Abstract
Imidacloprid (IMI) is an insecticide used worldwide, a neonicotinoid that could cause toxicity in non-target organisms. Zebrafish (Danio rerio) is a model organism widely used in different fields of research such as behavioral studies, biochemical parameters as well as neurotoxicity research. Here, we investigate whether the exposure to three concentrations (0.15, 15, and 45 μg/L) of IMI for 96 h alters responses in zebrafish. Oxidative stress parameters and acetylcholinesterase activity (AChE) as well as the behavioral responses of locomotion were measured. IMI exposure decreased distance traveled in fish exposed to the 45 μg/L. In the exploratory activity, time spent and transitions to the top area of the water column decreased in fish exposed to all concentrations of IMI. In addition, exposures to 45 and 15 μg/L of IMI decreased episodes of erratic movement in the zebrafish. Exposures to IMI at a concentration of 45 μg/L decreased the time spent in erratic movements and increased the time spent with no movement (i.e., “freezing”). Glutathione S-transferase (GST) activity was increased in the brain of zebrafish exposed for 96 h to concentrations of 0.15 and 45 μg/L. Brain AChE activity was reduced and the levels of carbonyl protein (CP) increased in brain of zebrafish at concentrations of 15 and 45 μg/L. Lipid peroxidation measured by TBARS and, also non-protein thiols (NPSH) did not show any variation in the brain of zebrafish exposed to IMI. Changes in the activity of cholinergic neurotransmitters in the brain tissues of zebrafish indicate IMI toxicity. Exposures of fish over 96 h to IMI at a nominal concentration of 45 μg/L caused more extensive sublethal responses in zebrafish, but this concentration is well above those expected in the aquatic environment. Studies are warranted to evaluate the effects on behavior and biomarker responses in fish exposed over longer periods to IMI at environmentally relevant concentrations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Neonicotinoid insecticides include one of the most important classes of synthetic pesticides used in agricultural practices, representing around 25% of total insecticide sales worldwide (Casida 2011; Bass et al. 2015). These pesticides are used to protect crops from insects with piercing-sucking mouthparts, and are also used as seed treatments to reduce damage from invertebrates in the soil (Godfray et al. 2014; Wintermantel et al. 2020). The extensive use of neonicotinoid pesticides in agriculture is a global problem, since it can cause impacts in non-target organisms like fish and birds (Blacquière et al. 2012; Husak et al. 2014; Simon-Delso et al. 2015; Soydan et al. 2017). IMI (1-(6-chloro-3-pyridylmethyl)-N-nitroimadazolidin-2-ylide neamine) is a common neonicotinoid utilized for controlling sucking insects in farming, as well as leafhopper, aphids, thrips on rice, vegetables, and other crops (Tomizawa and Casida 2005; Liu et al. 2005). Furthermore, IMI is a potential surface-water contaminant since IMI and its transformation products enter water bodies in runoff, spray drift or accidental spills (Jemec et al. 2007; Jeschke et al. 2011). In insects, neonicotinoids, including IMI acts as an agonist of the nicotinic acetylcholine receptor (nAChR). The mode of action also includes the opening of cation channels as, such as voltage-gated calcium channels. The opening of channels induced by binding of neonicotinoids to nAChRs leads to insecticidal activity. The neonicotinoids action in non-target organisms may frequently impair the central nervous system (CNS) homeostasis (Tomizawa and Casida 2011; Simon-Delso et al. 2015). A range of biomarkers have been used in toxicological studies to monitor for the negative effects of pesticides on non-target aquatic organisms as fish. The measurement of Acetylcholinesterase (AChE) activity is an example of a neuronal marker. AChE is an enzyme responsible for the hydrolysis of the acetylcholine neurotransmitter (ACh) in cholinergic synapses. However, inhibition of this enzyme may be linked to the mechanisms of action of pesticides (Topal et al. 2017; Cheghib et al. 2020). Furthermore, oxidative stress parameters are also used as biomarkers of chemical stress, which is considered as one of the key mechanisms involved in adverse effects caused by pesticides (Pearson and Patel 2016; Wang et al. 2018; Vieira et al. 2018). Prolonged oxidative damage is detrimental to biological systems and can contribute to lipid, DNA, protein damage and even cell death. When animals are exposed over the long-term, these sublethal changes can trigger pathophysiological responses, including respiratory, renal, endocrine, and reproductive disorders (Jabłońska-Trypuć 2017; Cheghib et al. 2020). Tišler et al. (2009) reported that the 96 h-LC50 of Danio rerio exposed to IMI was 241 mg/L, which is several orders of magnitude above the Canadian water quality guideline for IMI of 0.23 μg/L. However, fish in the natural environment are potentially chronically exposed to lower concentrations of IMI and its transformation products (Anderson et al. 2015). Therefore, it is important to understand the sublethal effects on fish species exposed to environmentally relevant concentrations of IMI. Concerning the model test organism in the present study, the zebrafish is a widely used fish species for studies in genetics, pharmacology, and toxicological research, with a genome that has about 70% similarity with the human genome (Howe et al., 2013; Fontana et al., 2018) and an evolutionarily conserved neurotransmitter system (Gonçalves et al., 2020a).
