Abstract
The imidacloprid-based insecticides (IBIs) are among the most used insecticides worldwide, and chronic and acute toxic effects (days exposure protocols) have been reported in several species in studies of IBIs at lethal concentrations. However, there is little information on shorter time exposures and environmentally relevant concentrations. In this study, we investigated the effect of a 30-min exposure to environmentally relevant concentrations of IBI on the behavior, redox status, and cortisol levels of zebrafish. We showed that the IBI decreased fish locomotion and social and aggressive behaviors and induced an anxiolytic-like behavior. Furthermore, IBI increased cortisol levels and protein carbonylation and decreased nitric oxide levels. These changes were mostly observed at 0.013 and 0.0013 µg·L−1 of IBI. In an environmental context, these behavioral and physiological disbalances, which were immediately triggered by IBI, can impair the ability of fish to evade predators and, consequently, affect their survival.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The worldwide contamination of aquatic environments with chemical compounds, even at low concentrations, is one of the main environmental issues (Schwarzenbach et al. 2006). Among these contaminants are insecticides, which are substances used in agriculture for insect control that can contaminate aquatic habitats through direct application, terrestrial runoff, or windborne drift (Relyea and Hoverman 2006; de Souza et al. 2020; Tudi et al. 2021). These agrochemical residues in the water can affect nontarget organisms, such as fish, and act as endocrine disruptors and modify their behavior, such as sexual behavior (Sanchez-Bayo 2011; Köhler and Triebskorn 2013).
Neonicotinoids are a class of insecticides that agonistically bind to the postsynaptic nicotinic acetylcholine receptors (nAChRs) in the invertebrate central nervous system (Morrissey et al. 2015). These compounds are mainly used to control sucking insects on crops (Tomizawa and Casida 2003; Sánchez-Bayo et al. 2016), although they are also used in spot-on insecticides for ectoparasite control in dogs and cats (Mencke and Jeschke 2002) and against household insects (Sadaria et al. 2017; Anthe et al. 2020). The imidacloprid (1-(6-chloro-3-pyridylmethyl)-N-nitro-imidazolidin-2-ylideneamine) (CAS number 138261–41-3) is one of the most commonly used insecticides worldwide (Van Dijk et al. 2013; Albuquerque et al. 2016; Zhang et al. 2019). Although it specifically targets invertebrates and exhibits low toxicity in mammals, birds, and fish (Tomizawa and Casida 2003), the toxic and sublethal effects of imidacloprid have been observed in several vertebrate species (Wang et al. 2018). The effects are abortion, fetus malformation, and immunotoxicity in rats (Gawade et al. 2013); nervous disorders and deaths in birds (Millot et al. 2017); reduction in growth, development, and reproduction of wild animals (Gibbons et al. 2015); dyspnea, diaphoresis, drooling, and multiple oral ulcers in humans (Lin et al. 2013); and decrease of larval survival and hatching rates and oxidative damage, neurotoxic effects, and genotoxicity in adult fish (Crosby et al. 2015; Frew et al. 2018; Islam et al. 2019; Almeida et al. 2021). In addition, imidacloprid-based insecticides (IBIs) are banned in the European Union (Gross 2013) for their direct and indirect deleterious effects on bees, which are important nontarget pollinator species (Cresswell 2011; Delkash-Roudsari et al. 2020). The previously cited toxicity studies used concentrations or doses based on LC50 (lethal concentration that causes the death of 50% of the tested animal population), although several studies have used environmental concentrations of imidacloprid (Van Dijk et al. 2013; Sánchez-Bayo and Hyne 2014; Morrissey et al. 2015; Zhang et al. 2019). Considering that the half-life of imidacloprid in an aquatic environment that suffers from solar radiation (photolysis) is 1–4 h (Kagabu and Medej 1995; Moza et al. 1998; Bonmatin et al. 2015), it is necessary to investigate its damage to aquatic life in short exposure times.
Biochemical factors, such as enzymes and oxidative damage products, are important tissue biomarkers of pesticide injuries (Agrawal and Sharma 2010; Ge et al. 2015; Jabłońska-Trypuć 2017). These biomarkers can reflect the balance or imbalance between pro- and antioxidation events in the organism and the interference of the pollutants in these processes (Valavanidis et al. 2006; Slaninova et al. 2009). If an imbalance in the mechanism of antioxidation and generation of reactive oxygen and nitrogen species (ROS and RNS, respectively) occurs, oxidative cell damages may result in that can alter several cellular and tissular functions (Comporti 1989; Gracy et al. 1999; Fedorova et al. 2014). The redox status of an organism is measured and evaluated through the quantification of damages caused by ROS and RNS, such as protein carbonylation (PC), lipoperoxidation, and imbalance in the production of nitric oxide, and through the analysis of antioxidant enzymes, such as superoxide dismutase (SOD), catalase (CAT), and glutathione S-transferase (GST). Antioxidant enzymes are directly linked to the detoxification of ROS (SOD and CAT) (Yang and Lee 2015; Hoseinifar et al. 2020) and xenobiotics, and the same was observed for GST (Ulrich and Jakob 2019). Furthermore, nonprotein thiols may also act in the detoxification of ROS (Baba and Bhatnagar 2018). Regarding the damage caused by ROS and RNS, biomarkers, such as lipid peroxidation and PC, can be analyzed. Carbonylation is considered irreversible damage of proteins through chemical modifications (Parvez and Raisuddin 2005; Dalle-Donne et al. 2006; Suzuki et al. 2010; Wong et al. 2010; Fedorova et al. 2014). Lipoperoxidation is an injury mainly caused to the cell membrane and, similar to PC, can lead to tissue damage and cell aging. This is measured through malondialdehyde (MDA), the end-product of this lipid oxidation process (Aslan et al. 2008; EL-Gendy et al. 2010). The enzyme, acetylcholinesterase (AChE), which is involved in cholinergic neurotransmission, is also considered an important biomarker of toxicity as several substances can alter its activity and trigger neurological damage in several species (Fu et al. 2018). Finally, nitric oxide (NO) is a substance that acts in different places and has different functions. Regarding the oxidative state, NO has scavenger activity in ROS (Pierini and Bryan 2015) and is correlated with endothelial dysfunctions (Förstermann 2010).
The behavior, which results from an interaction between the animal and the environment (Orger and De Polavieja 2017), is also used as a biomarker of toxicity. An intact behavioral repertoire and an appropriate stress response in a risk assessment situation are crucial for fish survival and fitness (Gerhardt 2007). Residues arising from anthropomorphic activity, such as agrochemicals, can have an endocrine disruptor effect (Ullah and Zorriehzahra 2015) and impair both the behavior and aspects related to the stress axis of these animals, which has cortisol as its final product (Mommsen et al. 1999; Fuzzen et al. 2010).
As the zebrafish (Danio rerio) is an established animal model for behavioral (Egan et al. 2009; Maximino et al. 2010) and toxicological (Bailey et al. 2013; Dai et al. 2014) studies, we evaluated in this study the effect of sublethal and environmentally relevant concentrations of IBI on the hypothalamus–pituitary–interrenal (HPI) axis, behavior, and redox status of adult zebrafish in an acute short-term protocol of exposure.
Material and methods
Reagents
The IBI was purchased from Nortox® (Arapongas, Brazil; imidacloprid, 480 g·L−1; other ingredients, 728.4 g·L−1). The imidacloprid analytical standard (≥ 98% purity, PESTANAL®) was purchased from Sigma-Aldrich® (São Paulo, Brazil). Methanol (high-performance liquid chromatography (HPLC) grade), formic acid, ethyl ether, and dibasic and monobasic potassium and sodium phosphates were purchased from Dinâmica Química Contemporânea® (Indaiatuba, Brazil); acetonitrile was purchased from Êxodo Científica® (Sumaré, Brazil), and ethyl ether was purchased from Neon® (Suzano, Brazil). Thiobarbituric acid (TBA), acetylthiocholine iodide, bovine albumin, Coomassie Blue, hydrogen peroxide, epinephrine, glycine, reduced glutathione, 1-chloro-2,4-dinitrobenzene (CDNB), dinitrophenyl hydrazine (DNPH), trichloroacetic acid (TCA), MDA, 5,5-dithio-bis(2-nitrobenzoic acid) (DTNB), l-cystein, and sodium azide were obtained from Sigma-Aldrich® (St. Louis, USA).
