Abstract
This study was designed to investigate the molecular (transcriptional expression), biochemical (oxidative stress and neurotoxicity), and histopathological effects of metal contamination in the gill of clams (Ruditapes decussatus) sampled from the Tunis lagoon. The concentrations of five heavy metals (Cd, Pb, Hg, Cu, and Zn) in surface sediments and their accumulation in soft tissues of R. decussatus were evaluated in three sites (Z1, Z2, and Z3). A metal contamination state of Tunis lagoon sediments was noted with spatial variations with relatively high levels at Z2. Biomarker analyses showed an increase in glutathione S-transferase and catalase activities and lipid peroxidation levels and a decrease in acetylcholinesterase activity in the studied sites. Molecular investigation showed a significant overexpression of: cytochrome c oxidase subunit I, ribosomal RNA 16S, Cu/Zn superoxide dismutase, heat shock protein 70, and metallothioneins in the three sampling sites. Moreover, our data were correlated to severe and diverse histopathological alterations in the clam gills. The principal component analysis showed that the Z2 region is more affected by metal contamination than Z1 and Z3 regions. Current field results suggest the use of several combined biomarkers at different cell levels instead of individual ones in monitoring programs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Coastal lagoons are complex and dynamic ecosystems, characterized by constant changes in environmental conditions (Porter et al. 2001; Kamel et al. 2014). Some Mediterranean lagoons are among the most extensively modified and threatened ecosystems mainly due to urbanization, industrialization, and tourism (Ben Khedher et al. 2013; Matozzo et al. 2010). Therefore, several contaminants are continuously released into these systems deteriorating water quality, imposing severe restrictions to organisms and possibly causing a decrease in natural resources (Cravo et al. 2012; Ben Khedher et al. 2014). Trace metals are among the most relevant contaminants that may affect both biotope and biota quality in marine ecosystems in general and lagoon ecosystems in particular (Sfriso et al. 2008; Ennouri et al. 2010; Ben Khedher et al. 2013).
Trace metals naturally occur in marine environments. However, human activities have introduced high amounts of these elements into the environment, making it difficult to distinguish natural contributions from anthropogenic ones. Heavy metals, which readily accumulate in marine sediments, can reach significant accumulation levels in the tissues of aquatic organisms (Fu et al. 2014). Various trace metals, such as copper (Cu) and zinc (Zn), are essential for aquatic organisms, but they are toxic above a given threshold (Kucuksezgin et al. 2006). Nonessential metals, such as cadmium (Cd), lead (Pb), and mercury (Hg), are toxic even at trace levels. An in vivo study showed that the phagocytic capacity in mussel P. viridis was inhibited by exposure to high Cu levels (50–200 µg L−1) (Nicholson 2003). Zebrafish exposed to 1.9 µg L−1 of Cd showed a pro-apoptotic response and mitochondrial damage (Gonzalez et al. 2006). Acute lethality of Pb to aquatic invertebrates and fish occurs at concentrations of 1 to 500 mg g−1, but mortality and adverse effects on growth and biochemical responses have been observed in chronic studies at concentrations as low as 0.007–0.020 mg g−1 (Demayo et al. 1982).
These heavy metals are often concentrated and amplified in organisms at higher levels of the food chain, particularly in benthic animals. Heavy metal concentrations found in the tissues of marine organisms do not always reflect the quantity of metals present in a toxic form within the cell, as demonstrated for Cu, which can be complexed in nonactive inorganic complexes (Viarengo and Nott 1993). Thus, there is a need to develop strategies to assess whether a given environment is under stress or not. Therefore, techniques based on measuring the biological effects are critical for any pollution-monitoring program (Nasci et al. 2000; Viarengo et al. 2007). The application of biomarkers in field conditions has been proposed by many authors in order to assess chronic responses in exposed aquatic populations under environmental realistic conditions (De la Torre et al. 2005; Morales-Caselles et al. 2008).
Over the years, some marine bivalves, particularly mussels and clams, have been used widely and successfully as bioindicators for monitoring pollution in many coastal environments (O’Connor 2002; Geret et al. 2003). Many studies have demonstrated that several bivalve species have a great ability to accumulate organic pollutants and heavy metals and, thus, can be used as sensitive in situ indicators for pollution assessment (Wetzel and Van Vleet 2004; Oros and Ross 2005; Zhu et al. 2005). Molecular approaches have been investigated by many authors to monitor the contamination impact on aquatic organisms and to better understand the early cellular events associated with the sensing and protective responses that occur upon exposure to contaminants (Negri et al. 2013; Arini et al. 2014; Banni et al. 2014; Dedeh et al. 2015).
Located in northern Tunisia, the Tunis lagoon is of great economic importance to the country. It is a Mediterranean eutrophic coastal lagoon covering 40 km2. Tunis, Tunisia’s capital city where the lagoon in located, is a densely populated area characterized by the presence of a variety of economic activities, such as harbors, tourism, agriculture, and several industries (e.g., chemical manufacturing plants, metal processing factories and electronic and mechanic industries). The Tunis lagoon drains a watershed of 40 km2, including 15 km2 occupied by industrial areas. It thus receives approximately 5500 m3 day−1 of industrial wastewater, rich in heavy metals, and hydrocarbons. As a consequence, high levels of chemicals were found in the lagoon sediments. These chemicals include polycyclic aromatic hydrocarbons (levels varied from 452 to 1411 ng g−1) (Mzoughi and Chouba 2011) and trace metals, such as Pb (13.1–18.8 µg g−1), Cr (54.5–62 µg g−1), Ni (21–25.8 µg g−1), Cu (11.3–15.8 µg g−1), and Zn (213.7–231 µg g−1) (Hellal et al. 2011). To our knowledge, no studies have assessed the levels of metal compounds in this region and have investigated their effects on clams.