For instance, exposures of zebrafish to the herbicide, glyphosate increased the distance traveled and mean swimming speed, and caused impairment in memory (Bridi et al., 2017). In addition, propiconazole decreased the number of crossings, entries, and time spent in the top part of the water column, and so the time spent in the bottom area of the tank increased (Valadas et al. 2019). Although the behavioral repertoire in zebrafish has been described (Kalueff et al., 2013), it is not clear how neurobehavioral responses are associated with toxicological mechanisms. Therefore, the hypothesis of the present study is that exposures of zebrafish to IMI at environmentally relevant concentrations can disrupt behavior and induce sublethal biochemical responses in brain tissue, such as indicators of damage to lipids (thiobarbituric acid reactive substances) and proteins (carbonyl proteins) from oxidative stress, changes to the levels of antioxidant systems (i.e., glutathione-S- transferase and NPSH) and changes in the activity of cholinergic neurotransmitters (i.e., acetylcholinesterase).
Materials and Methods
Animals
Adult zebrafish (4–5 months-old) of shortfin wild-type (SF) were acquired from local commercial suppliers (Hobby Aquários, RS, Brazil). The proportion used for the experiments was 50:50 (male: female). Fish were maintained with a maximum density of one fish per liter in a 20 L tank (27 ± 1 °C), and the animals were acclimatized for 15 days in 40 L aquariums before the experiments. The water was kept under mechanical, biological, and chemical filtration, and de-chlorinated with AquaSafe ™ (Tetra, VA, USA). Illumination was provided by a photoperiod cycle, 14 h/10 h, light/dark, respectively, using fluorescent light tubes. Fish were fed three times daily with Alcon BASIC ™ flake fish food (Alcon, Brazil). This experiment fully adhered to the National Institute of Health Guide for Care and Use of Laboratory and the protocols were approved by the Ethics Commission on Animal Use of the Federal University of Santa Maria (Process Number: 1777051118).
Preparation and Administration of the IMI
IMI was purchased from Sigma-Aldrich (St. Louis, MO, USA) and the solution was prepared in ultrapure water. Exposure to IMI was performed at nominal concentrations of 0.15 μg/L, 15 μg/L, and 45 μg/L. The lowest nominal concentration was chosen to be within the same order of magnitude of the concentrations of IMI detected in a large Brazilian freshwater reservoir of 0.05 μg/L (Amaral et al., 2020) and in a large river on the Brazil-Argentina border of 0.07 μg/L (Gonçalves et al.2020b). We also considered the IMI concentrations previously used in toxicological studies with zebrafish by Crosby et al. (2015).
Water Analysis
Water samples were collected at the beginning, middle, and at the end of the experimental period in a CorningTM polypropylene Falcon tube (50 mL). All measurements were made in triplicate for each group (control) and IMI exposure. The water samples were processed in the laboratory for pesticide residue analysis (Laboratório de Análises de Resíduos de Pesticidas, LARP, UFSM). Solid-phase extraction (SPE) was applied for sample preparation prior to analysis by ultra-high performance liquid chromatography tandem mass spectrometry (UHPLC-MS/MS) based on the analytical methods described by Donato et al. (2015). On the same day that the samples arrived at the laboratory, 40 mL volumes of the samples were adjusted to pH 2.5 and were pre-concentrated with Oasis® HLB (200 mg; 3 mL) SPE cartridges purchased from Waters (Milford, MA, USA). After the samples had passed under vacuum through the cartridges, 3 mL of ultrapure water was added to wash the cartridge and 1 mL of acetonitrile with 1% (v/v) acetic acid was used to concentrate IMI for subsequent analysis elution. The final extract was diluted 1:1 (v/v) with ultrapure water and analyzed by UHPLC-MS/MS. The mobile phase (A) was water/methanol (98:2 v/v) and B (methanol), both containing 0.1% of formic acid (v/v) and ammonium formate 5 mmol/L. The chromatographic gradient program started at 5% of solvent B (held 0.25 min), then increased linearly to reach 100% solvent B in 7.75 min. The selected flow rate was 0.225 mL min−1 and the injection volume was 10 μL. The MS/MS was operated using electrospray ionization and IMI was quantified by selected reaction monitoring (SRM). The selected ion transitions were 256 > 175 and 256 > 209 m/z for sition, used for quantification and identification, respectively. The MS/MS parameters for analyses were: source temperature, 150 °C; desolvation temperature, 500 °C; desolvation gas (nitrogen) flow, 400 L/h; cone gas (nitrogen) flow, 80 L/h; capillary voltage 2.0 kV and collision gas (argon) flow, 0.15 mL/min. The limit of quantification was 0.01 µg/L for IMI.
Experimental Design
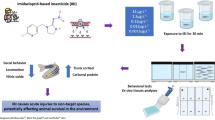
After the acclimation period, each fish was weighed (0.3–0.4 g) in order to ensure that the density in the exposure tanks was approximately 1,590 g of fish per liter of water. Twelve fish were transferred to 20 L tanks for exposure (in duplicate). Four groups were tested: control, IMI (0.15 μg/L), IMI (15 μg/L), and IMI (45 μg/L). Fish were exposed to IMI on the first day and remained in the tank for 96 h. After the exposure period, behavioral tests were performed, and then animals were anesthetized in the water at 4 °C and euthanized by the cervical section.
