Abstract
Ochratoxin A (OTA) is a mycotoxin produced by species of filamentous fungi widely found as a contaminant in food and with high toxic potential. Studies have shown that this toxin causes kidney and liver damage; however, data on the central nervous system effects of exposure to OTA are still scarce. Thus, this study aimed to investigate the effects of exposure to OTA on behavioral and neurochemical parameters in adult zebrafish. The animals were treated with different doses of OTA (1.38, 2.77, and 5.53 mg/kg) with intraperitoneal injections and submitted to behavioral evaluations in the open tank and social interaction tests. Subsequently, they were euthanized, and the brains were used to assess markers associated with oxidative status. In the open tank test, OTA altered distance traveled, absolute turn angle, mean speed, and freezing time. However, no significant effects were observed in the social interaction test. Moreover, OTA also increased glutathione peroxidase (GPx), glutathione-S-transferase (GST), and glutathione reductase (GR) levels and decreased non-protein thiols (NPSH) levels in the zebrafish brain. This study showed that OTA can affect behavior and neurochemical levels in zebrafish.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
It is estimated that 200 thousand people are added daily to the world’s demand for food (Nellemann et al. 2009). With the projections that by 2050 the world will reach 9.8 billion inhabitants (United Nations 2017), the search for solutions to meet those needs becomes urgent. Currently, the tools used to solve this issue are responsible for creating other problems. For example, the increase in pesticide use in large crops is already causing serious environmental and public health impacts (World Health Organization 2006; Langley and Mort 2012; Rani et al. 2021). Improperly tampering with livestock products may put consumers’ lives in danger (Xin and Stone 2008; Cavin et al. 2018), and industry investment in processed foods has been linked to the incidence of obesity, diabetes, celiac disease, and heart disease (Canella et al. 2014; Anand et al. 2015; Aguayo-Patrón and Calderón de la Barca 2017). Although for a long time environmental conditions and inadequate storage of food products have been ignored, today it is already clear that these conducts are responsible for the increasing presence of mycotoxins (Marroquín-Cardona et al. 2014).
Mycotoxins are naturally occurring compounds in species of fungi and are potentially toxic (Tola and Kebede 2016). Ochratoxin A (OTA) is a mycotoxin produced by filamentous fungi and belongs to the ochratoxin subgroup, along with ochratoxins B and C. However, OTA has more natural occurrence and higher toxicity than other ochratoxins. OTA has become a very common contaminant in food and the ecosystem. There is evidence of the presence of OTA in water sources (Mata et al. 2015; Hu et al. 2017) and sea animals (Sun et al. 2015). The highest incidence of detection, however, occurs in food. OTA has already been found in many types of food in the world, including meats found in Croatia (Pleadin et al. 2015), Brazilian and European coffee (v. d. Stegen et al. 1997; de Almeida et al. 2007), wines and beers from Chile and Hungary (Vega et al. 2012; Varga et al. 2014), fruits in Argentina and Canada (Magnoli et al. 2004; Lombaert et al. 2004), European juices (Jørgensen 2005), and several other types of products across the globe. The contamination of feed commodities also affects the preparation of animal feeds with the mycotoxin having already been identified in fish and poultry feed (Abidin et al. 2017; Pietsch et al. 2020; Nogueira et al. 2020).
The exportation market moves around billions of dollars per year (Food and Agriculture Organization (FAO) 2019a) and billions of food tons (Food and Agriculture Organization (FAO) 2019b) are transported to countries with varying laws and cultures. Most nations have protocols and specific regulations for the tolerable limits of contaminants in the food, including OTA (Official Journal of the European Union 2006; Bureau of Chemical Safety et al. 2009; Ministério da Saúde and Agência Nacional de Vigilância Sanitária 2011). However, there is no consensus on acceptable limits for this contaminant among countries and, as the trade develops, the OTA present in food crosses borders and easily spreads around the world due to a lack of consent between health inspection standards.
The mechanism of OTA toxicity is not clear yet. It is believed to be related to the inhibition of protein synthesis caused by the competition between the phenylalanine group of OTA and phenylalanine amino acid (Kőszegi and Poór 2016). The effects of OTA have already been evaluated in rodents (Kanisawa and Suzuki 1978; Castegnaro et al. 1998), birds (Stoev 2010), and fish (Doster et al. 1974; Manning et al. 2003). The toxin has been associated with immune modulation (Lea et al. 1989), hepatic (Qi et al. 2015), and kidney diseases (Abid et al. 2003; Fuchs and Peraica 2005). OTA has also been increasingly associated with neuropsychiatric disorders (Sava et al. 2006a, b; Yoon et al. 2009; Brewer et al. 2013). However, despite the importance of these reports, there is still little information regarding the behavioral and neurochemical effects related to OTA on non-target organisms. Therefore, OTA is an important contaminant for both environment and food commodities, but there are still several gaps in the knowledge about the effects of this toxin in organisms.
Native from Asia, zebrafish is a teleost that has high genetic and physiological homology with humans (Lieschke and Currie 2007). For this reason, this species has been used as a research animal model for different fields such as embryology and development (Keller et al. 2008; Hao et al. 2013), oxidative stress (Choi et al. 2010; Marcon et al. 2018), behavior (Abozaid et al. 2020; Reis et al. 2020; Nabinger et al. 2021), and genetics (Nasevicius and Ekker 2000; Pimentel Falcão et al. 2021). Moreover, this aquatic animal is a very interesting environmental bioindicator used in toxicology and ecotoxicology research due to its capacity to simulate the conditions of an animal in its natural ecosystem (Asharani et al. 2008; Valadas et al. 2019; Park et al. 2020). In this context, since zebrafish is a suitable environmental bioindicator used in toxicology research, this study aimed to investigate the behavior and neurochemical effects of OTA in adult zebrafish.
Materials and methods
Animals
The experiments were performed using 96 adult (4–6-month-old) short-fin wild-type zebrafish (Danio rerio, Hamilton, 1822) obtained from a local commercial supplier. Animals were of both sexes (50:50 male:female ratio) with the detailed ratio per group presented in Online Resource 1.
The animals were housed in a maximum density of two fish per liter of water in 16-L tanks (40 × 20 × 24 cm) and under a 14–10-h day/night cycle for 10 days before any procedure. Water parameters such as pH (7.0 ± 0.3), chlorine, ammonia (< 0.01 mg/L), and temperature (26 °C ± 2) were controlled. Fish were fed twice a day with commercial flake food (Poytara®, Brazil) and supplementation of brine shrimp (Artemia salina). After the behavioral tests, the animals were euthanized by hypothermic shock (2–4 °C) followed by decapitation, according to the AVMA Guidelines for the Euthanasia of Animals (Leary and Johnson, 2020). All procedures were approved by the Universidade Federal do Rio Grande do Sul ethical committee (#37761/2020). The protocols were reported following ARRIVE Guidelines 2.0 (Percie du Sert et al. 2020).