The purpose of the present study was to investigate the potential use of the biochemical, transcriptomic, and histopathological responses of wild clams (Ruditapes decussatus) as biomarkers of environmental pollution and water quality assessment in productive ecosystems, such as the Tunis lagoon.
Materials and Methods
Study Area
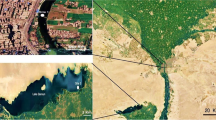
Three sampling sites were chosen in the Tunis lagoon because they are geographically located near contamination sources (Fig. 1). The sampling site Z1 (36°48′02.2″N 10°16′55.5″E) is located near a chemical industrial area, the sampling site Z2 (36°48′32.0″N 10°16′58.0″E) is located in the navigation canal and near the industrial area, and Z3 (36°48′13.0″N 10°16′36.1″E) is very close to Rades harbour—one of the largest harbours in Tunisia, which has the most important industrial complexes and intense commercial transport activities.
Control samples of surface sediments and clams were collected from Louza site (35°01′11.1″N 11°00′24.6″E). This site was chosen in another region 258 km far from the Tunis lagoon. Louza site has been considered as a reference site in monitoring programs along the Tunisian coasts (Banni et al. 2009).
Water quality was assessed at sampling sites. Temperature (°C) and salinity (psu) were measured with a WTW LF325 conductivity meter and pH was measured using a pH 330i WTW pH meter (Table S1).
Clam and Sediment Sampling
Clams R. decussatus of similar sizes (30–34 mm shell length) were collected from Z1, Z2, Z3, and Louza sites in April 2009. According to the literature, spawning events for R. decussatus in Tunisian coast occur irregularly from June to December and gametogenesis starts in January and February (Hamida et al. 2004). Therefore, the favorable sampling time is situated in the middle of period from January to May. The clams (n = 90 to 100 for each site) were immediately transported to the laboratory. From each site, 40 clams were dissected and soft bodies were removed, washed briefly in ultrapure water, pooled (4 individuals/pool), and stored at −80 °C until metal analysis (n = 10). For biomarkers approach, the gills of 30 clams were removed, pooled (3 individual gills/pool) into 10 pools for each sampling site, and stored at −80 °C for catalase (CAT), glutathione S-transferase (GST), acetylcholinesterase (AChE), and malondialdehyde (MDA) analysis (n = 10 for each biochemical biomarker). A second set of individual gills was kept at −80 °C in a RNA-preserving solution (RNA Later; Sigma-Aldrich) for transcriptome analysis (n = 5), and a third set of individual gills was dissected out and fixed in Bouin’s fixative (750 mL of picric acid-saturated aqueous solution, 250 mL of 40 % formaldehyde, and 50 mL of glacial acetic acid) for 48 h for histopathological analysis (n = 10).
Samples of surface sediments (up to 20 cm in depth) were collected from the three sites in the Tunis lagoon and from the Louza site (reference) at the same period. In the laboratory, sediment samples were homogenized, freeze-dried, passed through a stainless steel sieve (100 μm), and finally stored at 4 °C until analysis.
Heavy Metal Analysis
Freeze-dried clam soft bodies and sediments were digested in 3 mL of nitric acid at 100 °C for 3 h. The liquid underwent sixfold dilution with ultrapure water. Within each digestion series, appropriate blanks with no clam tissues or sediments also were subjected to the same procedure to account for background contamination levels and to validate the entire process. Standard biological and marine sediment reference materials with certified metal content (Tort-2: lobster hepatopancreas; Dolt-4: dogfish liver; PACS-1, MESS-2, and MESS-3: marine sediments from National Research Council of Canada, Ottawa, Canada) were treated and analysed under the same conditions (Dedeh et al. 2014). Metal concentrations (Ag, As, Cd, Mn, Ni, Pb, V, Zn, and Cu) in digests of clam tissues and sediments were analysed by inductively coupled plasma/atomic emission spectrometry (ICP/AES) (700 Series, Agilent Technologies). Quantification of mercury (Hg) in freeze-dried tissues was performed by flameless atomic absorption spectrometry (AMA 254, Altec, Prague, Czech Republic). All metal concentrations are reported in micrograms per gram of sample dry weight.
The bioaccumulation factor (BAF), relating the concentration of metal in surface sediments to its level in organism, was used to estimate each metal’s accumulation propensity in R. decussatus. It was calculated using the following equation:
BAF = [metal]clam (µg g−1)/[metal]sediment (µg g−1), where [metal]clam and [metal]sediment are the average metal concentrations in clams and sediments, respectively.
Biochemical Analysis
Before biochemical analysis, samples of gills were homogenized in phosphate buffer (0.1 M, pH 7.5). The homogenate obtained was centrifuged at 9000×g for 25 min to obtain the cytosolic fraction (S9). The quantities of proteins present in S9 fraction were determined according to the Bradford (1976) method using Coomassie Blue reagent (BioRad) and bovine serum albumin as standard protein.