Behavioral Measurements
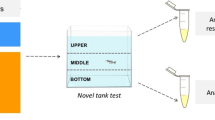
Novel Tank Test
After the period of exposure to the pesticide, zebrafish were placed individually in a novel apparatus (25 cm length × 15 cm height × 11 cm width) as illustrated in Fig. 1, divided, into horizontal portions (bottom and top). The tank was filled with 2 L of control tank water and the swimming behavior was recorded using a webcam connected to a laptop at a rate of 30 frames/s, and subsequently, the behavioral parameters were analyzed using automated video tracking system ANY-maze™ (Stoelting CO, USA). We used the novel tank test to analyze both locomotor and exploratory activity of zebrafish in a novel environment, which may reflect habituation to novelty stress (Rosemberg et al. 2011). Locomotor activity was measured by distance traveled (m) and angular velocity (◦/s). Fear/anxiety-related behaviors were determined by quantifying the number and duration (s) of stoppage of locomotory activity (i.e., “freezing”). The number and duration (s) of erratic movements also were verified. Vertical exploration was assessed by the following endpoints: the number of entries and time spent (s) in the bottom area, latency (s) to enter the top, the number of entries and time spent (s) in the top area. The habituation profile was evaluated by assessing the number of entries and time spent (s) on top during the 6-min trial (Gerlai et al. 2000). All behavioral experiments were performed between 09:00 am and 4:00 pm, according to Paredes et al. (2019) that confirm the extensive daily activity of zebrafish. During the exposure protocol, the animals were handled with all precautions to minimize stress,
Biochemical Parameters
Tissue Preparation
After the behavioral assays, fish were anesthetized in ice water at 4 °C and subsequently euthanized by decapitation and the brain was dissected on ice and transferred to microtubes for storage at − 80 °C. For each independent sample, two brains (one of each duplicate) were homogenized in 300 μL of Tris-HCl 50 mM buffer, pH 7.4. Samples were centrifuged (3000 × g for 10 min, − 4 °C) and the supernatant was removed for further testing. All tests were performed in duplicate and the biomarker analyses were conducted using a SpectraMax 96-well plate reader with UV–Vis detector purchased from Molecular Devices (San Jose, CA, USA).
Oxidative Damage to Biomolecules
The lipid redox status of the samples was estimated by thiobarbituric reactive substance (TBARS) production (Draper and Hadley 1990) adapted by Nunes et al. (2017). Briefly, 150 μL of 10% trichloroacetic acid (TCA) was added in 75 μL of the homogenized brain (70−100 μg protein) and subsequently centrifuged at 10,000 × g for 10 min. After 100 μL of the supernatant was added to 100 μL of 0.67% thiobarbituric acid (TBA, 4,6-dihydroxypyrimidine-2-thiol) and heated for 30 min at 100 °C. The determination of TBARS levels was performed with malondialdehyde (MDA) reaction at 532 nm. Data were expressed as nmol MDA/milligram protein and MDA was utilized as standard.
Carbonyl protein (CP) levels were determined using the method described elsewhere (Parvez and Raissudin 2005) adapted by Müller et al. (2017). A 200 µL subsample of protein was added to 10 mM 2,4-dinitrophenylhydrazine (DNPH) in 2 N hydrochloric acid. In a dark environment, samples were incubated for 1 h. Later, 0.15 mL of denaturing buffer (150 mM sodium phosphate buffer, pH 6.8 containing sodium dodecyl sulfate (SDS) 3.0%), 0.5 mL of heptane (99.5%) and 0.5 mL of ethanol (99.8%) were added and kept under continuous agitation for 30 s, and finally centrifuged for 15 min at 3000 × g. After isolation, the protein was washed twice by resuspension in ethanol/ethyl acetate (1:1) and resuspended in 0.25 ml of denaturing buffer. Data were calculated using the molar extinction coefficient of 22,000 M/cm. Total carbonylation was expressed as nmol carbonyl/milligram protein.
Antioxidant Parameters
Glutathione S-transferase (GST) activity was measured according to the literature (Habig et al. 1974), using 1 mM 1-chloro-2,4-dinitrobenzene (CDNB) in ethanol, 10 mM reduced glutathione (GSH), 20 mM potassium phosphate buffer (pH 6.5) and 10 μL of tissue homogenates (40–60 μg protein). Enzyme activity was measured using a molar extinction coefficient of 9.6 mM/cm. GST activity was determined according to the amount of enzyme required to catalyze the 1 mol CDNB conjugate with GSH/min at 25 °C. Results were expressed in micromol GS-DNB/min/milligram protein. For non-protein thiols quantification, we utilized an aliquot of supernatant (100 μL) mixed with 100 μL of 10% TCA and later centrifuged (3000 × g for 10 min at 4 °C). Supernatants (60–80 μg protein) were mixed with 5,5'-dithio-bis[2-nitrobenzoic acid] DTNB (0.01 M dissolved in ethanol) and the intense yellow color developed was measured at 412 nm after 1 h (Ellman 1959). Results were expressed as nmol SH/mg of protein.
Acetylcholinesterase (AChE) Activity
AChE activity was measured as described previously (Ellman et al. 1961) with some modifications. Aliquots of supernatants (10 µL) were pre-incubated at 30 °C for 2 min with 0.1 M phosphate buffer, pH 7.5, and 1 mM DTNB. The reaction was started by the addition of acetylthiocholine (1 mM). The AChE was measured at 412 nm and activity expressed as µmol of acetylthiocholine (ACh) hydrolyzed/mg protein/min using the SpectraMax from Molecular Devices (San Jose, CA, USA).
Protein Quantification
Protein was determined using the Coomassie blue method and bovine serum albumin as standard (Bradford 1976). Samples were run in duplicate and the absorbance was measured at 595 nm. All biochemical analysis including total protein was made using SpectraMax from Molecular Devices (San Jose, CA, USA).
Statistical Analyses
The normality of data and homogeneity of variances were analyzed using Kolmogorov–Smirnov and Bartlett’s tests, respectively. Results were expressed as a means ± standard error of the mean (S.E.M.) and analyzed by one-way analysis of variance (ANOVA), followed by Tukey’s multiple comparison test whenever appropriate. Data were significant when p ≤ 0.05. The “n” number used was 12. For biochemical analyzes, “n” number was 12, a pool of 2 brains (one from each duplicate).