Assessment of imidacloprid in water
We quantified the imidacloprid concentration in the exposure water according to Li et al. (2019) and Costa (2014), with modifications. We used Agilent® SPE Bond Elut C18 cartridges (500 mg, 3 mL; Agilent Technologies, CA, USA). The cartridges were preconditioned with 10 mL of methanol followed by 10 mL of purified water. In a manifold, 2000 mL of exposure water was passed through the cartridges at a flow rate of 9 mL·min−1. The cartridges were then washed with 10 mL of purified water and dried in a vacuum. The analytes were eluted in a glass tube with 10 mL of methanol and evaporated with a gentle stream of dry nitrogen. The analytes were reconstituted with 1 mL of methanol/water (1: 1, v: v) and filtered through a 0.22-mm nylon membrane filter (Filtrilo®, Paraná, Brazil). The chromatographic analyses were performed on the Shimadzu® LC-20AT Prominence liquid chromatography (HPLC–DAD (high performance liquid chromatography coupled with diode array detector); Shimadzu, Kyoto, Japan) with 270-nm wavelength (ultraviolet lamp). Chromatographic separation was performed with the C18 Zorbax Eclipse Plus column (4.6 mm × 250 mm, 5 µm, Agilent) using a mobile phase of water/acetonitrile (70: 30, v: v) at a flow rate of 1 mL·min−1 via a volume injection of 20 µL. The process was performed in triplicates. The recovery percentage calculation was calculated concerning the nominal concentration, considered as 100%.
Fish samples
One hundred eighty adults (average age, 300 days; average size, 2.5–3.0 cm; average weight, 0.15–0.25 g) of mixed-sex wild-type zebrafish (D. rerio) were housed in glass tanks at a density of 0.15 to 0.25 g per liter, with constant aeration under artificial photoperiod (14 h light: 10 h dark). During the preexperimental and acclimation periods (except on the experiment day), the fish were fed twice a day (08:00 h and 17:00 h) (Dametto et al. 2018) with a commercial flaked food (Tropical® Supervit; raw protein, 48%; fat extract and crude oils, 8%). The water of the aquariums was recorded to be at 26 ± 2 °C and pH 7.0 ± 0.2, with dissolved oxygen levels of 6 ± 0.5 mg·L−1, total ammonia levels of < 0.01 mg·L−1, total hardness of 45 ± 5 mg·L−1, and alkalinity of 40 ± 3 mg·L−1 CaCO3, as analyzed via colorimetric tests (Alcon®, Camboriú, Brazil) and using the YSI® 550A oximeter (YSI Life Sciences, Yellow Springs, USA).
Experimental design and procedures
To simulate acute exposure (Nema and Bhargava 2018), we exposed adult zebrafish for 30 min to five different concentrations of IBI based on the geometric mean determined by Morrissey et al. (2015). After exposure, behaviors related to stress, anxiety, sociability, and aggressiveness were evaluated. After the in vivo behavioral assessment, the fish were euthanized, and ex vivo analysis was performed by measuring the trunk cortisol levels and cerebral and trunk oxidative status. The initial procedures are detailed in the text below, and the details of each analysis are specified in the next sections.
The fish were initially acclimated in schools of four adults (two males and two females) (Pagnussat et al. 2013) for 1 week in the test room in 3-L aquariums (15 cm height × 15 cm width × 20 cm depth). The animals were randomly distributed in each aquarium using the application, RANDOM.ORG (Barcellos et al. 2020). On the day of exposure, three animals from the same aquarium were individually placed for 30 min in 2-L beakers with 1 L of the test solutions (the IBI at different concentrations dissolved in dechlorinated water or dechlorinated water only (for the control group)). After exposure, the animals were carefully removed simultaneously from the beakers and placed in the test aquariums, and their behavior was recorded. The animal that remained in the aquarium was relocated to the maintenance tanks. After the tests were performed and after 15 min from the beginning of the tests (for cortisol analysis (Ramsay et al. 2009)), the fish were removed from the test tanks and placed in ice-cold water, and euthanasia was performed via the spinal cord section (Leary and Cartner 2013). We then dissected fish for sex confirmation, and the brain and trunk were separated for ex vivo analysis. Before the ex vivo analysis, we analyzed the behavior of the animals exposed and sexed on the software. After confirming that there was no difference between the behavior of males and females exposed to IBI, we mixed the trunks and brains of both sexes for ex vivo analysis, following the 3R concept (replacement, reduction, and refinement) (Russell and Burch 1959; Tannenbaum and Bennett 2015). All experiments were performed under blinded conditions; the researchers who performed the tests and assays did not know to which group the animals belonged. This study was approved by the Ethics Commission for Animal Use (CEUA) at Universidade de Passo Fundo (UPF), Passo Fundo, Brazil (Protocol 036/2019, Ethics Declaration Section).
The experiment had a total of six groups (0 (control, dechlorinated water), 0.0013, 0.013, 0.13, 1.3, and 13 µg·L−1 (geometric mean determined by Morrissey et al. (2015), 0.13 µg·L−1) with 15 animals in each group in each behavioral test. One animal was used for the novel tank test (NTT) and social preference test (SPT) in the same record; one animal per apparatus was used in the mirror-induced aggression test (MIAT). Therefore, 30 animals were used per concentration in the behavioral tests. After being euthanized, the tissues were randomly separated within their groups for brain biochemistry analysis (pools of three brains, n = 5 pools per group), trunk biochemical parameters (n = 7 animals per group), and whole-body cortisol analysis (n = 8 animals per group). The behavioral tests were performed in a quiet room, and the operator left the room during the recording. The water in the tank test was completely replaced before each animal test. About the IBI concentrations, we prepared a stock solution of 1 g·L−1 from the commercial form (480 g·L−1) and serial dilutions (1 mg·L−1, 13, 1.3, 0.13, 0.013 and 0.0013 µg·L−1). The commercial form and the stock solution were stored at 4 ºC. The stock solution was prepared weekly in amber glass bottles, and the dilutions tested were prepared fresh. The contaminated water from the experiments was kept for at least 30 days in fiberglass tanks and then percolated in septic ponds, according to Kreutz et al. (2008). A schematic image of the experimental design is represented in Fig. 1.
Behavioral tests
Novel tank test (NTT) and social preference test (SPT)
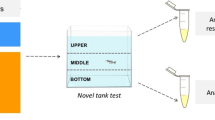
We performed NTT and SPT, according to Kirsten et al. (2018). The locomotor and exploratory parameters were evaluated in the NTT, which was performed first in a test tank (24 × 6 × 15 cm, width × depth × height; filled with 2 L of water; fish density, 0.075 to 0.125 g per liter) with opaque partitions in lateral sides and filled with water from the acclimation aquariums. The fish were individually placed in the test tank, and their behaviors were recorded for 6 min with the Logitech® c920 HD 1080p webcam (Logitech®, CA, USA). Next, using the ANY-maze® software (Stoelting Co., IL, USA), we virtually divided the test tank into three horizontal segments (bottom, middle, and top) (Rosemberg et al. 2011) and in three-time intervals (0–120 s, 120–240 s, and 240–360 s) to analyze the total distance traveled (m), the number of lines crossing between zones, and time spent in the top zone (s).
To perform the SPT, we removed the lateral partitions, and the fish had access to two tank lateral views: a tank with water and no fish on one side and a tank with water and 15 mixed-sex conspecifics on the other side. The animal test behavior was recorded for 60 s in a similar method as in the NTT. In this test, the aquarium was virtually divided into vertical mode: first (nearest to conspecifics), second, and third (next to tank without fish) segments. The measured parameters were the time the animal spent in the first (interpreted as a response to social stimuli), second, and third segments.
Mirror-induced aggression test (MIAT)
The MIAT was performed as described previously by Gerlai et al. (2000) and Fontana et al. (2016). In this test, the fish were individually placed in a glass aquarium (30 cm length × 20 cm height × 15 cm width; filled with 5 L of water; fish density, 0.03 to 0.05 g per liter) with three lateral opaque partitions and one lateral partition with a mirror laterally inclined at 22.5°. On the side where the mirror is attached to the aquarium, we considered that the fish was closer to its image. On the other side of the mirror, we considered that the fish was farther from its reflected image. The fish were recorded as in NTT, with the camera above the aquarium, which allowed the simultaneous filming of two animals. The behavior of the fish was recorded for 60 s after a 30-s habituation period and repeated for 60 s after a 10-min habituation period. In ANY-maze® software, the test tank was divided into four zones: q1, q2, q3, and q4. q1 represents the close area, and q4 is the far area relative to the mirror. The evaluated parameters were the times the animal spent in q1 and q4 quadrants in the first (after 30- s habituation) and second (after 10-min habituation) recordings.
Tissue preparation and analysis
For brain oxidative status evaluation immediately after euthanasia, the brain was carefully removed from the ice bath, and pools of three brains were homogenized in 750 µL of 50-mM potassium phosphate buffer solution (n = 5 per group) for 1 min in a Potter mixer on an ice bath. Afterward, the homogenate was centrifuged for 10 min at 15000xg at 4 °C (for acetylcholinesterase assay) or 700xg for 10 min at 4 °C (for GST, CAT, and SOD). The supernatants were collected and used in the tests (Pompermaier et al. 2022, with modifications). For mensuration of trunk hormonal and biochemical parameters, the animals were flash-frozen in liquid nitrogen (Oliveira et al. 2014; Freddo et al. 2020). The fish trunks for cortisol analysis were prepared according to Oliveira et al. (2014) (n = 8 per group), and the fish trunk for oxidative damage analysis was prepared according to Freddo et al. (2020) (n = 7 per group).