GST activity was measured in gills cytosol by the method of Habig et al. (1974) using 10 μg of cytosolic proteins, 1 mM 1-chloro-2,4-dinitrobenzene (CDNB) (Sigma-Aldrich, St. Louis, MO) as substrate, and 4 mM of reduced glutathione (GSH), in 100 mM of sodium phosphate buffer, pH 7.5. GST activity was determined by kinetic measurement at 25 °C using a Spectro UV–Vis Double Beam PC Scanning Spectrophotometer UVD-2960 (λ = 340 nm). Results were expressed as µmoles GS-CDNB produced per min and per mg proteins.
CAT activity was determined according to Claiborne’s method (1985). Reaction mixture (final volume of 1 mL) contained 0.78 mL 0.1 M phosphate buffer (pH 7.5) and 0.2 mL 0.5 mM H2O2. After 30 s preincubation, the reaction was started by the addition of 0.02 mL of the S9 fraction. CAT activity was evaluated by kinetic measurement at 25 °C using a Spectro UV–Vis Double Beam PC Scanning Spectrophotometer UVD-2960 (λ = 240 nm). Results were expressed as μmoles hydrogen peroxide transformed per min and per mg proteins.
Lipid peroxidation (LPO) was estimated in terms of thiobarbituric acid reactive species (TBARS), with the use of 1,1,3,3-tetramethoxypropane as standard. The reaction was assessed at 532 nm using thiobarbituric acid (TBA) reagent as described by Buege and Aust (1978). MDA content was expressed as nanomoles equivalent MDA per milligram proteins.
AChE activity was measured at 25 °C according to the colorimetric method of Ellman et al. (1961). In a typical assay, 1.05 mL of 0.1 M phosphate buffer, 50 μL of 8 mM dithiobisnitrobenzoate, 50 μL of supernatant S9, and 50 μL of 45 mM acetylthiocholine substrate were successively added. The enzymatic reaction rate was quantified spectrophotometrically at 412 nm against a blank without substrate for each activity measurement. In order to subtract the spontaneous hydrolysis of substrate, a second blank was performed without a sample. Enzyme activity was recorded over 5 min after the addition of the substrate concentration. AChE activity was expressed as specific activity (nanomole of substrate hydrolysed per minute per milligram of proteins).
Gene Expression Analysis
Total RNA was extracted from 30 to 50 mg of gills using the Absolutely RNA Miniprep Kit (Agilent technologies), according to the manufacturer’s instructions. However, to eliminate the maximum of lipids and proteins, we added a step of phenol:chloroform:isoamylic alcohol (25:24:1, Sigma) extraction (Dedeh et al. 2014). The elution volume was 30 µL and the concentration of RNA was quantified using a microplate spectrophotometer (Epoch, Biotek). The RNA quality in each sample was checked by measuring the 260/280 nm ratios and by 1 % agarose gel electrophoresis. First-strand cDNA was synthesized from total RNA using the AffinityScript cDNA Synthesis Kit (Agilent technologies) according to the manufacturer’s instructions. Specific primer pairs were designed with the Lightcycler probe designer software (Table S2). RT-qPCR reactions were performed using Brillant III Ultrafast SYBR QPCR kits (Agilent Technologies) according to manufacturer’s instruction in a Stratagene Mx3000P thermocycler (Agilent Technologies). The amplification program consisted of one cycle at 95 °C for 10 min and 50 amplifications cycles at 95 °C for 30 s, 55 °C for 30 s, and 72 °C for 30 s. The specificity of the amplification products was confirmed by size estimations on an agarose gel and by analyzing their melting curves to confirm that only one PCR product was amplified and detected. The melting curve was obtained by following the SybrGreen fluorescence level during gradual heating of the PCR products from 60 to 95 °C. Standard curves were generated using tenfold dilutions of a cDNA template on the LightCycler apparatus, and using each couple of gene specific primers (one standard curve per couple). Each dilution was assayed in triplicate for each couple of primers. Each standard curve was made by plotting the Ct against the log of the starting quantity of template for each dilution. The equation for the regression line and the r value was calculated. From that equation the slope of the standard curve was deduced and used to calculate the PCR efficiency, E, for each couple of primers, as follows: E = 10−1/slope (Table S2). The relative expression ratios of target genes were calculated according to Pfaffl (2001). Relative expression data were normalized to ribosomal RNA 18S (18S rRNA), a housekeeping gene. The 18S rRNA gene was chosen as the reference gene because of its stability across sampling sites, as highlighted by the fact that the mean C t values were the same regardless of the sampling site. Indeed, the C t collected in gill tissue were (n = 5, mean ± SD): Control: 11.7 ± 1.2; site Z1: 10.9 ± 1.8; site Z2: 11.6 ± 0.9 and site Z3: 12.5 ± 1.3. The differential expression factor (DEF) of a gene is the ratio of its relative expression in clams collected from the polluted site over that of control animals.
All RT-qPCR experiments were performed according to the Minimum Information for publication of Quantitative real-time PCR Experiments (MIQE) guidelines (Bustin et al. 2009). MIQE checklist is found in the Supplementary materials section.