Results
Measured Concentrations of IMI
As indicated in Table 1, the measured concentrations of IMI in exposure tanks were generally in good agreement with the nominal concentrations. The initial measured concentrations of IMI at the highest nominal concentration were about 80% lower than the nominal concentrations. The levels of IMI declined slightly over the 96 h exposure period (Table 1).
Behavioral Analysis
Exposure to IMI altered locomotion parameters. IMI exposure to 45 µg/L decreased the distance traveled when compared to the control and the other concentrations (F3.52 = 13.29, p < 0.0001; Fig. 2a). At all tested concentrations both transitions and time spent in the top area decreased as compared to the control (F3.52 = 11.82, p < 0.0001; F3.50 = 12.67, p < 0.0001, respectively; Fig. 2b) and no differences were observed among concentrations. Conversely, the absolute turn angle, maximum speed, and the latency to enter the top did not change. Zebrafish exposed to 45 µg/L of IMI showed a significant decrease in the episodes of erratic movements when compared to the control (F3.52 = 10.62, p < 0.0001) (Fig. 3a). Both the duration of erratic movements (F3.52 = 5.491, p = 0.0024) (Fig. 3b) and the freezing duration (F3.52 = 10.62, p < 0.0001) (Fig. 3d) decreased in the animals exposed to 45 µg/L of IMI when compared to the control and lower concentrations.
The influence of exposure to different concentrations (µg/L) of Imidacloprid (IMI) on responses in the novel tank in zebrafish. a locomotor parameters and (b) exploration parameters. The data were expressed as means ± S.E.M and analyzed by one–way ANOVA by Newman-Keuls multiple comparisons test. (****p < 0.0001, n = 12 per group)
Aversive behaviors triggered by different concentrations (µg/L) of Imidacloprid (IMI) in zebrafish. The defensive reactions (freezing and erratic movements). The scores obtained per group were depicted as representative heat maps (a, b) episodes and number of erratic movements, respectively (c, d) episodes and time of freezing, respectively. The data were expressed as means ± S.E.M and analyzed by one–way ANOVA by Newman-Keuls multiple comparisons test. (****p < 0.0001, n = 12 per group)
Biochemical Analyses
IMI exposure altered oxidative parameters in the brain of zebrafish. Concentrations of 0.15 and 45 µg/L increased GST activity in relation to the control (F3.20 = 1.216, p = 0.00012) (Fig. 4a). CP levels in brain tissue increased in zebrafish exposed to 15 and 45 μg/L IMI when compared to fish from the control treatment (F3.20 = 1.349, p < 0.0001), as shown in Fig. 4a. NPSH and TBARS levels in brain tissue were not significantly different from controls (Fig. 4b). There was a decrease in the AChE activity in the brains of zebrafish exposed to IMI at concentrations of 15 and 45 μg/L when compared to the control (F3.22 = 1.266, p < 0.0001), as shown in Fig. 5.
Effects of exposure to different concentrations (µg/L) of Imidacloprid (IMI) on oxidative stress parameters of the zebrafish brain. a Glutathione S-transferase (GST) activity and carbonyl protein. b lipid peroxidation (TBARS) and non-protein thiol (NPSH) levels. Data were expressed as means ± S.E.M and analyzed by one–way ANOVA by Newman-Keuls multiple comparisons test. (****p < 0.0001, n = 12 per group)
Discussion
Although the use of IMI is restricted in the European Union, in Brazil it is one of the most widely used insecticides currently sold (IBAMA, 2020). In addition, it has been found in surface water from Brazilian rivers and reservoirs (Sposito et al., 2018; Acayaba et al., 2020; Amaral et al., 2020; Marins et al., 2020; Severo et al., 2020). IMI could affect fish physiology and biochemical parameters at environmentally relevant concentrations in the low µg/L range (Bartlett et al. 2019; Cheghib et al., 2020). In the present study, we observed effects from exposure to IMI on the behavioral profile of zebrafish at the lowest nominal concentration of 0.15 µg/L, as well as biochemical effects in brain tissue of zebrafish at higher nominal concentrations of 15 and 45 μg/L. At these higher concentrations, we observed an increase in oxidative damage to proteins (i.e., elevated carbonyl protein) although lipid damage (i.e., elevated TBARS) was not observed. The changes in behavior could be related to the reduction in brain AChE activity. These fish showed impaired locomotion in behavior parameters that could be related to the brain AChE reduction. Animals showed impaired locomotion and “freezing” behavior in the bottom area of the tank, with decreased erratic movements when exposed to the highest IMI concentration of 45 µg/L. Some authors consider that after pesticide exposure, persisting effects on neurobehavioral function in zebrafish can be registered (Crosby et al. 2015; Castro et al. 2018). In the context, the observed hyperlocomotion could be a symptom of IMI toxicity. Our results are in agreement with previous findings, where fish exposed to IMI spend more time at the bottom of an aquarium and showed also reduced vertical swimming activity (Champagne et al. 2010). Schmidel et al. (2014) observed that exposure to the herbicide, atrazine at 1,000 µg/L impaired the behavior of zebrafish and also these fish showed reduced brain AChE activity. The fish also spent significantly more time near the aquarium walls when exposed to high atrazine concentrations. The results observed in the present study with zebrafish clearly indicate that behavior impairment of zebrafish could be related to changes in brain cholinergic neurotransmission. Another commonly observed response of fish exposed to low concentrations of pesticides is an increase in oxidative damage to lipid proteins (Vieira et al. 2018; Wu et al. 2018; Clasen et al. 2018). Carbonyl protein (CP) formation is an indicator of oxidative damage to proteins and TBARS is related to lipid damage (Parvez and Raissudin 2005; Lushchak 2016). Increased CP levels in zebrafish exposed to 15 and 45 μg/L of IMI indicated protein damage in brain tissue. Probably the CP increase could be due to the highest concentrations of IMI tested. Alterations to AChE activity are considered a biomarker of pesticide toxicity, and in addition, behavioral alterations in affected organisms have also been observed (Beauvais et al. 2000; Rodríguez-Fuentes et al. 2015; Pamanji et al. 2015; Cheghib et al. 2020; Pullaguri et al. 2020). Swimming alterations recorded in the present study in fish exposed to the highest concentration of IMI are common symptoms related to overstimulation of nicotinic and muscarinic receptors as noted by Pullaguri et al. (2020) in zebrafish exposed to 0.6 mg/L of the antibacterial compound, triclosan.