Brain biochemical analysis
We measured the activity of the enzymes, AChE (Ellman et al. 1961), GST (Habig et al. 1974; Habig and Jakoby 1981), CAT (Johansson and Håkan Borg 1988; Góth 1991), and SOD (Misra and Fridovich 1972) and total protein content (Bradford method, Kruger 2009). The detailed methodology for these assays is described in our previous study (Pompermaier et al. 2022). The samples were evaluated using the Bel® UV-M51 spectrophotometer (Bel engineering, São Paulo, Brazil). AChE activity was expressed in µmol of acetylthiocholine (AcSChl)·h·mg of protein−1 and evaluated at 412 nm; GST activity was expressed in mmol of the substrate, CDNB·mg of protein−1 and evaluated at 340 nm; CAT activity was expressed in units CAT·mg·protein−1 and evaluated at 240 nm, and the SOD was expressed in units SOD·mg of protein−1 and evaluated at 480 nm.
Trunk biochemical parameter mensuration
We analyzed the total protein content in the animal trunk homogenate via the Bradford method (Kruger 1994), measuring the levels of PC, NO, nonprotein thiols, CAT (Aebi 1984), and TBA reactive species (TBARS) reaction. The readings of the samples in all assays were performed using the Femto® cirrus 80 (Femto, São Paulo, Brazil) spectrophotometer. We evaluated PC through quantifying the reaction of DNPH with protein carbonyls to form protein hydrazones. The reaction was evaluated at 370 nm (Yan et al. 1995; Aparna and Patri 2021), calculated using the molar extinction coefficient of 22000 M·cm−1, and expressed as nmol·mg protein−1 (Carvalho-Silva et al. 2019). We colorimetrically evaluated the NO using the Griess reagent (Bracht and Ishii-iwamoto 2003) and quantified the product at 540 nm. The results were expressed as µgmol·g−1 of protein. Nonprotein thiol levels were measured as an indirect measure of intracellular glutathione content (Ellman 1959), and the product of the reaction was quantified at 412 nm. The results were expressed as µgmol·g−1 of protein. The CAT analysis was performed as described by Pompermaier et al. (2022). TBARS reaction, which is a parameter of lipid peroxidation, was measured using the pink chromogen produced in the assay reaction, evaluated at 535 nm, and expressed as ngmol·g−1 of protein (Ohkawa et al. 1979; Carvalho-Silva et al. 2019; Aparna and Patri 2021).
Whole-body cortisol analysis
We measured cortisol levels in the animal trunk homogenates using an enzyme-linked immunosorbent assay (ELISA) kit (DBC® serum human cortisol test, Diagnostics BioChem Canada Inc, Ontario, Canada) (Sink et al. 2008). The ELISA was performed according to the manufacturer’s manual. The absorbance of the samples was measured at 490 nm (UVM 340; Biochrom®, Cambridge, UK), and the results were expressed as ng·g−1 of body weight.
Statistics
Normality and homoscedasticity of the data were assessed via the Brown–Forsythe and Bartlett tests, respectively. When the data distribution was classified as normal and/or homoscedastic, we performed parametric analyses; when the data distribution was not classified as normal and/or homoscedastic, we performed nonparametric analyses. Comparisons between concentrations and the control were analyzed via a one-way analysis of variance (ANOVA) followed by Dunnett’s post hoc test with parametric data. With nonparametric data, a similar analysis was performed with the Kruskal–Wallis test, followed by Dunn’s post hoc test. For the analysis of sex effects on the behavioral tests, two-way ANOVA was performed, with sex and concentrations as factors. The p-value was set at < 0.05 for all tests. For MIAT, we performed the unpaired T-test or Mann–Whitney test between the 30-s and 10-min habituation periods, depending on the normality and homoscedasticity of the data. Parametric data were expressed as mean ± standard error of the mean (SEM), and nonparametric data were expressed as median ± interquartile range. All statistical analyses were performed in GraphPad Prism® software 7.00 version (GraphPad Software, CA, USA).
Results
Detection of IBI in water
All quantified concentrations were lower than the nominal concentrations, with IBI concentrations ranging from 12 to 0.0012 µg·L−1. The data with recovery percentages are presented in Table 1. The equation and r2 data were inserted in the supplementary material (Fig. 1A).
Behavioral tests
In the NTT, no difference was detected between all groups in the total distance traveled at the 0–120-s interval time (Fig. 2A). Fish exposed to 0.13 and 13 µg·L−1 of IBI traveled less distance than the animals in the control group at the 120–240 s interval, with a decrease of 32.1% and 37.7% relative to the control group, respectively (Fig. 2B). No difference was detected between all groups using a similar parameter at the 240–360 s interval time (Fig. 2C). No differences were observed in the crossing between zones at the 0–120 s interval time (Fig. 2D). The animals exposed to 0.013 and 0.13 µg·L−1 of IBI performed fewer crossings between the zones than the animals in the control group at the 120–240-s interval, with a decrease of 85.8% and 77.7% relative to the control group, respectively (Fig. 2E). No differences were observed in a similar parameter at the 240–360-s interval (Fig. 2F). The zebrafish exposed to 0.013 µg·L−1 of IBI also remained longer at the top of the tank than the control zebrafish at the 0–120-s interval, with an increase of 7000% relative to the control group (Fig. 2G). No differences were observed in a similar parameter at the 120–240 (Fig. 2H) and 240–360-s intervals (Fig. 2I).
Locomotor and behavior parameters of zebrafish exposed to different concentrations of imidacloprid-based insecticide (IBI) in the novel tank test. Total distance traveled at 0–120-s (A), 120–240-s (B), and 240–360-s (C) intervals; crossing number between zones at 0–120-s (D), 120–240-s (E), and 240–360-s (F) intervals; time spent in the top zone at 0–120-s (G), 120–240-s (H), and 240–360-s (I) intervals. The data are expressed as the mean ± SEM (ANOVA followed by Dunnett’s post hoc test; A) and median ± interquartile range (Mann–Whitney test followed by Dunn’s post hoc test; B–I). Asterisks indicate statistical difference against the control group (*p < 0.05); n = 15 animals per group
In the SPT, the animals exposed to the 0.13 µg·L−1 of IBI remained for a shorter time in the first segment when compared with the control animals (a decrease of 59.6% relative to the control group, Fig. 3A). No differences were detected between groups in time spent in the second (Fig. 3B) and third segments (Fig. 3C).
Behavior and locomotor parameters of zebrafish exposed to different concentrations of IBI in the social preference test. Time spent in first (A), second (B), and third (C) segments. Data were expressed as the median ± interquartile range. The comparisons were performed via the Kruskal–Wallis followed by Dunn’s post hoc test. Asterisks indicate statistical difference against the control group (*p < 0.05); n = 15 animals per group
In the MIAT, after the 30 s of habituation, the animals exposed to the 0.13 µg·L−1 of IBI remained for a shorter time in the segment nearest to the mirror, with a decrease of 53% relative to the control group (Fig. 4A) and longer in the segment further to the mirror, with an increase of 431% relative to the control group (Fig. 4B). The animals in the control and 0.0013 µg·L−1 IBI groups remained longer in the segment nearest to the mirror after 10 min of habituation compared with those after the 30 s habituation (increase of 172% and 141%, respectively) (Fig. 4A). Relative to the segment further to the mirror, the fish in the 0.13 µg·L−1 IBI group remained for a shorter time (decrease of 34.5%) in this area after 10 min than after 30 s of habituation (Fig. 4B). No differences were detected between all other groups relative to the time in segments and to the different times of evaluation (Fig. 4A and B).
Behavior parameters of zebrafish exposed to different concentrations of IBI in the mirror-induced aggression test. Time spent in the segment nearest to the mirror (A) and in the farthest segment to the mirror after 30 s and 10 min of habituation (B). Data were expressed as the median ± interquartile range. The comparisons between groups in each minute were performed via the Kruskal–Wallis test, followed by Dunn’s post hoc test. The comparisons between similar groups in the different time intervals were performed via unpaired T-test or Mann–Whitney, depending on the normality of the data. Symbols indicate statistical difference: *p < 0.05, ****p < 0.0001, control group versus 0.13 µg·L−1 IBI group; #p < 0.05, control group 30-s habituation versus control group 10-min habituation and 0.013 µg·L−1 IBI group 30-s habituation versus 0.013 µg·L−1 IBI group 10-min habituation; ##p < 0.01, 0.13 µg·L−1 IBI group 30-s habituation versus 0.13 µg·L−1 IBI group 10-min habituation); n = 15 animals per group
Regarding the possible sex effects on the IBI-exposed animals, no difference was observed between the behavior of the males and females exposed to all concentrations of IBI in the NTT, SPT, and MIAT (Supplementary Material, Table 3A).