Histological Methods
The gills preserved in Bouin’s fixative were dehydrated in a graded ethanol solution and embedded in paraffin. Embedded tissues were cut into sections of 5-μm thickness by a rotary microtome (Leitz WETZLAR 1512). The thin sections of the gill tissues were stained by Trichrome Masson for observation by a light microscope. Sections were photographed with a microscope (LEICA DM 750) equipped with a numerical camera (LEICA ICC50 HD) and examined for lesions. For each individual gill, seven slides were examined with four sections per slide. Seven alterations were found in the gills of clams.
Histopathological alterations were semi-quantitatively evaluated by ranking the severity of lesions (grades 0 (−), 0.5 (+/−), 1 (+), 2 (++) and 3 (+++)) as described in previous studies (Riba et al. 2004). A general index of damages was established to permit comparison of histopathological responses between sampling sites. An arithmetic average value was obtained from the original semi-quantitative assessment of the lesions.
Statistical Analysis
The results for metal accumulation and biochemical markers are presented as means ± SD. Relative gene expressions are presented as means ± SEM. Experimental data were initially tested for normality and homogeneity of variance to meet statistical demands. Data statistical analysis was performed using one-way analysis of variance (ANOVA) and Duncan’s test for multiple range comparison; p < 0.05 was considered significant. Pearson correlation matrix also was calculated to study the relationships between the biochemical and transcriptomic biomarkers measured and metal accumulation in clams. SigmaStat 3.5 (SYSTAT Software, Inc.) was used for statistical analysis. Principal component analysis (PCA) of biomarkers and metal data was applied to discriminate between different sites, using XLSTAT software 7.5.2.
Results
Levels of Heavy Metals
Mean concentrations of heavy metals (Cd, Pb, Hg, Cu, and Zn) in the surface sediments of the Tunis lagoon and results of metal bioaccumulation in R. decussatus are shown in Table 1. The sediment samples collected from different sites in the Tunis lagoon presented significantly higher metal concentrations than control sediments (site of Louza). Spatial variation of metal concentrations was observed among the three sampling sites. The levels of Cd, Pb, Cu, and Zn were higher in the sediments of Z2 than in those of the other sampling sites, whereas Hg concentration maximum value was recorded in Z1 sediments. Results of metal accumulation in soft tissues of R. decussatus showed significantly higher concentrations in clams sampled from the three sites in the Tunis lagoon compared with those in the controls, except for Cu at site Z3. There were no significant differences of Cd, Pb, Cu, and Zn concentrations between the three sampling sites, while the highest Hg level was recorded in clams sampled from site Z2 compared with those sampled from the other sites. Cd concentrations were not detectable in sediments from the control site and control clams. Analysis of each element showed the absence of correlation between metal concentration in sediments and their bioaccumulation in soft tissues of R. decussatus. The results of chemical analysis showed lower metal concentrations in the soft tissue of clams from the Tunis lagoon sites than in surface sediments, except for Hg level at site Z3 and Cd level at site Z1. Metal bioaccumulation factors (BAF) in R. decussatus from the Tunis lagoon through surface sediments are presented in Table S3. Control clams showed a higher BAF values (>1) for Hg, Cu, and Zn. However, clams sampled from Tunis lagoon sites showed a lower BAF values (<1) for the five metals, except for Hg at site Z3 and Cd at site Z1. Moreover, Pb showed the lowest BAF values (0.28 at reference site and <0.1 at Tunis lagoon) compared with the other analyzed metals.
Biochemical Analysis
Biomarker responses in the gill of R. decussatus sampled from the Tunis lagoon are shown in Fig. 2. The oxidative stress was assessed using GST and CAT activities and MDA content. Variations in GST activity in clam gills were observed among the sampling sites in the Tunis lagoon (Fig. 2a). GST activity ranged from 1036 to 2388 μmol min−1 mg−1 proteins. Clams sampled from site Z2 presented the highest value of GST level. Bivalves from the three sampling sites presented a significantly greater GST activity with 2.7-, 6.3-, and 3.9-fold increases for sites Z1, Z2, and Z3, respectively, compared with controls.
Responses of glutathione S-transferase (a), catalase (b), acetylcholinesterase (d) activities, and lipid peroxidation levels (c) in gills of clams collected from the three sites in Tunis lagoon and controls. Results are expressed as mean ± SD; n = 10. Different superscripts indicate significant difference (p < 0.05) between sampling sites according to the ANOVA, multiple comparison and Duncan’s test
The sites Z1, Z2, and Z3 exhibited an increase in CAT level with, respectively, 1.9-, 3.7-, and 2.4-fold increases compared with controls (Fig. 2b). A statistically significant difference in CAT activity was also measured in bivalves collected at the three sampling sites. The highest value (138 μmol min−1 mg−1 proteins) was recorded at site Z2.
Lipid oxidative alteration was investigated through the evaluation of the gill MDA accumulation (Fig. 2c). MDA level in the gills of clams was significantly higher at Z1, Z2, and Z3 in comparison with that in controls. Results showed the absence of a significant difference of MDA levels between sampling sites. MDA accumulation values in clams from the Tunis lagoon ranged from 3.2 to 4.1 nmol mg−1 proteins.