On the other hand, there was no increase observed in the levels of brain lipid peroxidation, as indicated by TBARS assay. In a previous study, IMI did not change MDA production in zebrafish (Alvim and Martinez 2019). Previous studies have shown that several pesticides induce an increase in the production of reactive oxygen species (ROS) and can cause lipoperoxidation (Lushchak 2016). Contrary to the results showed in the present results, Prochilodus lineatus exposed to IMI showed brain lipid peroxidation Vieira et al. 2018). There is a clear relationship between increased GST activity and the absence of lipid peroxidation, so it is possible that lipid peroxidation in the brain was inhibitedas a result of the increase in brain GST levels. The same hypothesis was previously proposed by (Vieira et al. 2018). Glutathione (GSH) is also an important component of Phase II detoxification. Therefore, the protective effects of glutathione may be related to its ability to form conjugates with IMI and its transformation products, as suggested by Sillapawattana and Schäffer (2017). However, in the present study, brain non-protein thiols (NPSH) levels did not show any variation. Consistent with the results of present study, when mosquitofish Gambusia affinis were exposed to a formulation of the neonicotinoid insecticide, thiamethoxam (Actara®) for 28 days, an increase in GST activity, carbonyl protein formation and a decrease in AChE activity were observed (Cheghib et al. 2020). In addition, many studies have shown that oxidative stress has negative effects on the nervous system and physiological development (Rahal et al. 2014; Ge et al., 2015; Parlak 2018; Wu et al., 2018).
Overall, we demonstrated in the present study that exposures to IMI at nominal concentrations higher than those reported in surface waters (i.e., 15 and 45 µg/L) altered the behavior of zebrafish and reduced AChE activity. However, exposures at the environmentally relevant concentration of 0.15 µg/L caused subtle changes to the antioxidant system (i.e., reduced GST activity). Since the zebrafish in the present study were only exposed to IMI for 96 h prior to assessing behavior and biomarkers response, further studies are warranted to evaluate effects in fish chronically exposed to this and other neonicotinoid insecticides. It also must be recognized that neonicotinoid insecticides are often present as mixtures in surface waters and may have additive effects or greater than additive effects.
References
Acayaba RD, Albuquerque AF, Ribessi RL, Umbuzeiro GA, Montagner CC (2020) Occurrence of pesticides in waters from the largest sugar cane plantation region in the world. Environ Sci Pollut R. https://doi.org/10.1007/s11356-020-11428-1
Alvim TT, Martinez CBR (2019) Genotoxic and oxidative damage in the freshwater teleost Prochilodus lineatus exposed to the insecticides lambda-cyhalothrin and imidacloprid alone and in combination. Mut Res 842:85–93. https://doi.org/10.1016/j.mrgentox.2018.11.011
Amaral AMB, Moura LK, Pellegrin J, Guerra LJ, Cerezer FO, Saibt N, Prestes OD, Zanella R, Loro VL, Clasen B (2020) Seasonal factors driving biochemical biomarkers in two fish species from a subtropical reservoir in southern Brazil: an integrated approach. Environ Pollut 266:115168. https://doi.org/10.1016/j.envpol.2020.115168
Anderson JC, Dubetz C, Palace VP (2015) Neonicotinoids in the Canadian aquatic environment: a literature review on current use products with a focus on fate, exposure, and biological effects. Sci Total Environ 505:409–422. https://doi.org/10.1016/j.scitotenv.2014.09.090
Bartlett AJ, Hedges AM, Intini KD, Brown LR, Maisonneuve FJ, Robinson SA, Gillis PL, de Solla SR (2019) Acute and chronic toxicity of neonicotinoid and butenolide insecticides to the freshwater amphipod, Hyalella azteca. Ecotoxicol Environ Saf 175:215–223. https://doi.org/10.1016/j.ecoenv.2019.03.038
Bass C, Denholm I, Williamson MS, Nauen R (2015) The global status of insect resistance to neonicotinoid insecticides. Pestic Biochem Physiol 121:78–87. https://doi.org/10.1016/j.pestbp.2015.04.004
Beauvais SL, Jones SB, Brewer SK, Little EE (2000) Physiological measures of neurotoxicity of diazinon and malathion to larval rainbow trout (Oncorhynchus mykiss) and their correlation with behavioral measures. Environ Toxicol 19:1875–1880. https://doi.org/10.1002/etc.5620190722
Bridi D, Altenhofen S, Gonzalez JB, Reolon GK, Bonan CD (2017) Glyphosate and roundup® alter morphology and behavior in zebrafish. Toxicology 392:32–39. https://doi.org/10.1016/j.tox.2017.10.007