Chemicals
Ochratoxin A (OTA) (CAS 303-47-9), dimethyl sulfoxide (DMSO) (CAS 67-68-5), and tricaine (MS-222) (CAS 886–86-2) were obtained from Sigma-Aldrich (St. Louis, MO, USA). Sodium chloride solution 0.9% (saline, ADV Farma, SP, Brazil) was obtained from a local commercial supplier. OTA was dissolved into DMSO (final concentration of 10% DMSO). The OTA doses were based on the LD50 for intraperitoneal injection on rainbow trout (Salmo gairdneri or Oncorhynchus mykiss) (Doster et al. 1972) of 5.53 mg/kg since there are no similar studies on adult zebrafish.
Experimental procedures
After the period of acclimation to the laboratory environment, the animals were divided into the following experimental groups: control (CTRL), 10% DMSO, OTA (1.38, 2.77, and 5.53 mg/kg). Allocation to experimental groups followed randomization procedures with a computerized random number generator (random.org) and the procedure was performed by researchers blinded to the experimental group. The drugs for each experimental group were administered at the beginning of the experiment (at 0 h) by intraperitoneal injections and the control group received saline. Briefly, the intraperitoneal injections were performed using a Hamilton Microliter™ Syringe (701 N 10 μL SYR 26 s/2″/2) × Epidurakatheter 0.45 × 0.85 mm (Perifix® Katheter, Braun, Germany) × Gingival Needle 30G/0.3 × 21 mm (GN Injecta, SP, Brazil). The injection volume was 1 μL/100 mg of animal weight. The animals were previously anesthetized by immersion in a solution of tricaine (300 mg/L) until loss of motor coordination and reduced respiratory rate. After the anesthesia, the animals were placed in a sponge soaked in water exposing the abdomen and the needle was gently inserted parallel to the spine in the abdomen’s midline posterior to the pectoral fins. This procedure was conducted in approximately 10 s (Fig. 1A) (Bertelli et al. 2021).
Following drug administration, the fish were kept in 4-L static tanks (17 × 17 × 17 cm) with two tanks for each concentration to minimize potential tank effects and remained there for 96 h. After 96 h of exposure, the animals were individually submitted to the open tank test (OTT). After this, the animals returned to the experimental tank and remained for 24 h. Then, the animals were submitted to the social interaction test (SIT). Immediately after the SIT, the animals were euthanized, and the brains were dissected and homogenized for the neurochemical assays of the parameters associated with oxidative status. The neurochemical parameter analyses were as follows: thiobarbituric acid reactive substance (TBARS), non-protein thiol (NPSH), glutathione peroxidase (GPx), glutathione-S-transferase (GST), and glutathione reductase (GR). The sex of the animals was confirmed after euthanasia by dissecting and analyzing the gonads.
Open tank test (OTT)
The OTT consists of a white circular arena (24 cm diameter, 8 cm height, and 2 cm water level). In this test, the animals were placed in the center of the arena and the behavior was individually recorded for 10 min (Fig. 1B). The videos were obtained from an upper view and for the analyses, the arena was virtually divided into two zones: center and periphery (Benvenutti et al. 2020). The following parameters were quantified using ANY-Maze software (Stoelting Co., USA): distance, crossings, absolute turn angle, mean speed, freezing episodes, and freezing duration.
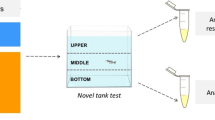
Social interaction test (SIT)
In the SIT, fish were placed individually in a central tank (30 × 10 × 15 cm) flanked by two identical tanks (15 × 10 × 13 cm) and filmed from a frontal view for 7 min (Fig. 1C). One of the two tanks positioned beside the central tank (test tank) contained only water (neutral stimulus), and the other contained 10 zebrafish (social stimulus). All tanks were filled with water at a level of 10 cm and in the same conditions. The side of the social stimulus tank was counterbalanced to avoid any eventual bias (Benvenutti et al. 2020). The analyses were carried out with the aid of the ANY-Maze software (Stoelting Co., USA), with the test tank virtually divided into three equal vertical zones (interaction, middle, and neutral). The interaction zone was considered to be next to the tank that contained the social stimulus, while the neutral zone was considered to be next to the neutral stimulus. Animals were placed in the middle zone and had 2 min to habituate to the tank test. After this, the behavior was analyzed for 5 min. The parameters quantified were distance traveled, number of crossings, and time in the interaction zone.
Neurochemical analysis
Following the behavioral tests, the animals were euthanized by hypothermic shock (2–4 °C) and decapitation. The brain samples were then collected to evaluate the oxidative status (Fig. 1D). For each independent sample, four brains were collected right after the euthanasia, pooled, and homogenized in 600 μL of phosphate-buffered saline (PBS). The mixture was centrifuged at 3000 g at 4 °C in a cooling centrifuge and collected the supernatant, which was kept in microtubes on ice until the assays were performed. The protein was quantified according to the Coomassie blue method using bovine serum albumin.
Thiobarbituric acid reactive substances (TBARS)
The lipid peroxidation was evaluated by analyses of the production of TBARS. Samples (50 μg of proteins) were mixed with thiobarbituric acid (TBA, 0.5%) and trichloroacetic acid (TCA, 20%). The mixture was heated at 100 °C for 30 min. The absorbance was determined at 532 nm in a microplate reader. Malondialdehyde (MDA, 2 mM) was the standard. The detailed protocol is available at Sachett et al. (2020a).
Non-protein thiols (NPSH)
The quantity of NPSH in the samples was determined by mixing the brain tissue preparation (50 μg of proteins) and trichloroacetic acid (TCA, 6%). Then, it was centrifugated (10,000 g, 10 min at 4° C), and the supernatants were added to potassium phosphate buffer (TFK, 1 M). After that, the mixture was added to 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB, 10 mM) and the absorbance of 5-thio-2-nitrobenzoic acid (TNB) formed was analyzed at 412 nm after 1 h. The detailed protocol is available at Sachett et al. (2020b).
Glutathione peroxidase activity (GPx)
The GPx levels were determined by a two-step method involving (I) neutralization of hydrogen peroxide (H2O2) by GPx in the presence of glutathione reduced (GSH) and (II) recycling of resulting glutathione oxidized (GSSG) by glutathione reductase (GR) in the presence of nicotinamide adenine dinucleotide phosphate (NADPH). For the determination, the sample (30 μg of protein) was mixed with a reaction medium containing TFK + ethylenediaminetetraacetic acid (EDTA) (0.5 M, pH 7.0), NADPH (1.6 mM), GSH (10 mM), GR (2.5 U/mL), and 10 mM azide. Then, H2O2 (4 mM) was added and the decrease of NADPH absorbance per minute was read at 340 nm. The detailed protocol is available at Sachett et al. (2021a).
Glutathione reductase activity (GR)
The GR levels were determined as the second part of the aforementioned GPx assay. The sample (30 μg of protein) was mixed with a reaction medium containing TFK + EDTA (154 mM, pH 7.0) and NADPH (2 mM). Then, oxidized glutathione (GSSG, 20 mM) was added and the decrease of NADPH absorbance per minute was read at 340 nm. The detailed protocol is available at Sachett et al. (2021b).