AChE was significantly inhibited in clams from all the sampling sites compared with controls (Fig. 2d). AChE level at sites Z1, Z2, and Z3 displayed 1.6-, 2.4-, and 1.9-fold decreases, respectively, compared with controls. AChE activity differed significantly between the clams from site Z2 and those from sites Z1 and Z3. The lowest AChE activity (4.75 nmol min−1 mg−1 proteins) was detected in clams sampled from site Z2.
Table 2 displays the correlation obtained amongst the investigated biochemical and transcriptomic biomarkers in gills and metal levels in soft tissue of clams. Significant correlations were recorded between the GST activity in gills and Hg, Cu, and Zn levels in clam soft body. Gills CAT activity was correlated with Hg and Zn accumulation in the tissue of clams and GST activity in gills. Lipid peroxidation level was positively correlated with GST and CAT activities in gills and metal concentrations (Cd, Pb, Hg, Cu, and Zn) in soft bodies of clams. Negative correlations were recorded between AChE level in gills, on the one hand, and the other biochemical parameters (GST and CAT activities and LPO level) in gills and chemical parameters (Cd, Pb, Hg, Cu and Zn) in soft body of clams, on the other hand.
Gene Expression Variations
Expression analysis of various genes (cox1, 16S rRNA, Cu/Zn-sod, hsp70, and mt) encoding proteins involved in mitochondrial metabolism, antioxidant stress, and detoxification system was performed by real time quantitative PCR on gill transcripts using 18S rRNA as reference gene (Table 3). A significant increase in cox1 and 16S rRNA transcription was observed in the gills of clams sampled from the three sampling sites compared with controls. No significant spatial variations were observed. Cox1 and 16S rRNA transcription levels were associated with the accumulation of five analysed metals in clam tissue. Compared with that in controls, the sod gene expression was higher in Tunis lagoon sites with a maximum expression level in clams from site Z2 in which it was 16-times higher than that in controls. The same pattern was observed for hsp70 and mt genes with a maximum expression level obtained in clams sampled from site Z2, which was 40- and 15-times higher than that in controls for hsp70 and mt, respectively. No significant differences between site Z1 and Z3 were observed. Positive correlations were noted between sod gene expression level in gills and Hg, Cu, and Zn levels in soft body of clams. The same holds true with the hsp70 gene expression level that was correlated with Hg and Zn accumulation. The expression of mt gene was associated with the accumulation of Cd, Hg, Cu, and Zn in clams. Moreover, positive correlations were observed between expression levels of all studied genes and the biochemical biomarkers (GST and CAT activities and MDA content); on the other hand, a negative correlation was recorded with AChE activity.
Histological Alterations
Control clams presented normal gills with well-defined lamellae formed by a single layer of epithelial cells of different types (e.g., ciliated and mucous-secreting cells) attached to a rod of chitinous cartilage-like material. Little ciliary erosion cases of gill lamellae were observed contrarily to clams sampled from the Tunis lagoon. Haemolymph sinuses of undamaged gills contained some haemocytes. Gills of control animals presented moderate haemocytic infiltration (Fig. 3a–c). In contrast, the most recurrent alterations observed in clams sampled from the three sites in the Tunis lagoon consisted of gill inflammation revealed by a variable degree of haemocytic infiltration (HI). The most damaged gills in clams from sites Z2 and Z3 presented diffuse inflammation, resolved by extensive haemocytic infiltration (Fig. 3h, l). Clams from Tunis lagoon sites showed histological lesions of gill lamellae, including ciliary erosion (CE) (Fig. 3e, g, k, n), fusion of lamellae (FL) (Fig. 3d–f), peculiar malformations (MF) (Fig. 3d, j), and epithelium alteration (EA) (Fig. 3e, f, g, k, l). Dramatic lamellae alterations (LA) were observed in gills of clams from the three sampling sites (Fig. 3e, h, i, l, m). Developing fibromas (Fig. 3l) were observed in few clams causing severe gill damage in animals sampled from Z2 and Z3.