Blacquière T, Smagghe G, Van Gestel CAM, Mommaerts V (2012) Neonicotinoids in bees: a review on concentrations, side-effects and risk assessment. Ecotoxicology 21:1581–1581. https://doi.org/10.1007/s10646-012-0890-7
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Casida JE (2011) Neonicotinoid metabolism: compounds, substituents, pathways, enzymes, organisms and relevance. J Agric Food Chem 59:2923–2931. https://doi.org/10.1021/jf102438c
Castro TFD, Souza JGS, de Carvalho AFS, Assis IL, Palmieri MJ, Vieira LFA, Marcussi S, Machado MRF, Murgas LDS (2018) Anxiety-associated behavior and genotoxicity found in adult Danio rerio exposed to tebuconazole-based commercial product. Environ Toxicol Pharmacol 62:140–146. https://doi.org/10.1016/j.etap.2018.06.011
Champagne DL, Hoefnagels CCM, Kloet RE, Richardson MK (2010) Translating rodent behavioral repertoire to zebrafish (Danio rerio): relevance for stress research. Behav Brain Res 214:332–342. https://doi.org/10.1016/j.bbr.2010.06.001
Cheghib Y, Chouahda S, Soltani N (2020) Side-effects of a neonicotinoid insecticide (Actara®) on a non-target larvivorous fish Gambusia affinis: growth and biomarkers response. Egypt J Aquat Res 46:167–172. https://doi.org/10.1016/j.ejar.2019.12.007
Clasen B, Loro VL, Murussi CR, Tiecher TL, Moraes B, Zanella R (2018) Bioaccumulation and oxidative stress caused by pesticides in Cyprinus carpio reared in a rice-fish system. Sci Total Environ 626:737–743
Crosby EB, Bailey JM, Oliveri AN, Levin ED (2015) Neurobehavioral impairments caused by developmental imidacloprid exposure in zebrafish. Neurotoxicol Teratol 49:81–90. https://doi.org/10.1016/j.ntt.2015.04.006
Donato FF, Martins ML, Munaretto JS, Prestes OD, Adaime MB, Zanella R (2015) Development of a multiresidue method for pesticide analysis in drinking water by solid phase extraction and determination by gas and liquid chromatography with triple quadrupole tandem mass spectrometry. J Braz Chem Soc 26(10):2077–2087. https://doi.org/10.5935/0103-5053.20150192
Draper HH, Hadley M (1990) Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol 186:421–431. https://doi.org/10.1016/0076-6879(90)86135-I
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77. https://doi.org/10.1016/0003-9861(59)90090-6
Ellman GL, Courtney KD, Andres V, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95. https://doi.org/10.1016/0006-2952(61)90145-9
Fontana BD, Mezzomo NJ, Kalueff AV, Rosemberg D (2018) The developing utility of zebrafish models of neurological and neuropsychiatric disorders: a critical review. Exp Neurol 299:157–171. https://doi.org/10.1016/j.expneurol.2017.10.004
Ge W, Yan S, Wang J, Zhu L, Chen A, Wang J (2015) Oxidative stress and DNA damage induced by imidacloprid in zebrafish (Danio rerio). J Agric Food Chem 63(6):1856–1862. https://doi.org/10.1021/jf504895h
Gerlai R, Lahav M, Guo S, Rosenthal A (2000) Drinks like a fish: zebra fish (Danio rerio) as a behavior genetic model to study alcohol effects. Pharmacol Biochem Behav 67:773–782. https://doi.org/10.1016/s0091-3057(00)00422-6
Godfray HCJ, Blacquière T, Field LM, Hails RS, Petrokofsky G, Potts SG, Raine NE, Vanbergen AJ, McLean AR (2014) A restatement of the natural science evidence base concerning neonicotinoid insecticides and insect pollinators. Proc R Soc B 281:20140558. https://doi.org/10.1098/rspb.2014.0558
Gonçalves C, Marins AT, do Amaral AMB, Nunes MEM, Müller TE, Severo E, Feijó A, Rodrigues CCR, Zanella R, Prestes OD, Clasen B, Loro VL (2020) Ecological impacts of pesticides on Astyanax jacuhiensis (Characiformes: Characidae) from the Uruguay river, Brazil. Ecotoxicol Environ Saf 205:111314. https://doi.org/10.1016/j.ecoenv.2020.111314
Gonçalves JFS, Souza TM, Vieira LR, Marchi FC, Nascimento AP, Farias DF (2020b) Toxicity testing of pesticides in zebrafish a systematic review on chemicals and associated toxicological endpoints. Environ Sci Pollut Res Int 27(10):10185–10204. https://doi.org/10.1007/s11356-020-07902-5
Habig WH, Pabst MJ, Jacoby WB (1974) Glutathione S-transferase, the first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Howe K, Clark MD, Torroja CF, Torrance J, Berthelot C, Muffato M et al (2013) The zebrafish reference genome sequence and its relationship to the human genome. Nature 496:498–503. https://doi.org/10.1038/nature12111
Husak VV, Mosiichuk NM, Maksymiv IV, Sluchyk IY, Storey JM, Storey KB, Lushchak V (2014) Histopathological and biochemical changes in goldfish kidney due to exposure to the herbicide sencor may be related to induction of oxidative stress. Aquat Toxicol 155:181–189. https://doi.org/10.1016/j.aquatox.2014.06.020