Glutathione-s-transferase activity (GST)
The GST levels were determined by the conjugating reaction of 1-chloro-2,4-dinitrobenzene (CDNB) with GSH GST-mediated (Habig and Jakoby 1981). The sample (30 μg of protein) was mixed with a reaction medium containing 230 μL of TFK + EDTA (100 mM, pH 6.5) and 10 μL of GSH (75 mM). After that, 10 μL of CDNB (30 mM) dissolved in ethanol 95% was added and the increase of absorbance per minute was read at 340 nm.
Statistical analysis
The sample size was calculated using G*Power 3.1.9.2 for Windows. Normality and homogeneity of variances were confirmed for all datasets using D’Agostino-Pearson and Levene tests, respectively. Student’s t-test was performed to compare the control and DMSO groups. One-way ANOVA followed by Tukey’s post hoc test was used for the analyses. For behavioral data, the outliers were identified based on distance traveled using the ROUT statistical test (GraphPad® software) and were removed from the analyses. This resulted in 3 outliers (2 animals from the DMSO group and 1 animal from OTA 2.77 mg/kg group) removed from the OTT and 3 outliers (1 animal from the DMSO group, 1 from the 2.77 mg/kg group, and 1 from the 5.53 mg/kg group) removed from the SIT. The tank and sex effects were tested in all comparisons, and no significant differences were observed. The data were expressed as mean ± standard deviation (S.D.). Differences were considered significant at p<0.05.
Results
DMSO did not show important modulation on behavior (Online Resource 2) or oxidative damage (Online Resource 3) compared with sodium chloride control. Therefore, we only used DMSO as a control group.
Open tank test
Figure 2 shows the acute effects of OTA in adult zebrafish in the open tank test. There was a significant decrease in the distance (Fig. 2A, p = 0.0105), absolute turn angle (Fig. 2C, p = 0.0090), mean speed (Fig. 2D, p = 0.0110), and an increase in freezing time (Fig. 2F, p = 0.0052) at the 1.38 mg/kg dose, indicating locomotor impairment. The parameters of crossings and freezing episodes were not altered by any dose.
Social interaction test
Figure 3 shows the acute effects of OTA on adult zebrafish at the SIT. OTA, in the tested doses, did not alter social behavior in any of the analyzed parameters.
Neurochemical analysis
Figure 4 shows the effects of OTA on neurochemical parameters. OTA at 1.38 mg/kg increased the GPx (Fig. 4C, p < 0.0001), GST (Fig. 4D, p < 0.0001), and GR (Fig. 4E, p = 0.0397) levels. The intermediate dose of 2.77 mg/kg decreased NPSH levels (Fig. 4B, p = 0.0006) and increased GPx (Fig. 4C, p = 0.0016) and GST (Fig. 4D, p = 0.0146) levels. The dose of 5.53 mg/kg increased GPx (Fig. 4C, p < 0.0001) and GR (Fig. 4E, p = 0.0238) levels.
Discussion
This study showed the deleterious effects of ochratoxin A in adult zebrafish. Briefly, the toxin decreased the total distance traveled, average speed, absolute turn angle, and increased the freezing time. However, in the social interaction test, there were no behavioral changes in the evaluated parameters. Neurochemical analysis showed that the compound was able to alter the oxidative status by triggering the oxidative defense system without damage as measured by TBARS.
In zebrafish, OTA has been studied with different emphases, especially with larvae and embryo models (Juan-García et al. 2020). Increased mortality due to malformations has been seen (Csenki et al. 2019). There was a negative effect on the formation of the hepatic and coagulation systems (Wu et al. 2018), occurrence of intracerebral hemorrhage (Wu et al. 2020), and genetic alteration related to nephrotoxicity in a study with OTA individually (Wu et al. 2016) and another that combined OTA with other mycotoxins (Csenki et al. 2021). In other fish species, intestinal disruptions were also seen (Liu et al. 2020), in addition to cell damage and immunosuppression (Zhao et al. 2022).
Although the toxin has been studied before, there are little data in the literature on the behavioral effects of OTA exposure not only in fish but also extending to other animals. In zebrafish larvae, OTA decreased the animals’ swimming speed but did not change parameters of distance and time spent active (Khezri et al. 2018). In marine water-reared sea bass (Dicentrarchus labrax L.), slow movement, loss of balance, and rapid movement of the operculum as respiratory manifestations were seen (El-Sayed et al. 2009). In what concerns behavior, another study investigated the toxic effects of OTA on Nile tilapia (Oreochromis niloticus) and showed sluggish swimming in the animals and off food patterns (Diab et al. 2018). With rodents, it was shown that OTA injected intraperitoneally was able to cause behavioral changes in gait analysis, spontaneous activity, cylinder test, and pole test, similar to Parkinsonian symptoms that were stabilized with the use of L-dopa (Bhat et al. 2018). In our study, the interference of OTA on locomotion parameters in zebrafish was shown in the open tank test similar to the results previously cited in other models. A possibility for these findings could be the link between locomotion and the nigrostriatal pathway that has already been reported to be affected by OTA in rodent models (Sava et al. 2006a, b). However, there were no changes in social interaction parameters. The social behavior in zebrafish presents a schooling cohesion that aims to search for food, escape from predators, and reproduce (Pitcher 1993). Thus, being a model closely linked to social functions, the zebrafish has been extensively studied for this type of behavior (Buske and Gerlai 2011; Scerbina et al. 2012; Dreosti et al. 2015). However, precisely because socialization is genetically preserved and has an ontogenic nature in zebrafish, it may be a parameter less vulnerable to milder modulations such as those shown in this study, since the lowest concentration used was about 25% of the LD50 established in another species. Another aspect to be considered is related to the cues provided by the apparatus since previous studies have already demonstrated the multifactorial character of social behavior in zebrafish being linked to visual cues (Engeszer et al. 2007), olfactory cues (Gerlach et al. 2007), and also sensitive to alarm substances released by co-specifics (Canzian et al. 2017). The apparatus used in this study, however, only allowed the visual cues to be transmitted to the animal, so it is uncertain to say what the effects of OTA would be under other parameters involved in the animal’s social behavior.