Brain oxidative stress parameters
No difference was detected between the AChE, GST, CAT, and SOD of the animals in the IBI-exposed and control groups (Table 2).
Trunk oxidative stress parameters
The animals exposed to 0.0013, 0.013, and 0.13 µg·L−1 of IBI showed increased PC (Fig. 5A; 395%, 349%, and 225%, respectively) when compared with those in the control group. They also showed decreased nitric oxide levels (Fig. 5B; 12.6%, 10.3%, and 8.7%) compared with those in the control group. No differences were found between groups in the nonprotein thiol (Fig. 5C), CAT (Fig. 5D), and lipid peroxidation levels (Fig. 5E).
Trunk oxidative stress parameters of zebrafish exposed to different concentrations of IBI: protein carbonylation (A), nitric oxide (B), nonprotein thiols (C), catalase (D), and lipid peroxidation–TBARS (E). The data are expressed as the mean ± SEM (ANOVA followed by Dunnett’s post hoc test; A–D) and median ± interquartile range (Mann–Whitney test followed by Dunn’s post hoc test; E). Statistical differences are indicated by asterisks (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001). n = 7 animals per group
Trunk cortisol
The trunk cortisol levels increased in fish exposed to 0.013 and 0.13 µg·L−1 (223% and 251%, respectively) of IBI compared with those of the control group (Fig. 5). All statistics values (F-, H-, T-, U-, and p-values) are shown in Supplementary Tables 1A and 2A).
Discussion
Here, we show that the presence of IBI at environmentally relevant concentrations in the water affects the behavior and physiology of zebrafish, even in acute short-term exposure. Fish exposed to some of the tested IBI concentrations presented a slight anxiolytic-like behavior (Fig. 2), hypomotility (Fig. 2), and decreased social interaction (Fig. 3) and aggressiveness (Fig. 4). IBI-exposed fish also presented increased whole-body cortisol levels (Fig. 6) and PC and decreased NO levels (Fig. 5). Observing the specific concentrations in which the effects were detected, we noted that most of the changes were observed at the 0.13 µg·L−1 IBI group, which characterized a nonmonotonic curve response. The highest concentration did not trigger any effects, whereas the lowest concentration induced different physiological responses. These data support the complexity of nicotinic agonists, such as nicotine, which can have anxiogenic or anxiolytic effects depending on the model, route of administration, dose or concentration, and exposure time (Picciotto et al. 2002). Furthermore, no differences in behavioral responses between the sexes were observed, which leads us to hypothesize that the damage caused by IBI affects the species, regardless of the sex of the animal.
In the SPT, fish exposed to 0.13 µg·L−1 of IBI remained for a shorter time near the conspecific segment (Fig. 3A). This result is the opposite of the zebrafish basal response to remain close to the conspecifics, which is a behavior observed in the laboratory (Miller and Gerlai 2011; Pham et al. 2012) and environment (Engeszer et al. 2007; Spence et al. 2007; Suriyampola et al. 2016). In the environment, the shoaling behavior is advantageous with communication among conspecifics, such as passing information on predators and social issues (Krause and Ruxton 2002; Miller and Gerlai 2011).
In the MIAT, fish exposed to 0.13 µg·L−1 of IBI remained for a shorter time near the mirror and longer far from the mirror in the first few seconds of the test (Fig. 4A). The entry of the animal in the segment farthest to the mirror can be interpreted as avoidance (Gerlai et al. 2000) or nonaggressive behavior (Way et al. 2015).
Regarding the effects of nicotinic receptor agonists, Miller et al. (2013) detected a decreased shoal cohesion in fish exposed to nicotine. Cheeta et al. (2001) detected that 0.45 mg·Kg−1 of nicotine decreased the social interaction in group-housed rats and found an increase in social interaction in nicotine-exposed gerbils (0.5 mg·Kg−1) (File et al. 2001). Although the mechanisms and effects of nicotinic agonists on the sociability and aggressiveness of various animal species are yet to be clarified, we emphasize our findings on the antisocial effect of IBI in zebrafish, because social behavior and aggressiveness are part of its basic strategies to survive in the environment.
In the NTT, the fish behavior followed a slightly anxiolytic-like pattern at the beginning of the test, which is characterized by the increased time spent at the top segment in the fish exposed to 0.013 µg·L−1 IBI (Fig. 2G), with hypomotility, as fish exposed to 0.13 and 13 µg·L−1 IBI presented a reduced number of crossings (Fig. 2B), and fish exposed to 0.013 and 0.13 µg·L−1 of IBI (Fig. 2E) showed a decreased total distance traveled. This increase in the top segment time is the opposite of the expected protective behavior of this animal, which at the beginning of the test should remain far from the top (Cachat et al. 2011; Kysil et al. 2017). The hypomotility presented by the animals, similar effects have been reported in imidacloprid-exposed larvae (Crosby et al. 2015), rats (Abd-Elhakim et al. 2018), nicotine-exposed fish (Levin et al. 2007), and mice (Lefever et al. 2017). The hypothesis about this hypomotility would agree with the multiplicity of effects of nicotine reported by Picciotto et al. (2002), and this locomotor alteration would be related to the brain region and receptors activated by this substance (Picciotto et al. 2000; Domino 2001).
About the increase of trunk cortisol levels in IBI-exposed animals (0.013 and 0.13 µg·L−1) (Fig. 6), similar IBI effects in the stress axis have been reported in rodents (Annabi et al. 2015; Khalil et al. 2017). In addition, the endocrine-disrupting potential of IBI has been reported that the pituitary–thyroid axis in birds (Pandey and Mohanty 2015) and the gonadal axis in rodents (Mikolić and Karačonji 2018). However, the concentrations found in natural habitats are often lower than those used in these experiments (Relyea and Hoverman 2006), which are the LD/LC50 (dose or concentration able to kill 50% of the population tested) in toxicity tests within 96 h. Nevertheless, it is important to highlight that IBIs are more toxic to fish than the individual compound (Tišler et al. 2009; Iturburu et al. 2018).
When we observed the behavioral and endocrine responses of IBI, we noted similarities with nicotine, which triggers different locomotor and behavioral responses for its stimulating or sedating effects (Picciotto et al. 2002; Klee et al. 2011; Wronikowska et al. 2020). While the SPT and cortisol results corroborate an anxiety-like condition in animals observed in nicotine-exposed rats (Cheeta et al. 2001), in the NTT, the IBI triggered an anxiolytic effect in the fish. The possible cause of this divergence between some behavioral and endocrine responses of IBI-exposed zebrafish might be related to the different time course of these responses. While behavioral changes are showed generally immediately after the stressor, the HPI axis activation lasts for some minutes (Schreck et al. 1997; Mommsen et al. 1999).
From an ecological point of view, the fish remain longer on the tank top segment (NTT) and remain indifferent to the shoal (SPT), even to the point of not performing agonistic interactions (MIAT). This can make the animals more susceptible to predators through the decreased shoal cohesion, and because the fish stays closer to the surface of the aquatic environment, it becomes more visible to predators (Miller and Gerlai 2011; Maximino et al. 2012; Suriyampola et al. 2016; Spinello et al. 2019; Ogi et al. 2021).
In the analysis of the oxidative status, the brain parameters were not affected by IBI exposure (Table 2). We hypothesized that the IBI had not caused damage to the central nervous system (CNS) due to the short exposure time of 30 min. In longer exposure protocols (hours and days) and at higher sublethal concentrations, alterations in AChE were reported both in acute and chronic exposure (Topal et al. 2017; Guerra et al. 2021). In addition, an increase in cerebral GST and CAT levels was also reported (Vieira et al. 2018).
However, when we analyzed the oxidative stress parameters in the trunk of animals exposed to IBI, we detected changes in the carbonyl protein and nitric oxide contents (Fig. 5A and B). PC could lead to a loss of protein function (Dalle-Donne et al. 2006; Wong et al. 2010). The fish exposed to IBI (0.0013, 0.013, and 0.13 µg·L−1) showed an increased PC, which can lead to cell death, tissue injury, and the development of diseases (Parvez and Raisuddin 2005; Dalle-Donne et al. 2006; Suzuki et al. 2010; Fedorova et al. 2014). PC is correlated with muscle issues, such as a decrease in functional activity (Vikhoreva et al. 2009), which could be related to the hypomotility observed in IBI-exposed animals (0.013 and 0.13 μg.L−1) (Fig. 2B and E). In addition, Vignet et al. (2019) observed muscle damage in fish exposed to imidacloprid. Furthermore, one of the reaction products of NO scavenger activity can induce PC (Trujillo et al. 2010; Moro et al. 2013). Guerra et al. (2021), Vieira et al. (2018), and Almeida et al. (2021) observed a similar increase in PC at sublethal concentrations of imidacloprid, however, in 96 h exposure protocols.