IBAMA (Brazilian Institute of the Environment and Renewable Natural Resources) (2020). Annual pesticide production, import, export and sales bulletins in Brazil. http://www.ibama.gov.br/agrotoxicos/relatorios-de-comercializacao-de- agrotoxicos#historicodecomercializacao
Jabłońska-Trypuć A (2017) Pesticides as inducers of oxidative stress. React Oxyg Species 3:96–110. https://doi.org/10.20455/ros.2017.823
Jemec A, Tisler T, Drobne D, Sepcic K, Fournier D, Trebse P (2007) Comparative toxicity of imidacloprid, of its commercial liquid formulation and of diazinon to a non-target arthropod, the microcrustacean Daphnia magna. Chemosphere 68(8):1408–1418. https://doi.org/10.1016/j.chemosphere.2007.04.015
Jeschke P, Nauen R, Schindled M, Elbert A (2011) Overview of the status and global strategy for neonicotinoids. J Agric Food Chem 59:2897–2908. https://doi.org/10.1021/jf101303g
Kalueff AV, Gebhardt M, Stewart AM, Cachat JM, Brimmer M, Chawla JS et al (2013) Towards a comprehensive catalog of zebrafish behavior 1.0 and beyond. Zebrafish 10:70–86. https://doi.org/10.1089/zeb.2012.0861
Liu H, Song J, Zhang S, Qu L, Zhao Y, Wu Y, Liu H (2005) Analysis of residues of imidacloprid in tobacco by high-performance liquid chromatography with liquid–liquid partition cleanup. Pest Manag Sci 61(5):511–514. https://doi.org/10.1002/ps.987
Lushchak VI (2016) Contaminant-induced oxidative stress in fish: a mechanistic approach. Fish Physiol Biochem 42:711–747. https://doi.org/10.1007/s10695-015-0171-5
Marins AT, Severo ES, Leitemperger JW, Cerezer C, Müller TE, Costa MD, Weimer GH, Bandeira NMG, Prestes OD, Zanella R, Loro VL (2020) Assessment of river water quality in an agricultural region of Brazil using biomarkers in a native neotropical fish, Astyanax spp. (Characidae). Bull Environ Contam Toxicol 104:575–581. https://doi.org/10.1007/s00128-020-02821-0
Müller TE, Nunes SZ, Silveira A, Loro VL, Rosemberg DB (2017) Repeated ethanol exposure alters social behavior and oxidative stress parameters of zebrafish. Mol Neurobiol 79:105–111. https://doi.org/10.1007/s12035-017-0441-6
Nunes ME, Müller TE, Braga MM, Fontana BD, Quadros VA, Marins A, Rodrigues C, Menezes C, Rosemberg DB, Loro VL (2017) Chronic treatment with paraquat induces brain injury, changes in antioxidant defenses system, and modulates behavioral functions in zebrafish. Mol Neurobiol 54:3925–3934. https://doi.org/10.1007/s12035-016-9919-x
Pamanji R, Bethu MS, Yashwanth B, Leelavathi S, Rao JV (2015) Developmental toxic effects of monocrotophos, an organophosphorus pesticide, on zebrafish (Danio rerio) embryos. Environ Sci Pollut Res 22:7744–7753. https://doi.org/10.1007/s11356-015-4120-8
Paredes JF, Cowan M, López-Olmeda JF, Muñoz-Cueto JA, Sánchez-Vázquez FJ (2019) Daily rhythms of expression in reproductive genes along the brain-pituitary-gonad axis and liver of zebrafish. Comp Biochem Physiol Part A Mol Integr Physiol 231:158–169. https://doi.org/10.1016/j.cbpa.2019.02.017
Parlak V (2018) Evaluation of apoptosis, oxidative stress responses, AChE activity and body malformations in zebrafish (Danio rerio) embryos exposed to deltamethrin. Chemosphere 207:397–403. https://doi.org/10.1016/j.chemosphere.2018.05.112
Parvez S, Raisuddin S (2005) Protein carbonyls: novel biomarkers of exposure to oxidative stress-inducing pesticides in freshwater fish Channa punctate (Bloch). Environ Toxicol Pharmacol 20:112–117. https://doi.org/10.1016/j.etap.2004.11.002
Pearson JN, Patel M (2016) The role of oxidative stress in organophosphate and nerve agent toxicity. Ann N Y Acad Sci 1378:17–24. https://doi.org/10.1111/nyas.13115
Pullaguri N, Nema S, Bhargava Y, Bhargava A (2020) Triclosan alters adult zebrafish behavior and targets acetylcholinesterase activity and expression. Environ Toxicol Pharmacol 75:103311. https://doi.org/10.1016/j.etap.2019.103311
Rahal A, Kumar A, Singh V, Yadav B, Tiwari R, Chakraborty S, Dhama K (2014) Oxidative stress, prooxidants, and antioxidants: the interplay. Biomed Res Int. https://doi.org/10.1155/2014/761264
Rodríguez-Fuentes G, Rubio-Escalante FJ, Norena-Barroso E, Escalante-Herrera KS, Schlenk D (2015) Impacts of oxidative stress on acetylcholinesterase transcription, and activity in embryos of zebrafish (Danio rerio) following Chlorpyrifos exposure. Comp Biochem Physiol C Toxicol Pharmacol 172–173:19–25. https://doi.org/10.1016/j.cbpc.2015.04.003
Rosemberg DB, Rico EP, Mussulini BH, Piato AL, Calcagnotto ME, Bonan CD, Dias RD, Blaser RE, Souza DO, Oliveira DL (2011) Differences in spatio-temporal behavior of zebrafish in the open tank paradigma after a short-period confinement into dark and bright environments. PLoS ONE 6(5):e19397. https://doi.org/10.1371/journal.pone.0019397