With increasing global concern about the spread of mycotoxins, the effect of these compounds on oxidative stress parameters has become a very debated issue (da Silva et al. 2018; Mavrommatis et al. 2021), with emphasis on ochratoxin A (Sorrenti et al. 2013; Tao et al. 2018). OTA can interact with peroxidases that produce a phenoxyl radical from OTA. Glutathione (GSH) is capable of turning the phenoxyl radical into OTA again by forming a superoxide anion radical (O2•−) that results in hydrogen peroxide (H2O2). H2O2 by Fenton reaction produces a hydroxyl radical (OH•) that is responsible for oxidative damage (Adlouni et al. 2000). Another common pathway for OTA is the formation of an OTA–Fe3+ complex that is reduced in OTA–Fe2+ by cytochrome P450 resulting in OH• (Rahimtula et al. 1988). Several studies report the imbalance of oxidative status caused by the compound. In zebrafish larvae, there was the formation of reactive oxygen species (ROS) proportional to the increase in OTA concentration (Tschirren et al. 2018). A study with tambaqui (Colossoma macropomum), a freshwater fish, found an increase in the ROS and lipid peroxidation in the animal’s muscles, as well as a decrease in the levels of antioxidant enzymes superoxide dismutase (SOD) and GPx (Baldissera et al. 2020). Similarly, an increase in lipid peroxidation and antioxidant enzymes activity catalase (CAT) and GR was seen with a decrease in SOD activity and GSH levels in the brain, kidney, and liver of rats (Nogaim et al. 2020). A study found an increase in ROS formation, lipid peroxidation, and decreased GSH levels in kidney cells (Lee et al. 2018). However, studies with birds have shown that in long-term exposure antioxidant defenses can increase against oxidative imbalance, especially the glutathione redox system (Kövesi et al. 2019; Fernye et al. 2021). Also, a study with Caenorhabditis elegans showed an increase in the expression of SOD and CAT in wine containing OTA (Schmidt et al. 2020). These studies corroborate with our results which showed that, in adult zebrafish, there was an increase in enzyme defenses with an elevation of GPx, GR, and GST, especially at the lowest dose. In the intermediate dose, there was no increase in GR as occurred in the other doses, which is consistent with the decrease in GSH levels (NPSH) in this group since GR is responsible for the recycling of glutathione, which is essential for the maintenance of antioxidant levels. The increase in GPx under these conditions indicates an attempt to control a possible increase in reactive oxygen species since GPx reduces H2O2 through the GSH oxidation, something quite common to occur in OTA exposures as mentioned in previous studies. The increase in GST levels also indicated an increase in OTA metabolization and elimination since GST catalyzes the conjugation of the reduced form of glutathione to xenobiotic substrates for detoxification. Likewise, this activation of defenses prevented the increase of ROS levels and consequently avoiding lipid peroxidation (TBARS levels) (Gandhi and Abramov 2012; Dasuri et al. 2013).
Despite the zebrafish being a model used for decades in research in several areas, many gaps still exist in the model, especially in the area of toxicology. In recent years there has been a considerable increase in studies in this field due to initiatives to standardize this type of analysis in fish (Gonçalves et al. 2020), including the OECD protocols (OECD Guidelines for the Testing of Chemicals 1992). However, for adult animals, the methodologies tend to be limited to direct exposure to the animals’ water, which is not suitable for all protocols. In the case of OTA, the formulation of the compound and the difficulty in storing or disposing of waste made this type of exposure impracticable so the intraperitoneal injection standardized in the laboratory was chosen. The use of intraperitoneal injection to assess the effects of OTA has already been used in other models, being effective in detecting deleterious effects on the metabolism mechanism in rats (Størmer et al. 1985), on the immune system (Prior and Sisodia 1982) and neurotoxicity in mice (Miki et al. 1994; Tamaru et al. 1988). In fish, OTA was injected peritoneally into rainbow trout (Salmo gairdneri) acutely (96 h) for toxicological evaluation by histology and determination of LD50 (5.53 mg/kg) (Doster et al. 1974). However, these data were never detailed in other species and the use of zebrafish to evaluate the effects of OTA remained limited with little information regarding the effects of the toxin in this species.
The large distribution of OTA among products also makes the toxin’s presence in the environment very important. Sun et al. determined a range of 0.0005 mg/kg to 0.0019 mg/kg of OTA found in fish, which is way below the doses used in this study. However, the main focus of OTA contamination is food, specialty cereals, wine, and coffee (Li et al. 2021). (Gruber-Dorninger et al. 2019) determined a large range of OTA on food and its maximum value was 2 mg/kg on cereals. The wide contamination of OTA allows many different dose ranges according to the commodities and in this context, the doses of this study (1.38 mg/kg, 2.77 mg/kg, and 5.53 mg/kg) are relevant to that matter, and the following previous findings.
Due to these important gaps in the literature, another point to be clarified is the dose-response reaction of zebrafish against OTA. In this study, the doses that were more behaviorally and neurochemically reactive were the lowest doses, with the highest dose changing a few parameters in oxidative status. Thus, in this study, we speculate that OTA showed a hormetic effect in adult zebrafish. Hormesis is a biphasic dose-response characterized by stimulation at low doses and inhibition at high doses (Calabrese and Baldwin 2002). For OTA, this type of curve has already been reported in an in vitro study (Li et al. 2014), however, this is the first time that this behavior has been seen in an in vivo model. A biphasic curve can indicate the biological plasticity of the target organism (Calabrese and Mattson 2011), and the zebrafish is a widely studied model precisely because of its capacity for neuroplasticity and regeneration (Cosacak et al. 2015; Ghosh and Hui 2016). Thus, it is possible that the hormetic behavior of OTA, in this case, is linked to the animal’s biological characteristics. Moreover, hormetic curves often occur with endocrine disruptors (Vandenberg et al. 2012) and other studies have demonstrated the potential of OTA to interfere with hormone production (Frizzell et al. 2013; Woo et al. 2013). For all these reasons, toxicological results for low doses should not be ignored.
Conclusion
Although concern about controlling OTA levels is increasing, more efforts are still needed. For this, understanding the effects of the toxin on organisms is essential. This study demonstrated the potential that the toxin has for causing deleterious effects in adult zebrafish through behavioral changes by locomotion impairment and neurochemical modulation of oxidative stress components; however, more studies are needed to elucidate the compound’s mechanism of action and its effects on other organisms to further contribute to the field of toxicology and environment.