The negative effects of IBI are also exhibited through the decrease in NO levels (0.0013, 0.013, and 0.13 µg·L−1) (Fig. 5B). Increased ROS levels lead to a reduction in NO levels via chemical inactivation as NO possesses a scavenger capacity (Pierini and Bryan 2015). The decrease in NO levels is related to endothelial dysfunction, such as thrombosis and vascular inflammation (Förstermann 2010). In chronic exposure and at sublethal concentrations of IBI, Ismael et al. (Ismael et al. 2021) also detected a decrease in NO levels.
We concluded that the 30-min acute short-term exposure to IBI could disrupt the HPI axis, impair locomotor and behavioral parameters, and unbalance the redox status of the exposed fish. This oxidative damage did not extend to the brain tissue, probably because of the short exposure time. Thus, we hypothesize that the IBI can act similarly to insect nAChR (Abbink 1991; Tomizawa and Casida 2003; Crossthwaite et al. 2017), irreversibly bind to receptors in the neuromuscular junctions of the fish (Vignet et al. 2019), and cause all of these changes. Furthermore, our study reinforces the importance of implementing and developing the 17 sustainable development goals (SDGs) of the United Nations (UN), especially SDGs 6 (guarantee of clean drinking water and sanitation) and 14 (maintenance of life below water). Therefore, we highlight that the animals exposed to IBI in an acute short-term protocol and at environmentally relevant concentrations, which can often occur in the environment, may have lost their protective instincts and consequently make them more susceptible to predation (Chaulet et al. 2019). These sublethal effects and the physiological damages in exposed animals can represent an imminent danger to nontarget species in the environment and aquatic habitats.
Data availability
Data will be available upon reasonable request to the authors.
References
Abbink J (1991) The biochemistry of imidacloprid. Pflanzenschutz-Nachrichten Bayer (Germany, FR) 44:183–194
Abd-Elhakim YM, Mohammed HH, Mohamed WAM (2018) Imidacloprid impacts on neurobehavioral performance, oxidative stress, and apoptotic events in the brain of adolescent and adult rats. J Agric Food Chem 66:13513–13524. https://doi.org/10.1021/ACS.JAFC.8B05793
Aebi H (1984) [13] Catalase in vitro. Methods Enzymol 105:121–126. https://doi.org/10.1016/S0076-6879(84)05016-3
Agrawal A, Sharma B (2010) Pesticides induced oxidative stress in mammalian systems. Int J Biol Med Res 1:90–104
Albuquerque AF, Ribeiro JS, Kummrow F et al (2016) Pesticides in Brazilian freshwaters: a critical review. Environ Sci Process Impacts 18:779–787. https://doi.org/10.1039/c6em00268d
Almeida EC, Passos LS, Vieira CED et al (2021) Can the insecticide imidacloprid affect the health of the neotropical freshwater fish Astyanax altiparanae (Teleostei: Characidae)? Environ Toxicol Pharmacol 85:103634. https://doi.org/10.1016/J.ETAP.2021.103634
Annabi A, Dhouib IE, Dkhili H et al (2015) Mechanisms of imidacloprid-induced alteration of hypothalamic-pituitary-adrenal (HPA) axis after subchronic exposure in male rats. Recent Adv Biol Med 01:51. https://doi.org/10.18639/rabm.2015.01.195931
Anthe M, Valles-Ebeling B, Achtenhagen J et al (2020) Development of an aquatic exposure assessment model for imidacloprid in sewage treatment plant discharges arising from use of veterinary medicinal products. Environ Sci Eur 32:1–21. https://doi.org/10.1186/S12302-020-00424-4/TABLES/3
Aparna S, Patri M (2021) Benzo[a]pyrene exposure and overcrowding stress impacts anxiety-like behavior and impairs learning and memory in adult zebrafish, Danio rerio. Environ Toxicol 36:352–361. https://doi.org/10.1002/TOX.23041
Aslan R, Şekeroǧlu MR, Gültekin F, Bayiroǧlu F (1997) Blood lipoperoxidation and antioxidant enzymes inhealthy individuals: relation to age, sex, habits, lifestyle and environment. J Environ Sci Health Part A 32(8):2101–2109. https://doi.org/10.1080/10934529709376670
Baba SP, Bhatnagar A (2018) Role of thiols in oxidative stress. Curr Opin Toxicol 7:133–139. https://doi.org/10.1016/j.cotox.2018.03.005
Bailey J, Oliveri A, Levin ED (2013) Zebrafish model systems for developmental neurobehavioral toxicology. Birth Defects Res C Embryo Today 99:14–23. https://doi.org/10.1002/BDRC.21027
Barcellos HH de A, Pompermaier A, Mendonça-Soares S et al (2020) Aripiprazole prevents stress-induced anxiety and social impairment, but impairs antipredatory behavior in zebrafish. Pharmacol Biochem Behav 189:172841. https://doi.org/10.1016/j.pbb.2019.172841
Bonmatin JM, Giorio C, Girolami V et al (2015) Environmental fate and exposure; neonicotinoids and fipronil. Environ Sci Pollut Res 22:35–67. https://doi.org/10.1007/S11356-014-3332-7/TABLES/5
Bracht A, Ishii-iwamoto EL (2003) Métodos de laboratório em bioquímica, First. Manole, São Paulo, São Paulo, Brasil
Cachat JM, Canavello PR, Elegante MF et al (2011) Modeling stress and anxiety in zebrafish. NeuroMethods 52:73–88
Carvalho-Silva M, Gomes LM, Gomes ML et al (2019) Omega-3 fatty acid supplementation can prevent changes in mitochondrial energy metabolism and oxidative stress caused by chronic administration of L-tyrosine in the brain of rats. Metabol Brain Dis 4:1207–1219. https://doi.org/10.1007/s11011-019-00411-6
Chaulet FC, Barcellos HHA, Fior D et al (2019) Glyphosate- and fipronil-based agrochemicals and their mixtures change zebrafish behavior. Arch Environ Contam Toxicol 77:443–451. https://doi.org/10.1007/s00244-019-00644-7
Cheeta S, Irvine E, File SE (2001) Social isolation modifies nicotine’s effects in animal tests of anxiety. Br J Pharmacol 132:1389. https://doi.org/10.1038/SJ.BJP.0703991
Comporti M (1989) Three models of free radical-induced cell injury. Chem Biol Interact 72:1–56. https://doi.org/10.1016/0009-2797(89)90016-1
Costa NR (2014) Determinação de fipronil e imidacloprido em mel utilizando cromatografia gasosa e líquida. Master Dissertation. Universidade de Brasília
Cresswell JE (2011) A meta-analysis of experiments testing the effects of a neonicotinoid insecticide (imidacloprid) on honey bees. Ecotoxicology 20:149–157. https://doi.org/10.1007/S10646-010-0566-0
Crosby EB, Bailey JM, Oliveri AN, Levin ED (2015) Neurobehavioral impairments caused by developmental imidacloprid exposure in zebrafish. Neurotoxicol Teratol 49:81–90. https://doi.org/10.1016/j.ntt.2015.04.006
Crossthwaite AJ, Bigot A, Camblin P et al (2017) The invertebrate pharmacology of insecticides acting at nicotinic acetylcholine receptors. J Pestic Sci 42:67. https://doi.org/10.1584/JPESTICS.D17-019
Dai YJ, Jia YF, Chen N et al (2014) Zebrafish as a model system to study toxicology. Environ Toxicol Chem 33:11–17. https://doi.org/10.1002/etc.2406
Dalle-Donne I, Aldini G, Carini M et al (2006) Protein carbonylation, cellular dysfunction, and disease progression. J Cell Mol Med 10:389–406. https://doi.org/10.1111/J.1582-4934.2006.TB00407.X
Dametto FS, Fior D, Idalencio R et al (2018) Feeding regimen modulates zebrafish behavior. PeerJ 2018:e5343. https://doi.org/10.7717/peerj.5343
de Souza RM, Seibert D, Quesada HB et al (2020) Occurrence, impacts and general aspects of pesticides in surface water: a review. Process Saf Environ Prot 135:22–37. https://doi.org/10.1016/j.psep.2019.12.035
Delkash-Roudsari S, Chicas-Mosier AM, Goldansaz SH et al (2020) Assessment of lethal and sublethal effects of imidacloprid, ethion, and glyphosate on aversive conditioning, motility, and lifsespan in honey bees (Apis mellifera L.). Ecotoxicol Environ Saf 204:111108. https://doi.org/10.1016/J.ECOENV.2020.111108
Domino EF (2001) Nicotine induced behavioral locomotor sensitization. Prog Neuropsychopharmacol Biol Psychiatry 25:59–71. https://doi.org/10.1016/S0278-5846(00)00148-2
Egan RJ, Bergner CL, Hart PC et al (2009) Understanding behavioral and physiological phenotypes of stress and anxiety in zebrafish. Behav Brain Res 205:38–44. https://doi.org/10.1016/j.bbr.2009.06.022