Example micrographs of histopathological lesions in the gill of R. decussatus collected in Tunis lagoon sites and control clams. a–c Histological section of a control gill. Normal structure of gills showing the regular arrangement of filaments (GF) and lamellae (GL) with frontal (FC) and lateral (LC) cilia and intact lamellar epithelium (Ep). HS haemolymphatic sinuses, Ct supporting cartilage, GC goblet (mucous-secreting) cells (a, ×100 magnification; b, c, ×1000 magnification). d–g Alterations in the histoarchitecture of the gills of R. decussatus from site Z1. Haemocytic infiltration (HI), fusion of gill lamellae, malformation (MF) at tip of the gill lamellae, lamellar epithelium alterations (EA), ciliary erosion (CE) and alterations of gill lamellae (LA) (d, e, ×400 magnification; f, g, ×1000 magnification). h–k Alterations in the histoarchitecture of the gills of R. decussatus from site Z2. Diffuse haemocytic infiltrations (HI) in the gill filaments indicating inflammation, malformations (MF) at tip of the gill, lamellar epithelium alterations (EA), ciliary erosion (CE), and lamellae alterations (LA) (h, i, ×100 magnification; j, k, ×1000 magnification). l–o Alterations in the histoarchitecture of the gills of R. decussatus from site Z3. Lamellar epithelium alteration (EA), ciliary erosions (CE), fusion of gill lamellae (FL), lamellae alterations (LA), diffuse haemocytic infiltration (HI) in the gill of clam, and developing fibroma (Fb) in the gill filaments (l, ×100 magnification; m, o, ×400 magnification; n, ×1000 magnification)
To establish the possible relationship between metal accumulation and the histopathological lesions in clams, we categorized the severity of lesions and calculated the correlation matrix. We noted positive correlations between HI and Cd (r = 0.671; p < 0.05), Pb (r = 0.643; p < 0.05), Hg (r = 0.796; p < 0.01), Cu (r = 0.600; p < 0.05), and Zn (r = 0.805; p < 0.01). The CE was positively correlated with Hg (r = 0.718; p < 0.01), Cu (r = 0.613; p < 0.05), and Zn (r = 0.785; p < 0.01). Other lesions, such as FL, also were correlated with the soft-tissue Cd (r = 0.840; p < 0.01), Pb (r = 0.633; p < 0.05), and Hg (r = 0.597; p < 0.05). MF was significantly correlated with Cd (r = 0.688; p < 0.05), Hg (r = 0.826; p < 0.01), Cu (r = 0,677; p < 0.05), and Zn (r = 0.638; p < 0.05). EA was positively correlated with Cd (r = 0.609; p < 0.05), Hg (r = 0.895; p < 0.01), Cu (r = 0.801; p < 0.01), and Zn (r = 0.834; p < 0.01). LA was positively correlated with Hg (r = 0.731; p < 0.01), Cu (r = 0.634; p < 0.05), and Zn (r = 0.781; p < 0.01).
The intensity of each lesion in the gills of clams and the index of damage (ID) are presented in Table 4. In fact, haemocytic infiltration, lamellae alteration, and ciliary erosion were more frequent at Z2 and Z3 than at Z1. The epithelium alterations were more abundant at Z2. The fusions of lamellae and the malformations at the tip of the gill were more frequent in clams from Z1. The developing fibromas were observed only in clams from Z2 and Z3. Concerning the index of damage (ID), site Z2 presented the highest ID compared with the other sampling sites. Moreover, ID observed at site Z3 was slightly higher compared with that of site Z1.
Principal Component Analysis
For a complete overview of the contamination pattern in the three sampling sites from the Tunis lagoon, a PCA was performed using all chemical data, biochemical responses, and transcriptomic biomarkers (19 variables) (Fig. 4; Table S4). The two principal components accounted for 86.5 % of the total variance. The first axis (F1), explaining 72 % of the total variance, was characterized by high positive contributions of 17 variables: Cd, Pb, Hg, Cu, and Zn levels in clams, Cd, Pb, Cu, and Zn levels in sediments, GST and CAT activities, MDA accumulation and cox1, 16S rRNA, sod, hsp70, and mt genes expression (Fig. 4a). Moreover, F1 presented a strong negative contribution due to AChE activity. On the other hand, the variance explained by the second axis (F2) (13 % of the total variance), represented a positive contribution of Hg level in sediments. Axis F1 discriminated between Tunis lagoon sites and the control group, whereas axis F2 discriminated between the three sampling sites in the Tunis lagoon (Fig. 4b). The scores plot showed that the control group was clearly separated from the other sampling sites for featuring lower values of metals in tissue and sediments, lower levels of biochemical and genetic biomarkers in gills and higher AChE activity. Both Z1 and Z3 sites shared the same pollution pattern. Site Z2 was clearly differed from the other sampling sites, because clams from that site presented the highest values of sod, hsp70, and mt gene expressions, CAT and GST activities and Cu, Cd, Pb, and Zn levels in sediments, and the lowest AChE activity.
Principal component analysis (PCA) based on chemical, biochemical and transcriptomic variables (F1 vs. F2) studied in sediments and clams sampled from Tunis lagoon and control group. a Distribution of the variables on the principal components. b Scattered plot of scores for each site from PCA. Projection points were grouped by drawing arbitrary ellipses representing each sampling site
Discussion
Metal Accumulation in Sediments and Soft Tissue of Clams
In coastal ecosystems, sediments are an important sink of heavy metals and also may serve as an enriched source of metal for benthic organisms (Pan and Wang 2012). Metal levels in surface sediments collected from Tunis lagoon sites were higher than those in the control sediments (site of Louza). This is related to the presence of different contamination sources around the lagoon. It is continually submitted to various anthropogenic inputs (sewage, industrial effluents, and agriculture runoff), as well as naval and commercial shipping harbours (La Goulette Harbour and Rades Harbour) (Ennouri et al. 2010). Concentrations of heavy metals in sediments collected from the sampling sites are relatively similar compared with some other studied lagoon systems. The concentrations of Cd, Pb, Cu, and Zn found in sediments of Bizerte lagoon (Tunisia) ranged between 0–1.6, 0–94, 0–51, and 0–485 µg g−1 dw, respectively (Ben Khedher et al. 2013). The sediments of Berre lagoon (France) presented metal concentrations ranging between 0.2–1.6, 0.15–0.4, 18–82, 11–48, and 50–151 µg g−1 dw for Cd, Hg, Pb, Cu, and Zn, respectively (Accornero et al. 2008). Surface sediments from site Z2 contained the highest levels of Cd, Pb, Cu, and Zn due to its proximity to industries and navigation canal. Furthermore, Z2 is the closest sampling site to the La Goulette Harbour. However, sediments from site Z1 presented the highest level of Hg. The increase in Hg load at site Z1 is due primarily to the existence of an electric power plant near this sampling site, in addition to the presence of an industrial area. The results showed a decrease in Hg concentrations in sediments as we move away from the electric power plant (Z1 > Z2 > Z3). Sediments sampled from site Z3 presented higher Cd and Pb levels than those from site Z1. The contamination at site Z3 is mainly due to the Rades Harbour activities.