References
Abid S, Hassen W, Achour A et al (2003) Ochratoxin a and human chronic nephropathy in Tunisia: is the situation endemic? Hum Exp Toxicol 22:77–84. https://doi.org/10.1191/0960327103ht328oa
Abidin Z, Khatoon A, Arooj N et al (2017) Estimation of ochratoxin A in poultry feed and its ingredients with special reference to temperature conditions. Br Poult Sci 58:251–255. https://doi.org/10.1080/00071668.2017.1293797
Abozaid A, Trzuskot L, Najmi Z et al (2020) Developmental stage and genotype dependent behavioral effects of embryonic alcohol exposure in zebrafish larvae. Prog Neuro-Psychopharmacol Biol Psychiatry 97:109774. https://doi.org/10.1016/j.pnpbp.2019.109774
Adlouni CE, Pinelli E, Azemar B, et al (2000) Phenobarbital increases DNA adduct and metabolites formed by ochratoxin A: role of CYP 2C9 and microsomal glutathione-S-transferase. 35:9. https://doi.org/10.1002/(SICI)1098-2280(2000)35:2<123::AID-EM7>3.0.CO;2-L
Aguayo-Patrón SV, Calderón de la Barca AM (2017) Old fashioned vs. ultra-processed-based current diets: possible implication in the increased susceptibility to type 1 diabetes and celiac disease in childhood. Foods 6:. https://doi.org/10.3390/foods6110100
Anand SS, Hawkes C, de Souza RJ et al (2015) Food Consumption and its impact on Cardiovascular Disease: Importance of Solutions focused on the globalized food system. J Am Coll Cardiol 66:1590–1614. https://doi.org/10.1016/j.jacc.2015.07.050
Asharani PV, Lian WY, Gong Z, Valiyaveettil S (2008) Toxicity of silver nanoparticles in zebrafish models. Nanotechnology 19:255102. https://doi.org/10.1088/0957-4484/19/25/255102
Baldissera MD, Souza CF, da Silva JA et al (2020) Dietary ochratoxin A (OTA) decreases growth performance and impairs muscle antioxidant system and meat fatty acid profiles in juvenile tambaqui (Colossoma macropomum). Comparative Biochem Physiol Part C: Toxicol Pharmacol 236:108803. https://doi.org/10.1016/j.cbpc.2020.108803
Benvenutti R, Gallas-Lopes M, Sachett A, et al (2020) How do zebrafish respond to MK-801 and amphetamine? Relevance for assessing schizophrenia-relevant endophenotypes in alternative model organisms. Neuroscience
Bertelli PR, Mocelin R, Marcon M et al (2021) Anti-stress effects of the glucagon-like peptide-1 receptor agonist liraglutide in zebrafish. Prog Neuro-Psychopharmacol Biol Psychiat 111:110388. https://doi.org/10.1016/j.pnpbp.2021.110388
Bhat PV, Anand T, Mohan Manu T, Khanum F (2018) Restorative effect of l-Dopa treatment against Ochratoxin A induced neurotoxicity. Neurochem Int 118:252–263. https://doi.org/10.1016/j.neuint.2018.04.003
Brewer JH, Thrasher JD, Straus DC et al (2013) Detection of mycotoxins in patients with chronic fatigue syndrome. Toxins 5:605–617. https://doi.org/10.3390/toxins5040605
Bureau of Chemical Safety, Food Directorate, Canada Health Products and Food Branch (2009) Information Document on Health Canada’s Proposed Maximum Limits (Standards) for the Presence of the Mycotoxin Ochratoxin A in Foods
Buske C, Gerlai R (2011) Shoaling develops with age in Zebrafish (Danio rerio). Prog Neuro-Psychopharmacol Biol Psychiatry 35:1409–1415. https://doi.org/10.1016/j.pnpbp.2010.09.003
Calabrese EJ, Baldwin LA (2002) Defining hormesis. Hum Exp Toxicol 21:91–97. https://doi.org/10.1191/0960327102ht217oa
Calabrese EJ, Mattson MP (2011) Hormesis provides a generalized quantitative estimate of biological plasticity. J Cell Commun Signal 5:25–38. https://doi.org/10.1007/s12079-011-0119-1
Canella DS, Levy RB, Martins APB et al (2014) Ultra-Processed Food Products and Obesity in Brazilian Households (2008–2009). PLoS One 9:e92752. https://doi.org/10.1371/journal.pone.0092752
Canzian J, Fontana BD, Quadros VA, Rosemberg DB (2017) Conspecific alarm substance differently alters group behavior of zebrafish populations: Putative involvement of cholinergic and purinergic signaling in anxiety- and fear-like responses. Behav Brain Res 320:255–263. https://doi.org/10.1016/j.bbr.2016.12.018
Castegnaro M, Mohr U, Pfuhl-Leszkowicz A et al (1998) Sex- and strain-specific induction of renal tumors by ochratoxin A in rats correlates with DNA adduction. Int J Cancer 77:70–75. https://doi.org/10.1002/(SICI)1097-0215(19980703)77:1<70::AID-IJC12>3.0.CO;2-D
Cavin C, Cottenet G, Cooper KM, Zbinden P (2018) Meat Vulnerabilities to Economic Food Adulteration Require New Analytical Solutions. CHIMIA Int J Chem 72:697–703. https://doi.org/10.2533/chimia.2018.697
Choi JE, Kim S, Ahn JH et al (2010) Induction of oxidative stress and apoptosis by silver nanoparticles in the liver of adult zebrafish. Aquat Toxicol 100:151–159. https://doi.org/10.1016/j.aquatox.2009.12.012
Cosacak MI, Papadimitriou C, Kizil C (2015) Regeneration, Plasticity, and Induced Molecular Programs in Adult Zebrafish Brain. Biomed Res Int 2015:1–10. https://doi.org/10.1155/2015/769763
Csenki Z, Garai E, Risa A et al (2019) Biological evaluation of microbial toxin degradation by microinjected zebrafish (Danio rerio) embryos. Chemosphere 227:151–161. https://doi.org/10.1016/j.chemosphere.2019.04.014
Csenki Z, Garai E, Faisal Z et al (2021) The individual and combined effects of ochratoxin A with citrinin and their metabolites (ochratoxin B, ochratoxin C, and dihydrocitrinone) on 2D/3D cell cultures, and zebrafish embryo models. Food Chem Toxicol 158:112674. https://doi.org/10.1016/j.fct.2021.112674
da Silva EO, Bracarense APFL, Oswald IP (2018) Mycotoxins and oxidative stress: where are we? World Mycotoxin J 11:113–134. https://doi.org/10.3920/WMJ2017.2267
Dasuri K, Zhang L, Keller JN (2013) Oxidative stress, neurodegeneration, and the balance of protein degradation and protein synthesis. Free Radic Biol Med 62:170–185. https://doi.org/10.1016/j.freeradbiomed.2012.09.016
de Almeida AP, Alaburda J, Shundo L et al (2007) Ochratoxin A in brazilian instant coffee. Braz J Microbiol 38:300–303. https://doi.org/10.1590/S1517-83822007000200022
Diab AM, Salem RM, Abeer E-LMS et al (2018) Experimental ochratoxicosis A in Nile tilapia and its amelioration by some feed additives. Int J Vet Sci Med 6:149–158. https://doi.org/10.1016/j.ijvsm.2018.09.004
Doster RC, Sinnhuber RO, Wales JH (1972) Acute intraperitoneal toxicity of ochratoxins A and B in rainbow trout (Salmo gairdneri). Food Cosmetics Toxicol 10:85–92. https://doi.org/10.1016/S0015-6264(72)80049-X