EL-Gendy KS, Aly NM, Mahmoud FH et al (2010) The role of vitamin C as antioxidant in protection of oxidative stress induced by imidacloprid. Food Chem Toxicol 48(215):221. https://doi.org/10.1016/J.FCT.2009.10.003
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82(1):70–77. https://doi.org/10.1016/0003-9861(59)90090-6
Ellman GL, Courtney KD, Andres V, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:. https://doi.org/10.1016/0006-2952(61)90145-9
Engeszer RE, Patterson LB, Rao AA, Parichy DM (2007) Zebrafish in the wild: a review of natural history and new notes from the field. Zebrafish 4:21–40. https://doi.org/10.1089/ZEB.2006.9997
Fedorova M, Bollineni RC, Hoffmann R (2014) Protein carbonylation as a major hallmark of oxidative damage: update of analytical strategies. Mass Spectrom Rev 33:79–97. https://doi.org/10.1002/MAS.21381
File SE, Cheeta S, Akanezi C (2001) Diazepam and nicotine increase social interaction in gerbils: a test for anxiolytic action. Brain Res 888:311–313. https://doi.org/10.1016/S0006-8993(00)03102-4
Fontana BD, Meinerz DL, Rosa LVC et al (2016) Modulatory action of taurine on ethanol-induced aggressive behavior in zebrafish. Pharmacol Biochem Behav 141:18–27. https://doi.org/10.1016/j.pbb.2015.11.011
Förstermann U (2010) Nitric oxide and oxidative stress in vascular disease. Pflügers Arch - Eur J Physiol 459:923–939. https://doi.org/10.1007/S00424-010-0808-2
Freddo N, Soares SM, Fortuna M et al (2021) Stimulants cocktail: methylphenidate plus caffeine impairs memory and cognition and alters mitochondrial and oxidative status. Prog Neuro-Psychopharmacol Biol Psych 106:110069. https://doi.org/10.1016/j.pnpbp.2020.110069
Frew JA, Brown JT, Fitzsimmons PN et al (2018) Toxicokinetics of the neonicotinoid insecticide imidacloprid in rainbow trout (Oncorhynchus mykiss). Comp Biochem Physiol C Toxicol Pharmacol 205:34. https://doi.org/10.1016/J.CBPC.2018.01.002
Fu H, Xia Y, Chen Y et al (2018) Acetylcholinesterase is a potential biomarker for a broad spectrum of organic environmental pollutants. Environ Sci Technol 52:8065–8074. https://doi.org/10.1021/ACS.EST.7B04004/ASSET/IMAGES/LARGE/ES-2017-04004S_0001.JPEG
Fuzzen MLM, Van Der Kraak G, Bernier NJ (2010) Stirring up new ideas about the regulation of the hypothalamic-pituitary- interrenal axis in zebrafish (Danio rerio). Zebrafish 7:349–358. https://doi.org/10.1089/zeb.2010.0662
Gawade L, Dadarkar SS, Husain R, Gatne M (2013) A detailed study of developmental immunotoxicity of imidacloprid in Wistar rats. Food Chem Toxicol 51:61–70. https://doi.org/10.1016/j.fct.2012.09.009
Ge W, Yan S, Wang J et al (2015) Oxidative stress and DNA damage induced by imidacloprid in zebrafish (Danio rerio). J Agric Food Chem 63:1856–1862. https://doi.org/10.1021/JF504895H
Gerhardt A (2007) Aquatic behavioral ecotoxicology - prospects and limitations. Hum Ecol Risk Assess 13:481–491. https://doi.org/10.1080/10807030701340839
Gerlai R, Lahav M, Guo S, Rosenthal A (2000) Drinks like a fish: zebra fish (Danio rerio) as a behavior genetic model to study alcohol effects. Pharmacol Biochem Behav 67:773–782. https://doi.org/10.1016/S0091-3057(00)00422-6
Gibbons D, Morrissey C, Mineau P (2015) A review of the direct and indirect effects of neonicotinoids and fipronil on vertebrate wildlife. Environ Sci Pollut Res 22:103–118. https://doi.org/10.1007/S11356-014-3180-5
Góth L (1991) A simple method for determination of serum catalase activity and revision of reference range. Clin Chim Acta 196:143–151. https://doi.org/10.1016/0009-8981(91)90067-M
Gracy RW, Talent JM, Kong Y, Conrad CC (1999) Reactive oxygen species: the unavoidable environmental insult? Mutat Res 428:17–22. https://doi.org/10.1016/S1383-5742(99)00027-7
Gross M (2013) EU ban puts spotlight on complex effects of neonicotinoids. Curr Biol 23(11):462–464. https://doi.org/10.1016/J.CUB.2013.05.030
Guerra LJ, do Amaral AMB, de Quadros VA et al (2021) Biochemical and behavioral responses in zebrafish exposed to imidacloprid oxidative damage and antioxidant responses. Arch Environ Contam Toxicol 81(255):264. https://doi.org/10.1007/S00244-021-00865-9/FIGURES/5
Habig WH, Jakoby WB (1981) Assays for differentiation of glutathione S-transferases. Methods Enzymol 77:398–405. https://doi.org/10.1016/S0076-6879(81)77053-8
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139. https://doi.org/10.1016/S0021-9258(19)42083-8
Hoseinifar SH, Yousefi S, Van Doan H et al (2020) Oxidative stress and antioxidant defense in fish: the implications of probiotic, prebiotic, and synbiotics. Rev Fish Sci Aquaculture 29(2):198–217. https://doi.org/10.1080/23308249.2020.1795616
Islam MA, Hossen MS, Sumon KA, Rahman MM (2019) Acute toxicity of imidacloprid on the developmental stages of common carp Cyprinus carpio. Toxicol Environ Health Sci 11:244–251. https://doi.org/10.1007/S13530-019-0410-8
Ismael NEM, Abd El-hameed SAA, Salama AM et al (2021) The effects of dietary clinoptilolite and chitosan nanoparticles on growth, body composition, haemato-biochemical parameters, immune responses, and antioxidative status of Nile tilapia exposed to imidacloprid. Environ Sci Pollut Res 28:29535–29550. https://doi.org/10.1007/s11356-021-12693-4
Iturburu FG, Bertrand L, Mendieta JR et al (2018) An integrated biomarker response study explains more than the sum of the parts: oxidative stress in the fish Australoheros facetus exposed to imidacloprid. Ecol Indic 93:351–357. https://doi.org/10.1016/J.ECOLIND.2018.05.019
Jabłońska-Trypuć A (2017) Pesticides as inducers of oxidative stress. React Oxyg Species 3:96–110. https://doi.org/10.20455/ros.2017.823
Johansson LH, Håkan Borg LA (1988) A spectrophotometric method for determination of catalase activity in small tissue samples. Anal Biochem 174:331–336. https://doi.org/10.1016/0003-2697(88)90554-4
Kagabu S, Medej S (1995) Stability Comparison of imidacloprid and related compounds under simulated sunlight, hydrolysis conditions, and to oxygen. Biosci Biotechnol Biochem 59:980–985. https://doi.org/10.1271/BBB.59.980
Khalil SR, Awad A, Mohammed HH, Nassan MA (2017) Imidacloprid insecticide exposure induces stress and disrupts glucose homeostasis in male rats. Environ Toxicol Pharmacol 55:165–174. https://doi.org/10.1016/j.etap.2017.08.017
Kirsten K, Soares SM, Koakoski G et al (2018) Characterization of sickness behavior in zebrafish. Brain Behav Immun 73:596–602. https://doi.org/10.1016/j.bbi.2018.07.004
Klee EW, Ebbert JO, Schneider H et al (2011) Zebrafish for the study of the biological effects of nicotine. Nicotine Tob Res 13:301–312. https://doi.org/10.1093/ntr/ntr010
Köhler HR, Triebskorn R (2013) Wildlife ecotoxicology of pesticides: can we track effects to the population level and beyond? Science (80- ) 341:759–765. https://doi.org/10.1126/SCIENCE.1237591
Krause J, Ruxton GD (2002) Living in groups. Oxford University Press (OUP), Oxford, p 224
Kreutz LC, Barcellos LJG, Silva TO et al (2008) Acute toxicity test of agricultural pesticides on silver catfish (Rhamdia quelen) fingerlings. Cienc Rural 38:1050–1055. https://doi.org/10.1590/s0103-84782008000400022
Kruger NJ (1994) The Bradford method for protein quantitation. Methods Mol Biol 32:9–15. https://doi.org/10.1385/0-89603-268-X:9
Kruger NJ (2009) The Bradford method for protein quantitation. Humana Press, Totowa, pp 17–24
Kysil EV, Meshalkina DA, Frick EE et al (2017) Comparative analyses of zebrafish anxiety-like behavior using conflict-based novelty tests. Zebrafish 14:197–208. https://doi.org/10.1089/zeb.2016.1415
Leary S, Cartner S (2013) AVMA Guidelines for the euthanasia of animals: 2013 edition members of the panel on euthanasia. American Veterinary Medical Association, Schaumburg
Lefever TW, Lee YOK, Kovach AL et al (2017) Delivery of nicotine aerosol to mice via a modified electronic cigarette device. Drug Alcohol Depend 172:80–87. https://doi.org/10.1016/J.DRUGALCDEP.2016.12.004