Analyses of metal loads in R. decussatus showed higher levels in animals from the Tunis lagoon compared with the control group. This is attributed to the contamination of Tunis lagoon environment by heavy metals. A higher tissular level of Zn and Cu than those of Cd, Pb, and Hg was observed. These results may be explained by the fact that both Cu and Zn for most living organisms are essential elements in metabolic processes and are required for maintaining cellular function involved in numerous enzymatic reactions (Tóth et al. 1996; Rejomon et al. 2010). The present study showed that clams accumulated metal levels lower than those recorded in surface sediments. The ability of heavy metals to accumulate in bivalves depends heavily on the bioavailability of these metals within the sediment, which refers to the state of the trace metal that is readily available for uptake by biota in the surrounding environment. The bioavailability of these metals from the sediment is affected by factors such as sediment characteristics. It has been reported that low bioavailability could result when heavy metals are adsorbed on ion exchange sites of fine silt/clays or within iron, aluminum, and manganese colloidal compound (Bendell-Young and Harvey 1991; Bryan and Langston 1992; Janssen et al. 1997). Furthermore, these authors have reported that incorporation of metals into lattice structure of clay can cause low bioavailability. The Tunis lagoon sediments are mainly calcareous sandy mud with much organic material characterized by high clay percentages (Harbridge et al. 1976; Ouertani et al. 2006), which may explain the low bioavailability of metals for clams. The lack of correlation between metal concentrations in clams and sediment and low bioaccumulation factors (<1) at Tunis lagoon sites suggest the absence of a direct contribution of the sediments in bivalve contamination. Moreover, the lack of relationship between metal concentrations in clams and sediments possibly reflects the importance of the dissolved phase (pore water and overlying water) as sources for these metals (Cheggour et al. 2005), since R. decussatus is a filter-feeding organism that filters high volume of water and suspended particles (Bebianno et al. 2004). BAF analysis showed a lower bioavailability of metals for clams in the Tunis lagoon than for those in the reference site, which may be due to the higher level of seawater salinity in the lagoon. It was reported that increases in salinity produce predictable decreases in metal uptake rate (Martín-Díaz et al. 2005).
Enzymatic Activities and Lipid Peroxidation Levels
The increased GST and CAT activities and MDA content in clams from the Tunis lagoon sites compared with control values indicate that these animals are facing an oxidative challenge, essentially associated with the presence of heavy metals in the environment. It is known that metals are involved in reactive oxygen species (ROS) generation, which can adversely affect cells by producing lipid peroxidation of intracellular membranes. The destruction of these membranes results in loss of cellular enzymes and dysfunction of cellular metabolism (Geret and Bebianno 2004). A spatial variation of GST and CAT activities was observed. The highest activities were noted in clams from site Z2 and seemed to be associated with Hg, Cu and Zn levels in clam tissues. It appeared that methylmercury toxicity was principally based on mitochondrial metabolism impairments due to ROS production in zebrafish exposed through diet (Cambier et al. 2010). The high affinity of mercury for binding to thiols suggests that depletion of intracellular thiols (especially glutathione) either directly or indirectly causes oxidative stress (Valko et al. 2005). Copper is a potent redox active metal able to generate reactive ROS through Fenton reaction (Valko et al. 2005). Stamler (1994) reported that the inhibition of glutathione reductase activity in cells could be a central aspect in zinc toxicity. Glutathione reductase is essential in the maintenance of glutathione in its reduced form, and the inhibition of this enzyme creates an imbalance in the GSH/GSSG ratio, contributing to oxidative stress (Stamler 1994). The highest correlation found between Cd tissue levels and lipid peroxidation suggests the involvement of this metal in inducing oxidative stress. Indeed, cadmium can indirectly contribute to oxidative cell stress by displacing Fe and Cu ions from ferritin and other proteins (Varotto et al. 2013). It also can displace Zn from metallothioneins and from the active sites of enzymes (e.g., metalloproteinases, lyases, dehydrogenases, SOD), impairing the catalytic, inhibitory, or accessory Zn functions in kinases/phosphatases and zinc-finger proteins (Moulis 2010). Increased CAT activity and MDA content has also been reported in R. decussatus sampled from contaminated Tunisian coastal areas (Bizerte lagoon and Gulf of Gabès) (Banni et al. 2009).
Several studies have demonstrated the usefulness of measuring AChE activity in evaluating the effects of exposure to neurotoxic compounds in aquatic organisms. AChE-inhibiting neurotoxic compounds can cause a serious dysfunction in aquatic organisms, e.g., behavioural changes, paralysis, and death (Fulton and Key 2001; Banni et al. 2010). AChE activity measured in the gills of clams sampled from the investigated sites appeared inhibited compared with controls. Heavy metals, and particularly Hg and Cu, present a pronounced preference for sulphur donor groups, and may therefore inhibit this enzyme by binding to thiol residues of proteins (Viarengo 1989). Clams from sampling site Z2 presented the more decreased AChE activity, which seemed to be due to the increased Hg and Cu levels in clams. Similarly, clam R. decussatus sampled from Bizerte lagoon (Tunisia) showed a lower AChE activity at the most affected site by anthropogenic contamination (Dellali et al. 2004).