Doster RC, Sinnhuber RO, Pawlowski NE (1974) Acute intraperitoneal toxicity of ochratoxin and b derivatives in rainbow trout (salmo gairdneri). Food Cosmetics Toxicol 12:499–505
Dreosti E, Lopes G, Kampff AR, Wilson SW (2015) Development of social behavior in young zebrafish. Front Neural Circuits 9. https://doi.org/10.3389/fncir.2015.00039
El-Sayed YS, Khalil RH, Saad TT (2009) Acute toxicity of ochratoxin-A in marine water-reared sea bass (Dicentrarchus labrax L.). Chemosphere 75:878–882. https://doi.org/10.1016/j.chemosphere.2009.01.049
Engeszer RE, Da Barbiano LA, Ryan MJ, Parichy DM (2007) Timing and plasticity of shoaling behaviour in the zebrafish, Danio rerio. Anim Behav 74:1269–1275. https://doi.org/10.1016/j.anbehav.2007.01.032
Fernye C, Ancsin Z, Balogh K et al (2021) Role of the glutathione redox system in the susceptibility of pheasants (Phasianus colchicus) to ochratoxin A. Acta Vet Hung 69:125–133. https://doi.org/10.1556/004.2021.00021
Food and Agriculture Organization (FAO) (2019a) FAOSTAT - Value of Agricultural Production
Food and Agriculture Organization (FAO) (2019b) FAOSTAT - Commodities by regions
Frizzell C, Verhaegen S, Ropstad E et al (2013) Endocrine disrupting effects of ochratoxin A at the level of nuclear receptor activation and steroidogenesis. Toxicol Lett 217:243–250. https://doi.org/10.1016/j.toxlet.2012.12.018
Fuchs R, Peraica M (2005) Ochratoxin A in human kidney diseases. Food Addit Contam 22:53–57. https://doi.org/10.1080/02652030500309368
Gandhi S, Abramov AY (2012) Mechanism of Oxidative Stress in Neurodegeneration. Oxidative Med Cell Longev 2012:1–11. https://doi.org/10.1155/2012/428010
Gerlach G, Hodgins-Davis A, MacDonald B, Hannah RC (2007) Benefits of kin association: related and familiar zebrafish larvae (Danio rerio) show improved growth. Behav Ecol Sociobiol 61:1765–1770. https://doi.org/10.1007/s00265-007-0409-z
Ghosh S, Hui SP (2016) Regeneration of Zebrafish CNS: Adult Neurogenesis. Neural Plasticity 2016:1–21. https://doi.org/10.1155/2016/5815439
Gonçalves ÍFS, Souza TM, Vieira LR, Marchi FC, Nascimento AP, Farias DF et al (2020) Toxicity testing of pesticides in zebrafish—a systematic review on chemicals and associated toxicological endpoints. Environ Sci Pollut Res 27:10185–10204. https://doi.org/10.1007/s11356-020-07902-5
Gruber-Dorninger C, Jenkins T, Schatzmayr G (2019) Global Mycotoxin Occurrence in Feed: A Ten-Year Survey. Toxins (Basel) 11:E375. https://doi.org/10.3390/toxins11070375
Habig WH, Jakoby WB (1981) Assays for differentiation of glutathione S-Transferases. Methods in Enzymology. Academic Press, In, pp 398–405
Hao R, Bondesson M, Singh AV et al (2013) Identification of Estrogen Target Genes during Zebrafish Embryonic Development through Transcriptomic Analysis. PLoS One 8:e79020. https://doi.org/10.1371/journal.pone.0079020
Hu M, Huang P, Suo L, Wu F (2017) Cetylpyridinium chloride functionalized silica-coated magnetite microspheres for the solid-phase extraction and pre-concentration of ochratoxin A from environmental water samples with high-performance liquid chromatographic analysis. J Sep Sci 40:2431–2437. https://doi.org/10.1002/jssc.201601464
Jørgensen K (2005) Occurrence of ochratoxin A in commodities and processed food – A review of EU occurrence data. Food Addit Contam 26–30. https://doi.org/10.1080/02652030500344811
Juan-García A, Bind M-A, Engert F (2020) Larval zebrafish as an in vitro model for evaluating toxicological effects of mycotoxins. Ecotoxicol Environ Saf 202:110909. https://doi.org/10.1016/j.ecoenv.2020.110909
Kanisawa M, Suzuki S (1978) Induction of renal and hepatic tumors in mice by ochratoxin A, a mycotoxin. Gann 69:599–600
Keller PJ, Schmidt AD, Wittbrodt J, Stelzer EHK (2008) Reconstruction of Zebrafish Early Embryonic Development by Scanned Light Sheet Microscopy. Science 322:1065–1069. https://doi.org/10.1126/science.1162493
Khezri A, Herranz-Jusdado JG, Ropstad E, Fraser TWK (2018) Mycotoxins induce developmental toxicity and behavioural aberrations in zebrafish larvae. Environ Pollut 242:500–506. https://doi.org/10.1016/j.envpol.2018.07.010
Kőszegi T, Poór M (2016) Ochratoxin A: Molecular Interactions, Mechanisms of Toxicity and Prevention at the Molecular Level. Toxins (Basel) 8. https://doi.org/10.3390/toxins8040111
Kövesi B, Cserháti M, Erdélyi M et al (2019) Long-Term Effects of Ochratoxin A on the Glutathione Redox System and Its Regulation in Chicken. Antioxidants 8:178. https://doi.org/10.3390/antiox8060178
Langley RL, Mort SA (2012) Human Exposures to Pesticides in the United States. Journal of Agromedicine 17:300–315. https://doi.org/10.1080/1059924X.2012.688467
Lea T, Steien K, Stormer FC (1989) Mechanism of ochratoxin A-induced immunosuppression. Mycopathologia 107:153–159
Lee HJ, Pyo MC, Shin HS et al (2018) Renal toxicity through AhR, PXR, and Nrf2 signaling pathway activation of ochratoxin A-induced oxidative stress in kidney cells. Food Chem Toxicol 122:59–68. https://doi.org/10.1016/j.fct.2018.10.004
Li Y, Zhang B, He X et al (2014) Analysis of Individual and Combined Effects of Ochratoxin A and Zearalenone on HepG2 and KK-1 Cells with Mathematical Models. Toxins (Basel) 6:1177–1192. https://doi.org/10.3390/toxins6041177
Li X, Ma W, Ma Z et al (2021) The Occurrence and Contamination Level of Ochratoxin A in Plant and Animal-Derived Food Commodities. Molecules 26:6928. https://doi.org/10.3390/molecules26226928
Lieschke GJ, Currie PD (2007) Animal models of human disease: zebrafish swim into view. Nat Rev Genet 8:353–367. https://doi.org/10.1038/nrg2091
Liu X, Wu P, Jiang W-D et al (2020) Effects of Dietary Ochratoxin A on Growth Performance and Intestinal Apical Junctional Complex of Juvenile Grass Carp (Ctenopharyngodon idella). Toxins (Basel) 13:11. https://doi.org/10.3390/toxins13010011
Lombaert GA, Pellaers P, Neumann G et al (2004) Ochratoxin A in dried vine fruits on the Canadian retail market. Food Addit Contam 21:578–585. https://doi.org/10.1080/02652030410001687681
Magnoli C, Astoreca A, Ponsone L et al (2004) Survey of mycoflora and ochratoxin A in dried vine fruits from Argentina markets. Lett Appl Microbiol 39:326–331. https://doi.org/10.1111/j.1472-765X.2004.01583.x