Levin ED, Bencan Z, Cerutti DT (2007) Anxiolytic effects of nicotine in zebrafish. Physiol Behav 90:54–58. https://doi.org/10.1016/j.physbeh.2006.08.026
Li X, Chen J, He X et al (2019) Simultaneous determination of neonicotinoids and fipronil and its metabolites in environmental water from coastal bay using disk-based solid-phase extraction and high-performance liquid chromatography-tandem mass spectrometry. Chemosphere 234:224–231. https://doi.org/10.1016/J.CHEMOSPHERE.2019.05.243
Lin PC, Lin HJ, Liao YY et al (2013) Acute poisoning with neonicotinoid insecticides: a case report and literature review. Basic Clin Pharmacol Toxicol 112:282–286. https://doi.org/10.1111/bcpt.12027
Maximino C, de Brito TM, Gouveia A (2010) Construct validity of behavioral models of anxiety: where experimental psychopathology meets ecology and evolution. Psychol Neurosci 3:117–123. https://doi.org/10.3922/j.psns.2010.1.015
Maximino C, Benzecry R, Oliveira KRM et al (2012) A comparison of the light/dark and novel tank tests in zebrafish. Behaviour 149:1099–1123. https://doi.org/10.1163/1568539X-00003029
Mencke N, Jeschke P (2002) Therapy and prevention of parasitic insects in veterinary medicine using imidacloprid. Curr Top Med Chem 2:701–715. https://doi.org/10.2174/1568026023393598
Mikolić A, Karačonji IB (2018) Imidacloprid as reproductive toxicant and endocrine disruptor: investigations in laboratory animals. Arh Hig Rada Toksikol 69:103–108. https://doi.org/10.2478/AIHT-2018-69-3144
Miller NY, Gerlai R (2011) Shoaling in zebrafish: what we don’t know. Rev Neurosci 22:17–25. https://doi.org/10.1515/RNS.2011.004
Miller N, Greene K, Dydinski A, Gerlai R (2013) Effects of nicotine and alcohol on zebrafish (Danio rerio) shoaling. Behav Brain Res 240:192–196. https://doi.org/10.1016/J.BBR.2012.11.033
Millot F, Decors A, Mastain O et al (2017) Field evidence of bird poisonings by imidacloprid-treated seeds: a review of incidents reported by the French SAGIR network from 1995 to 2014. Environ Sci Pollut Res 24:5469–5485. https://doi.org/10.1007/s11356-016-8272-y
Misra HP, Fridovich I (1972) The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247:317–3175. https://doi.org/10.1016/S0021-9258(19)45228-9
Mommsen TP, Vijayan MM, Moon TW (1999) Cortisol in teleosts: dynamics, mechanisms of action, and metabolic regulation. Rev Fish Biol Fish 9:211–268
Moro AM, Charão MF, Brucker N et al (2013) Genotoxicity and oxidative stress in gasoline station attendants. Mutat Res 754:63–70. https://doi.org/10.1016/J.MRGENTOX.2013.04.008
Morrissey CA, Mineau P, Devries JH et al (2015) Neonicotinoid contamination of global surface waters and associated risk to aquatic invertebrates: a review. Environ Int 74:291–303. https://doi.org/10.1016/j.envint.2014.10.024
Moza PN, Hustert K, Feicht E, Kettrup A (1998) Photolysis of imidacloprid in aqueous solution. Chemosphere 36:497–502. https://doi.org/10.1016/S0045-6535(97)00359-7
Nema S, Bhargava Y (2018) Quantitative assessment of cypermethrin induced behavioural and biochemical anomalies in adult zebrafish. Neurotoxicol Teratol 68:57–65. https://doi.org/10.1016/j.ntt.2018.05.003
Ogi A, Licitra R, Naef V et al (2021) Social preference tests in zebrafish: a systematic review. Front Vet Sci 7:1239. https://doi.org/10.3389/FVETS.2020.590057/BIBTEX
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358. https://doi.org/10.1016/0003-2697(79)90738-3
Oliveira TA, Koakoski G, da Motta AC et al (2014) Death-associated odors induce stress in zebrafish. Horm Behav 65:340–344. https://doi.org/10.1016/J.YHBEH.2014.02.009
Orger MB, De Polavieja GG (2017) Zebrafish behavior: opportunities and challenges. Annu Rev Neurosci 40:125–147. https://doi.org/10.1146/ANNUREV-NEURO-071714-033857
Pagnussat N, Piato AL, Schaefer IC et al (2013) One for all and all for one: the importance of shoaling on behavioral and stress responses in zebrafish. Zebrafish 10:338–342. https://doi.org/10.1089/ZEB.2013.0867
Pandey SP, Mohanty B (2015) The neonicotinoid pesticide imidacloprid and the dithiocarbamate fungicide mancozeb disrupt the pituitary-thyroid axis of a wildlife bird. Chemosphere 122:227–234. https://doi.org/10.1016/j.chemosphere.2014.11.061
Parvez S, Raisuddin S (2005) Protein carbonyls: novel biomarkers of exposure to oxidative stress-inducing pesticides in freshwater fish Channa punctata (Bloch). Environ Toxicol Pharmacol 20:112–117. https://doi.org/10.1016/J.ETAP.2004.11.002
Pham M, Raymond J, Hester J et al (2012) Assessing social behavior phenotypes in adult zebrafish: shoaling, social preference, and mirror biting tests. In: Kalueff AV, Stewart AM (eds), Zebrafish Protocols for Neurobehavioral Research, pp 231–246.https://doi.org/10.1007/978-1-61779-597-8_17
Picciotto MR, Caldarone BJ, King SL, Zachariou V (2000) Nicotinic receptors in the brain. Links between molecular biology and behavior. Neuropsychopharmacology 22:451–465. https://doi.org/10.1016/S0893-133X(99)00146-3
Picciotto MR, Brunzell DH, Caldarone BJ (2002) Effect of nicotine and nicotinic receptors on anxiety and depression. NeuroReport 13:1097–1106. https://doi.org/10.1097/00001756-200207020-00006
Pierini D, Bryan NS (2015) Nitric oxide availability as a marker of oxidative stress. Methods Mol Biol 1208:63–71. https://doi.org/10.1007/978-1-4939-1441-8_5
Pompermaier A, Varela ACC, Mozzato MT et al (2022) Impaired initial development and behavior in zebrafish exposed to environmentally relevant concentrations of widely used pesticides. Comp Biochem Physiol Part C Toxicol Pharmacol 257:109328. https://doi.org/10.1016/J.CBPC.2022.109328
Ramsay JM, Feist GW, Varga ZM et al (2009) Whole-body cortisol response of zebrafish to acute net handling stress. Aquaculture 297:157–162. https://doi.org/10.1016/j.aquaculture.2009.08.035
Relyea R, Hoverman J (2006) Assessing the ecology in ecotoxicology: a review and synthesis in freshwater systems. Ecol Lett 9:1157–1171. https://doi.org/10.1111/j.1461-0248.2006.00966.x
Rosemberg DB, Rico EP, Mussulini BM, et al (2011) Differences in spatio-temporal behavior of zebrafish in the open tank paradigm after a short-period confinement into dark and bright environments. PLoS One 6(5):e19397. https://doi.org/10.1371/journal.pone.0019397
Russell WMS, Burch RL (1959) The principles of humane experimental technique. Methuen & Co Ltd, London
Sadaria AM, Sutton R, Moran KD et al (2017) Passage of fiproles and imidacloprid from urban pest control uses through wastewater treatment plants in northern California, USA. Environ Toxicol Chem 36:1473–1482. https://doi.org/10.1002/ETC.3673
Sanchez-Bayo F (2011) Insecticides mode of action in relation to their toxicity to non-target organisms. J Environ Anal Toxicol. https://doi.org/10.4172/2161-0525.S4-002
Sánchez-Bayo F, Hyne RV (2014) Detection and analysis of neonicotinoids in river waters - development of a passive sampler for three commonly used insecticides. Chemosphere 99:143–151. https://doi.org/10.1016/j.chemosphere.2013.10.051
Sánchez-Bayo F, Goka K, Hayasaka D (2016) Contamination of the aquatic environment with neonicotinoids and its implication for ecosystems. Front Environ Sci 4:1–14. https://doi.org/10.3389/fenvs.2016.00071
Schreck CB, Olla BL, Davis MW (1997) Behavioral responses to stress. In: Iwama GK, Pickering AD, Sumpter JP, Schreck CB (eds) Fish stress and health in aquaculture. Cambridge University Press, London, p 278
Schwarzenbach RP, Escher BI, Fenner K et al (2006) The challenge of micropollutants in aquatic systems. Science (80- ) 313:1072–1077. https://doi.org/10.1126/science.1127291
Sink TD, Lochmann RT, Fecteau KA (2008) Validation, use, and disadvantages of enzyme-linked immunosorbent assay kits for detection of cortisol in channel catfish, largemouth bass, red pacu, and golden shiners. Fish Physiol Biochem 34:95–101. https://doi.org/10.1007/s10695-007-9150-9