Schmidel AJ, Assmann KL, Werlang CC, Bertoncello KT, Francescon F, Rambo CL, Beltrame GM, Calegari D, Batista CB, Blaser RE, Roman Júnior WA, Conterato GMM, Piato AL, Zanatta L, Magro JD, Rosemberg DB (2014) Subchronic atrazine exposure changes defensive behaviour profile and disrupts brain acetylcholinesterase activity of zebrafish. Neurotoxicol Teratol 44:62–69. https://doi.org/10.1016/j.ntt.2014.05.006
Severo ES, Marins AT, Cerezer C, Costa D, Nunes M, Prestes OD, Zanella R, Loro VL (2020) Ecological risk of pesticide contamination in a Brazilian river located near a rural area: a study of biomarkers using zebrafish embryos. Ecotox Environ Safe 190:110071. https://doi.org/10.1016/j.ecoenv.2019.110071
Sillapawattana P, Schäffer A (2017) Effects of imidacloprid on detoxifying enzyme glutathione S-transferase on Folsomia candida (Collembola). Environ Sci Pollut Res 24:11111–11119. https://doi.org/10.1007/s11356-016-6686-1
Simon-Delso N, Amaral-Rogers V, Belzunces LP, Bonmatin JM, Chagnon M et al (2015) Systemic insecticides (neonicotinoids and fipronil): trends, uses, mode of action and metabolites. Environ Sci Pollut Res Int 22:5–34. https://doi.org/10.1007/s11356-014-3470-y
Soydan E, Guler A, Bıyık S, Senturk M, Supuran CT, Ekinci D (2017) Carbonic anhydrase from Apis mellifera: purification and inhibition by pesticides. J Enzym Inhib Med Chem 32(1):47–50. https://doi.org/10.1080/14756366.2016.1232255
Sposito JCV, Montagner CC, Casado M, Navarro-Martín L, Solórzano JCJ, Piña B, Grisolia AB (2018) Emerging contaminants in Brazilian rivers: occurrence and effects on gene expression in zebrafish (Danio rerio) embryos. Chemosphere 209:696–704. https://doi.org/10.1016/j.chemosphere.2018.06.046
Tišler T, Jemec A, Mozetic B, Trebse P (2009) Hazard identification of imidacloprid to aquatic environment. Chemosphere 76(7):907–914. https://doi.org/10.1016/j.chemosphere.2009.05.002
Tomizawa M, Casida JE (2005) Neonicotinoid insecticide toxicology: mechanisms of selective action. Annu Rev Pharmacol Toxicol 45:247–268. https://doi.org/10.1146/annurev.pharmtox.45.120403.095930
Tomizawa M, Casida JE (2011) Neonicotinoid insecticides: highlights of a symposium on strategic molecular designs. J Agr Food Chem 59:2883–2886. https://doi.org/10.1021/jf103856c
Topal A, Alak G, Ozkaraca M, Yeltekin AC, Comaklı S, Acil G, Kokturk M, Atamanalp M (2017) Neurotoxic responses in brain tissues of rainbow trout exposed to imidacloprid pesticide: assessment of 8-hydroxy-2-deoxyguanosine activity, oxidative stress and acetylcholinesterase activity. Chemosphere 175:186–191. https://doi.org/10.1016/j.chemosphere.2017.02.047
Ulrich K, Jakob U (2019) The role of thiols in antioxidant systems. Free Radic Biol Med 140:14–27. https://doi.org/10.1016/j.freeradbiomed.2019.05.035
Valadas J, Mocelin R, Sachett A, Marcon M, Zanette RA, Dallegrave E, Herrmann AP, Piato A (2019) Propiconazole induces abnormal behavior and oxidative stress in zebrafish. Environ Sci Pollut R 26:27808–27815. https://doi.org/10.1007/s11356-019-05977-3
Vieira CED, Perez MR, Acayaba RD, Raimundo CCM, Martinez CBR (2018) DNA damage and oxidative stress induced by imidacloprid exposure in different tissues of the neotropical fish Prochilodus lineatus. Chemosphere 195:125–134. https://doi.org/10.1016/j.chemosphere.2017.12.077
Wang X, Anadón A, Wu Q, Qiao F, Ares I, Martínez-Larrañaga MR, Yuan Z, Martínez MA (2018) Mechanism of neonicotinoid toxicity: impact on oxidative stress and metabolism. Annu Rev Pharmacol Toxicol 58:471–507. https://doi.org/10.1146/annurev-pharmtox-010617-052429
Wintermantel D, Odoux JF, Decourtye A, Henry M, Allier F, Bretagnolle V (2020) Neonicotinoid-induced mortality risk for bees foraging on oilseed rape nectar persists despite EU moratorium. Sci Total Environ 704:135400. https://doi.org/10.1016/j.scitotenv.2019.135400
Wu S, Li X, Liu X, Yang G, An X, Wang Q, Wang Y (2018) Joint toxic effects of triazophos and imidacloprid on zebrafish (Danio rerio). Environ Pollut 235:470–481. https://doi.org/10.1016/j.envpol.2017.12.120
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
The current study was approved by the Ethics Commission on Animal Use of the Federal University of Santa Maria (Process Number: 1777051118).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Guerra, L.J., do Amaral, A.M.B., de Quadros, V.A. et al. Biochemical and Behavioral Responses in Zebrafish Exposed to Imidacloprid Oxidative Damage and Antioxidant Responses. Arch Environ Contam Toxicol 81, 255–264 (2021). https://doi.org/10.1007/s00244-021-00865-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-021-00865-9