. In: Amino acid biosynthesis—pathways, regulation and metabolic engineering. Springer, Berlin 5:259–272
Elišáková V, Pátek M, Holátko J, Nešvera J, Leyval D, Goergen J-L, Delaunay S (2005) Feedback-resistant acetohydroxy acid synthase increases valine production in Corynebacterium glutamicum. Appl Environ Microbiol 71:207–213
Gu P, Yang F, Su T, Li F, Li Y, Qi Q (2014) Construction of an L-serine producing Escherichia coli via metabolic engineering. J Ind Microbiol Biotechnol 41:1443–1450
Hou X, Chen X, Zhang Y, Qian H, Zhang W (2012) L-Valine production with minimization of by-products’ synthesis in Corynebacterium glutamicum and Brevibacterium flavum. Amino Acids 43:2301–2311
Hsiao HY, Wei T (1986) Enzymatic production of L-serine with a feedback control system for formaldehyde addition. Biotechnol Bioeng 28:1510–1518
Ikeda M, Takeno S (2013) Amino acid production by Corynebacterium glutamicum. In: Yukama H, Inui M (eds) Microbiology monographs 23, Corynebacterium glutamicum. Springer, Berlin, pp 107–147
Izumi Y, Yoshida T, Miyazaki SS, Mitsunaga T, Ohshiro T, Shimao M, Miyata A, Tanabe T (1993) L-Serine production by a methylotroph and its related enzymes. Appl Microbiol Biotechnol 39:427–432
Jojima T, Fujii M, Mori E, Inui M, Yukawa H (2010) Engineering of sugar metabolism of Corynebacterium glutamicum for production of amino acid L-alanine under oxygen deprivation. Appl Microbiol Biotechnol 87:159–165
Kennedy M, Krouse D (1999) Strategies for improving fermentation medium performance: a review. J Ind Microbiol Biotechnol 23:456–475
Lai S, Zhang Y, Liu S, Liang Y, Shang X, Chai X, Wen T (2012) Metabolic engineering and flux analysis of Corynebacterium glutamicum for L-serine production. Sci China Life Sci 55:283–290
Leuchtenberger W, Huthmacher K, Drauz K (2005) Biotechnological production of amino acids and derivatives: current status and prospects. Appl Microbiol Biotechnol 69:1–8
Leyval D, Uy D, Delaunay S, Goergen J, Engasser J (2003) Characterisation of the enzyme activities involved in the valine biosynthetic pathway in a valine-producing strain of Corynebacterium glutamicum. J Biotechnol 104:241–252
Li Y, Chen G-K, Tong X-W, Zhang H-T, Liu X-G, Liu Y-H, Lu F-P (2012) Construction of Escherichia coli strains producing L-serine from glucose. Biotechnol Lett 34:1525–1530
Marienhagen J, Eggeling L (2008) Metabolic function of Corynebacterium glutamicum aminotransferases AlaT and AvtA and impact on L-valine production. Appl Environ Microbiol 74:7457–7462
Marienhagen J, Kennerknecht N, Sahm H, Eggeling L (2005) Functional analysis of all aminotransferase proteins inferred from the genome sequence of Corynebacterium glutamicum. J Bacteriol 187:7639–7646
Netzer R, Peters-Wendisch P, Eggeling L, Sahm H (2004) Cometabolism of a nongrowth substrate: L-serine utilization by Corynebacterium glutamicum. Appl Environ Microbiol 70:7148–7155
Peters-Wendisch P, Stolz M, Etterich H, Kennerknecht N, Sahm H, Eggeling L (2005) Metabolic engineering of Corynebacterium glutamicum for L-serine production. Appl Environ Microbiol 71:7139–7144
Schäfer A, Tauch A, Jäger W, Kalinowski J, Thierbach G, Pühler A (1994) Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene 145:69–73
Stolz M, Peters-Wendisch P, Etterich H, Gerharz T, Faurie R, Sahm H, Fersterra H, Eggeling L (2007) Reduced folate supply as a key to enhanced L-serine production by Corynebacterium glutamicum. Appl Environ Microbiol 73:750–755
Van der Rest M, Lange C, Molenaar D (1999) A heat shock following electroporation induces highly efficient transformation of Corynebacterium glutamicum with xenogeneic plasmid DNA. Appl Microbiol Biotechnol 52:541–545
Wieschalka S, Blombach B, Eikmanns BJ (2012) Engineering Corynebacterium glutamicum for the production of pyruvate. Appl Microbiol Biotechnol 94:449–459
Xu G, Jin X, Guo W, Dou W, Zhang X, Xu Z (2014) Characterization, modification, and overexpression of 3-phosphoglycerate dehydrogenase in Corynebacterium glutamicum for enhancing L-serine production. Ann Microbiol. doi:10.1007/s13213-014-0936-6
Zhang X, Xu G, Li H, Dou W, Xu Z (2014) Effect of cofactor folate on the growth of Corynebacterium glutamicum SYPS-062 and L-serine accumulation. Appl Biochem Biotechnol 173:1607–1617
Zhang X, Zhang R, Bao T, Rao Z, Yang T, Xu M, Xu Z, Li H, Yang S (2014b) The rebalanced pathway significantly enhances acetoin production by disruption of acetoin reductase gene and moderate-expression of a new water-forming NADH oxidase in Bacillus subtilis. Metab Eng 23:34–41
Acknowledgments
This work was financially supported by the High Tech Development Program of China (863 Project, No. 2012AA022102) and the production project of Ministry of Education of Guangdong province (No. 2012B091000083).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhu, Q., Zhang, X., Luo, Y. et al. l-Serine overproduction with minimization of by-product synthesis by engineered Corynebacterium glutamicum . Appl Microbiol Biotechnol 99, 1665–1673 (2015). https://doi.org/10.1007/s00253-014-6243-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-014-6243-0