Slaninova A, Smutna M, Modra H, Svobodova Z (2009) A review: oxidative stress in fish induced by pesticides. Neuroendocrinol Lett 30:2–12
Spence R, Gerlach G, Lawrence C, Smith C (2007) The behaviour and ecology of the zebrafish, Danio rerio. Biol Rev 83:13–34. https://doi.org/10.1111/j.1469-185X.2007.00030.x
Spinello C, Yang Y, Macrì S, Porfiri M (2019) Zebrafish adjust their behavior in response to an interactive robotic predator. Front Robot AI 6:38. https://doi.org/10.3389/FROBT.2019.00038/FULL
Suriyampola PS, Shelton DS, Shukla R et al (2016) Zebrafish social behavior in the wild. Zebrafish 13(1):1–8. https://doi.org/10.1089/zeb.2015.1159
Suzuki YJ, Carini M, Butterfield DA (2010) Protein carbonylation. Antioxidants Redox. Signal 12:323–325. https://doi.org/10.1089/ars.2009.2887
Tannenbaum J, Bennett BT (2015) Russell and Burch’s 3Rs then and now: the need for clarity in definition and purpose. J Am Assoc Lab Anim Sci 54:120
Tišler T, Jemec A, Mozetič B, Trebše P (2009) Hazard identification of imidacloprid to aquatic environment. Chemosphere 76:907–914. https://doi.org/10.1016/J.CHEMOSPHERE.2009.05.002
Tomizawa M, Casida JE (2003) Selective toxicity of neonicotinoids attributable to specificity of insect and mammalian nicotinic receptors. Annu Rev Entomol 48:339–364. https://doi.org/10.1146/annurev.ento.48.091801.112731
Topal A, Alak G, Ozkaraca M et al (2017) Neurotoxic responses in brain tissues of rainbow trout exposed to imidacloprid pesticide: assessment of 8-hydroxy-2-deoxyguanosine activity, oxidative stress and acetylcholinesterase activity. Chemosphere 175:186–191. https://doi.org/10.1016/j.chemosphere.2017.02.047
Trujillo M, Alvarez B, Souza JM et al (2010) Mechanisms and biological consequences of peroxynitrite-dependent protein oxidation and nitration. In: Nitric Oxide 2nd edn. Elsevier Inc., pp 61–72
Tudi M, Ruan HD, Wang L et al (2021) Agriculture development, pesticide application and its impact on the environment. Int J Environ Res Public Health 18:1112. https://doi.org/10.3390/IJERPH18031112
Ullah S, Zorriehzahra MJ (2015) Ecotoxicology: a review of pesticides induced toxicity in fish. Adv Anim Vet Sci 3:40–57. https://doi.org/10.14737/journal.aavs/2015/3.1.40.57
Ulrich K, Jakob U (2019) The role of thiols in antioxidant systems. Free Radical Bio Med 140:14–27. https://doi.org/10.1016/j.freeradbiomed.2019.05.035
Valavanidis A, Vlahogianni T, Dassenakis M, Scoullos M (2006) Molecular biomarkers of oxidative stress in aquatic organisms in relation to toxic environmental pollutants. Ecotoxicol Environ Saf 64:178–189. https://doi.org/10.1016/j.ecoenv.2005.03.013
Van Dijk TC, Van Staalduinen MA, Van der Sluijs JP (2013) Macro-invertebrate decline in surface water polluted with imidacloprid. PLoS One 8:e62374. https://doi.org/10.1371/JOURNAL.PONE.0062374
Vieira CED, Pérez MR, Acayaba RDA et al (2018) DNA damage and oxidative stress induced by imidacloprid exposure in different tissues of the neotropical fish Prochilodus lineatus. Chemosphere 195:125–134. https://doi.org/10.1016/J.CHEMOSPHERE.2017.12.077
Vignet C, Cappello T, Fu Q et al (2019) Imidacloprid induces adverse effects on fish early life stages that are more severe in Japanese medaka (Oryzias latipes) than in zebrafish (Danio rerio). Chemosphere 225:470–478. https://doi.org/10.1016/J.CHEMOSPHERE.2019.03.002
Vikhoreva NN, Vikhorev PG, Fedorova MA et al (2009) The in vitro motility assay parameters of actin filaments from Mytilus edulis exposed in vivo to copper ions. Arch Biochem Biophys 491:32–38. https://doi.org/10.1016/J.ABB.2009.09.014
Wang X, Anadón A, Wu Q et al (2018) Mechanism of neonicotinoid toxicity: impact on oxidative stress and metabolism. Annu Rev Pharmacol Toxicol 58:471–507. https://doi.org/10.1146/ANNUREV-PHARMTOX-010617-052429
Way GP, Ruhl N, Snekser JL et al (2015) A comparison of methodologies to test aggression in zebrafish. Zebrafish 12:144–151. https://doi.org/10.1089/zeb.2014.1025
Wong CM, Marcocci L, Liu L, Suzuki YJ (2010) Cell signaling by protein carbonylation and decarbonylation. Antioxidants Redox Signal 12:393–404. https://doi.org/10.1089/ars.2009.2805
Wronikowska O, Michalak A, Skalicka-Woźniak K et al (2020) Fishing for a deeper understanding of nicotine effects using zebrafish behavioural models. Prog Neuropsychopharmacol Biol Psychiatry 98:109826. https://doi.org/10.1016/J.PNPBP.2019.109826
Yan LJ, Traber MG, Packer L (1995) Spectrophotometric method for determination of carbonyls in oxidatively modified apolipoprotein B of human low-density lipoproteins. Anal Biochem 228:349–351. https://doi.org/10.1006/abio.1995.1362
Yang HY, Lee TH (2015) Antioxidant enzymes as redox-based biomarkers: a brief review. BMB Rep 48:200. https://doi.org/10.5483/BMBREP.2015.48.4.274
Zhang Q, Lu Z, Chang CH et al (2019) Dietary risk of neonicotinoid insecticides through fruit and vegetable consumption in school-age children. Environ Int 126:672–681. https://doi.org/10.1016/J.ENVINT.2019.02.051
Acknowledgements
The authors thank all the chromatographic services provided by the Laboratório de Bioquímica e Biologia Molecular of the Instituto Federal de Educação, Ciência e Tecnologia do Rio Grande do Sul, Brazil. The authors would also like to thank Indianara Furlani (Universidade Federal de Ciências da Saúde de Porto Alegre). Lastly, the authors would like to provide a brief tribute to Prof. Dra. Rosilene Perin Kaizer (in memorian) for her support and assistance in the experimental design and execution of this study.
Funding
This study was financed by the grants from Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS, grant number 19/2551–0001-873–8) and Conselho Nacional de Pesquisa e Tecnologia (CNPq) (research productivity grant number 302167/2022–6 received by LJGB). The study was also suported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior-Brasil (CAPES)—Finance Code 001.
Author information
Authors and Affiliations
Contributions
LJGB and SMS conceived the studies. The laboratory experiments and analyses were performed by SMS, MF, NF, ACCV, AP, MTM, VCC, and WAT. The statistical analyses were performed by SMS and LJGB. The data was discussed, referenced, and reviewed by SMS, MF, NF, LGRG, and LJGB. LJGB and LGRG were responsible for funding acquisition. This work was under the supervision of LJGB. The original draft and review of writing were performed by SMS and LJGB. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethical approval
This study followed the guidelines of Conselho Nacional de Controle de Experimentação Animal (CONCEA) and was approved by the CEUA at UPF (protocol 036/2019). This study was also registered in SisGen (Sistema Nacional de Gestão do Patrimônio Genético e do Conhecimento Tradicional Associado) and complied with their guidelines (registration code A14E252).
Consent to participate
This is not applicable
Consent to publish
The author consent to publish this work. No other participant was involved.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Bruno Nunes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Environmental concentrations of IBI triggered behavioral and physiological disbalances.
• IBI triggered behavioral and physiological disbalances in a 30-minute exposure protocol.
• IBI decreased fish locomotion, social interaction, and aggressiveness.
• IBI induced a slight anxiolytic-like behavior in exposed fish.
• IBI-exposed animals presented an increase in trunk cortisol levels and protein carbonylation.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mendonça-Soares, S., Fortuna, M., Freddo, N. et al. Behavioral, biochemical, and endocrine responses of zebrafish to 30-min exposure with environmentally relevant concentrations of imidacloprid-based insecticide. Environ Sci Pollut Res 30, 73662–73676 (2023). https://doi.org/10.1007/s11356-023-27667-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-27667-x