Manning BB, Ulloa RM, Li MH et al (2003) Ochratoxin A fed to channel catfish ( Ictalurus punctatus ) causes reduced growth and lesions of hepatopancreatic tissue. Aquaculture 219:739–750. https://doi.org/10.1016/S0044-8486(03)00033-4
Marcon M, Mocelin R, Sachett A et al (2018) Enriched environment prevents oxidative stress in zebrafish submitted to unpredictable chronic stress. PeerJ 6. https://doi.org/10.7717/peerj.5136
Marroquín-Cardona AG, Johnson NM, Phillips TD, Hayes AW (2014) Mycotoxins in a changing global environment – A review. Food Chem Toxicol 69:220–230. https://doi.org/10.1016/j.fct.2014.04.025
Mata AT, Ferreira JP, Oliveira BR et al (2015) Bottled water: Analysis of mycotoxins by LC–MS/MS. Food Chem 176:455–464. https://doi.org/10.1016/j.foodchem.2014.12.088
Mavrommatis A, Giamouri E, Tavrizelou S et al (2021) Impact of mycotoxins on animals’ oxidative status. Antioxidants (Basel) 10:214. https://doi.org/10.3390/antiox10020214
Miki T, Fukui Y, Uemura N, Takeuchi Y (1994) Regional difference in the neurotoxicity of ochratoxin A on the developing cerebral cortex in mice. Brain Res Dev Brain Res 82(1–2):259–64. https://doi.org/10.1016/0165-3806(94)90168-6
Ministério da Saúde, Agência Nacional de Vigilância Sanitária (2011) Resolução No 7, de 18 de fevereiro de 2011
Nabinger DD, Altenhofen S, Peixoto JV et al (2021) Feeding status alters exploratory and anxiety-like behaviors in zebrafish larvae exposed to quinpirole. Prog Neuro-Psychopharmacol Biol Psychiatry 108:110179. https://doi.org/10.1016/j.pnpbp.2020.110179
Nasevicius A, Ekker SC (2000) Effective targeted gene ‘knockdown’ in zebrafish. Nat Genet 26:216–220. https://doi.org/10.1038/79951
Nellemann C, United Nations Environment Programme, GRID--Arendal (eds) (2009) The environmental food crisis: the environment’s role in averting future food crises: a UNEP rapid response assessment. UNEP, Arendal, Norway
Nogaim QA, Sai Pratyusha Bugata L, Pv P et al (2020) Protective effect of Yemeni green coffee powder against the oxidative stress induced by Ochratoxin A. Toxicol Rep 7:142–148. https://doi.org/10.1016/j.toxrep.2019.11.015
Nogueira WV, de Oliveira FK, Marimón Sibaja KV et al (2020) Occurrence and bioacessibility of mycotoxins in fish feed. Food Additives & Contaminants: Part B 13:244–251. https://doi.org/10.1080/19393210.2020.1766577
OECD Guidelines for the Testing of Chemicals (1992) Test No. 203: Fish, Acute Toxicity Test. OECD
Official Journal of the European Union (2006) COMMISSION REGULATION (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs
Park S, Lee J-Y, You S et al (2020) Neurotoxic effects of aflatoxin B1 on human astrocytes in vitro and on glial cell development in zebrafish in vivo. J Hazard Mater 386:121639. https://doi.org/10.1016/j.jhazmat.2019.121639
Percie du Sert N, Hurst V, Ahluwalia A et al (2020) The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol 18:e3000410. https://doi.org/10.1371/journal.pbio.3000410
Pietsch C, Müller G, Mourabit S et al (2020) Occurrence of Fungi and Fungal Toxins in Fish Feed During Storage. Toxins (Basel) 12:E171. https://doi.org/10.3390/toxins12030171
Pimentel Falcão MA, Banderó Walker CI, Rodrigo Disner G et al (2021) Knockdown of miR-26a in Zebrafish Leads to Impairment of the Anti-Inflammatory Function of TnP in the Control of Neutrophilia. Fish Shellfish Immunol. https://doi.org/10.1016/j.fsi.2021.04.029
Pitcher TJ (ed) (1993) Behaviour of Teleost Fishes, 2nd edn. Springer, Netherlands
Pleadin J, Malenica M, Vah N et al (2015) Survey of aflatoxin B 1 and ochratoxin A occurrence in traditional meat products coming from Croatian households and markets. Food Control 52:71–77. https://doi.org/10.1016/j.foodcont.2014.12.027
Prior MG, Sisodia CS (1982) The effects of ochratoxin A on the immune response of Swiss mice. Can J Comp Med 46:91–96
Qi X, Yang X, Chen S et al (2015) Ochratoxin A induced early hepatotoxicity: new mechanistic insights from microRNA, mRNA and proteomic profiling studies. Sci Rep 4:5163. https://doi.org/10.1038/srep05163
Rahimtula AD, Béréziat J-C, Bussacchini-Griot V, Bartsch H (1988) Lipid peroxidation as a possible cause of ochratoxin a toxicity. Biochem Pharmacol 37:4469–4477. https://doi.org/10.1016/0006-2952(88)90662-4
Rani L, Thapa K, Kanojia N et al (2021) An extensive review on the consequences of chemical pesticides on human health and environment. J Clean Prod 283:124657. https://doi.org/10.1016/j.jclepro.2020.124657
Reis CG, Mocelin R, Benvenutti R et al (2020) Effects of N-acetylcysteine amide on anxiety and stress behavior in zebrafish. Naunyn Schmiedeberg's Arch Pharmacol 393:591–601. https://doi.org/10.1007/s00210-019-01762-8
Sachett A, Gallas-Lopes M, Conterato GMM, Benvenutti R, Herrmann AP, Piato A (2020a) Quantification of thiobarbituric acid reactive species (TBARS) optimized for zebrafish brain tissue. Protocols.io. https://doi.org/10.17504/protocols.io.bjp8kmrw
Sachett A, Gallas-Lopes M, Conterato GMM, Benvenutti R, Herrmann AP, Piato A (2020b) Quantification of nonprotein sulfhydryl groups (NPSH) optimized for zebrafish brain tissue. https://doi.org/10.17504/protocols.io.bx8tprwn
Sachett A, Gallas-Lopes M, Conterato GMM, Herrmann AP, Piato A (2021a) Glutathione peroxidase (GPx) activity assessment for zebrafish brain tissue. https://doi.org/10.17504/protocols.io.bsujneun
Sachett A, Gallas-Lopes M, Conterato GMM, Herrmann AP, Piato A (2021b) Glutathione reductase (GR) activity assessment for zebrafish brain tissue. https://doi.org/10.17504/protocols.io.bsuuneww
Sava V, Reunova O, Velasquez A et al (2006a) Acute neurotoxic effects of the fungal metabolite ochratoxin-A. NeuroToxicology 27(27):82–92. https://doi.org/10.1016/j.neuro.2005.07.004
Sava V, Reunova O, Velasquez A, Sanchez-Ramos J (2006b) Can low level exposure to ochratoxin-A cause parkinsonism? J Neurol Sci 249:68–75. https://doi.org/10.1016/j.jns.2006.06.006
Scerbina T, Chatterjee D, Gerlai R (2012) Dopamine receptor antagonism disrupts social preference in zebrafish: a strain comparison study. Amino Acids 43:2059–2072. https://doi.org/10.1007/s00726-012-1284-0
Schmidt L, de Heck NV, Ferreira I et al (2020) Ochratoxin A presence in Cabernet Sauvignon wine changes antioxidant activity in vitro and oxidative stress markers in vivo. Food Additives & Contaminants: Part A 37:1755–1764. https://doi.org/10.1080/19440049.2020.1802067
Sorrenti V, Di Giacomo C, Acquaviva R et al (2013) Toxicity of ochratoxin A and its modulation by antioxidants: a review. Toxins 5:1742–1766. https://doi.org/10.3390/toxins5101742
Stoev SD (2010) Studies on carcinogenic and toxic effects of ochratoxin A in chicks. Toxins 2:649–664. https://doi.org/10.3390/toxins2040649
Størmer FC, Kolsaker P, Holm H et al (1985) Metabolism of ochratoxin B and its possible effects upon the metabolism and toxicity of ochratoxin A in rats. Appl Environ Microbiol 49:1108–1112
Sun W, Han Z, Aerts J et al (2015) A reliable liquid chromatography–tandem mass spectrometry method for simultaneous determination of multiple mycotoxins in fresh fish and dried seafoods. J Chromatogr A 1387:42–48. https://doi.org/10.1016/j.chroma.2015.01.071
Tamaru M, Hirata Y, Matsutani T (1988) Neurochemical effects of prenatal treatment with ochratoxin A on fetal and adult mouse brain. Neurochem Res 13:1139–1147. https://doi.org/10.1007/BF00971631
Tao Y, Xie S, Xu F et al (2018) Ochratoxin A: Toxicity, oxidative stress and metabolism. Food Chem Toxicol 112:320–331. https://doi.org/10.1016/j.fct.2018.01.002
Tola M, Kebede B (2016) Occurrence, importance and control of mycotoxins: A review. Cogent Food Agric 2. https://doi.org/10.1080/23311932.2016.1191103
Tschirren L, Siebenmann S, Pietsch C (2018) Toxicity of Ochratoxin to Early Life Stages of Zebrafish (Danio rerio). Toxins 10:264. https://doi.org/10.3390/toxins10070264
United Nations (2017) World population projected to reach 9.8 billion in 2050, and 11.2 billion in 2100 – says UN
v. d. Stegen G, Jörissen U, Pittet A et al (1997) Screening of European coffee final products for occurrence of ochratoxin A (OTA). Food Addit Contam 14:211–216. https://doi.org/10.1080/02652039709374518
Valadas J, Mocelin R, Sachett A et al (2019) Propiconazole induces abnormal behavior and oxidative stress in zebrafish. Environ Sci Pollut Res 26:27808–27815. https://doi.org/10.1007/s11356-019-05977-3
Vandenberg LN, Colborn T, Hayes TB et al (2012) Hormones and Endocrine-Disrupting Chemicals: Low-Dose Effects and Nonmonotonic Dose Responses. Endocr Rev 33:378–455. https://doi.org/10.1210/er.2011-1050
Varga J, Kiss R, Mátrai T et al (2014) Detection of Ochratoxin A in Hungarian Wines and Beers detection of ochratoxin a in hungarian. Acta Aliment 34:381–392. https://doi.org/10.1556/AAlim.34.2005.4.6
Vega M, Ríos G, von Baer D et al (2012) Ochratoxin A occurrence in wines produced in Chile. Food Control 28:147–150. https://doi.org/10.1016/j.foodcont.2012.04.032
Woo CSJ, Wan MLY, Ahokas J, El-Nezami H (2013) Potential endocrine disrupting effect of ochratoxin A on human placental 3β-hydroxysteroid dehydrogenase/isomerase in JEG-3 cells at levels relevant to human exposure. Reprod Toxicol 38:47–52. https://doi.org/10.1016/j.reprotox.2013.02.034
World Health Organization (ed) (2006) Pesticide residues in food - 2004: toxicological evaluations : joint meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Core Assessment Group, Rome, 20-29 September 2004. World Health Organization, Geneva
Wu T-S, Yang J-J, Wang Y-W et al (2016) Mycotoxin ochratoxin A disrupts renal development via a miR-731/prolactin receptor axis in zebrafish. Toxicol Res (Camb) 5:519–529. https://doi.org/10.1039/c5tx00360a
Wu T-S, Lin Y-T, Huang Y-T et al (2018) Disruption of liver development and coagulation pathway by ochratoxin A in embryonic zebrafish. Toxicol Appl Pharmacol 340:1–8. https://doi.org/10.1016/j.taap.2017.12.012
Wu T-S, Lin Y-T, Huang Y-T et al (2020) Ochratoxin A triggered intracerebral hemorrhage in embryonic zebrafish: Involvement of microRNA-731 and prolactin receptor. Chemosphere 242:125143. https://doi.org/10.1016/j.chemosphere.2019.125143
Xin H, Stone R (2008) TAINTED MILK SCANDAL: Chinese Probe Unmasks High-Tech Adulteration With Melamine. Science 322:1310–1311. https://doi.org/10.1126/science.322.5906.1310
Yoon S, Cong WT, Bang Y et al (2009) Proteome response to ochratoxin A-induced apoptotic cell death in mouse hippocampal HT22 cells. NeuroToxicology 30:666–676. https://doi.org/10.1016/j.neuro.2009.04.013
Zhao P, Liu X, Jiang W-D et al (2022) The multiple biotoxicity integrated study in grass carp (Ctenopharyngodon idella) caused by Ochratoxin A: Oxidative damage, apoptosis and immunosuppression. J Hazard Mater 436:129268. https://doi.org/10.1016/j.jhazmat.2022.129268
Data availability
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
Funding
This work was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, proc. 303343/2020-6), and Pró-Reitoria de Pesquisa (PROPESQ) at Universidade Federal do Rio Grande do Sul (UFRGS).
Author information
Authors and Affiliations
Contributions
All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Conceptualization: Jéssica Valadas and Angelo Piato; methodology: Jéssica Valadas, Adrieli Sachett, Matheus Marcon, Leonardo M. Bastos, Angelo Piato; investigation: Jéssica Valadas, Adrieli Sachett, Matheus Marcon, Leonardo M. Bastos; formal analysis Jéssica Valadas, Adrieli Sachett, Matheus Marcon, Leonardo M. Bastos, Angelo Piato; resources: Angelo Piato; writing—original draft: Jéssica Valadas; writing—review and editing: Jéssica Valadas, Adrieli Sachett, Matheus Marcon, Leonardo M. Bastos, Angelo Piato; supervision: Angelo Piato; funding acquisition: Angelo Piato. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
All procedures were approved by the Universidade Federal do Rio Grande do Sul ethical committee (#37761/2020) and were performed following relevant guidelines on the care and use of laboratory animals and following the Brazilian legislation regarding animal research.
Consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Bruno Nunes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 194 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Valadas, J., Sachett, A., Marcon, M. et al. Ochratoxin A induces locomotor impairment and oxidative imbalance in adult zebrafish. Environ Sci Pollut Res 30, 21144–21155 (2023). https://doi.org/10.1007/s11356-022-23692-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-23692-4