Transcriptional Response
Clams collected from the three sampling sites in the Tunis lagoon displayed an increased expression of cox1 gene, compatible with an impaired electron-transport chain, and increased expression of the mitochondrial 16S rRNA gene, indicating an increased synthesis of mitochondria. The overexpression of cox1 gene could be a compensatory mechanism to restore decreased mitochondrial activity. Overexpression of cox1 gene has been shown already in zebrafish Danio rerio contaminated with dietary methylmercury (Gonzalez et al. 2005) and in freshwater and marine bivalves exposed to Cd and Cd–Zn mixture (Achard-Joris et al. 2006). The overexpression of the 16S rRNA gene also fits well with a compensatory response, suggesting that cells increased the numbers of mitochondria to balance those that were not functioning correctly. The 16S rRNA induction also could reflect a higher energy demand in these organs likely due to detoxification mechanisms. Similarly, cox1 and 16S rRNA genes were found overexpressed in Ruditapes philippinarum exposed to Cd, Hg and Pb metals mixture (Dedeh et al. 2014). Genes involved in oxidative stress scavenging (sod), general stress response (hsp70), and detoxification mechanisms (mt) were upregulated in the gills of clams sampled from Tunis lagoon sites compared with controls. Bivalves from site Z2 showed the highest expression levels of sod, hsp70, and mt genes compared with sites Z1 and Z3, because it is exposed to several origins of pollution (industrial activities and shipping). The upregulation of sod, hsp70, and mt genes at site Z2 seemed to be more associated with Hg level in clams. Gene expression analysis in methylmercury-exposed zebrafish revealed that the expression levels of mt and both the cytoplasmic and mitochondrial sod genes were highly induced, suggesting an impact of methylmercury on detoxification process and the generation of oxidative stress (Gonzalez et al. 2005). The transcription levels of hsp70 and mt genes in Hg exposed fish (Gobiocypris rarus) increased in a dose-dependent manner (Li et al. 2014). Gene expression changes in bivalves have been used as effective early warning tools in aquatic environment monitoring (Arini et al. 2014). Transcriptional responses are typically rapid and thus considered highly sensitive indicators of stress, as the initial interaction takes place at the molecular level and builds the mechanistic basis for subsequent consequences at higher levels of biological organization (Schirmer et al. 2010). In this study, a correlation was found between molecular responses, on the one hand, and GST, CAT, and MDA levels on the other hand. This may explain that the cell is mobilized, involving various subcellular mechanisms, to deal with the metal contamination. Furthermore, it has been demonstrated that mt gene expression levels are upregulated not only by metal exposures but also by ROS generation (Viarengo et al. 2000; Fang et al. 2010). Our results suggest that the induction of investigated genes and antioxidant enzyme activity indicates an adaptive cellular response of the redox defense system and the mitochondrial function in R. decussatus after in situ metal exposure. According to multivariate analysis, Tunis lagoon sites seemed to be more affected by metal contamination compared with the reference site. Moreover, Z2 appeared to be the most disturbed site, indicating a higher anthropogenic impact.
Histopathological Lesions in Clams
In aquatic organisms, the gills represent a vital organ, because they play an important role in the transport of respiratory gases and regulate the osmotic and ionic balance. Toxic substances, such as heavy metals, may cause damage to gill tissues, thereby reducing the oxygen consumption and disrupting the osmoregulatory function of aquatic organisms (Ghate and Mulherkar 1979). In the present study, detailed histopathology of the clam gills from the Tunis lagoon revealed several structural alterations. Clams sampled from sites Z2 and Z3, which are localized near the Rades harbour and the navigation canal, displayed gills with greater inflammatory alterations (haemocytic infiltration, ciliary erosion, epithelium, and lamellae alterations) and fibroma development compared with those from site Z1, the latter being the furthest site from sources of pollution. However, lamellae fusion alteration was more frequent at site Z1 compared with Z2 and Z3 and seemed to be mainly related to the Cd level in clam tissues. The use of ID suggests that gill tissues of clams from site Z2 were the most altered. The metal-induced physical damages in gills may result from microtubules disassembly in ciliated epithelium. Metals, such as Cu, may affect the microtubule structures of the gills either by directing binding to tubulin thiol groups, or indirectly, by inducing alterations of redox balance and oxidative stress conditions in the tissue (Viarengo et al. 1994). Furthermore, it was considered that metal-induced degenerative alterations in tissues are related to autolytic processes as a consequence of lysosomal membrane destabilization (Krishnakumar et al. 1990).
According to previous works, inflammatory changes in gills tend to be nonspecific and reflect a physiological adaptation to stress (Mallatt 1985). These changes might be considered as a protective mechanism, because the vulnerable surface area of the gills is decreased to maintain the osmoregulatory functions (Saravana Bhavan and Geraldine 2000). In the present study, the inflammatory changes (haemocytic infiltration, malformation and fusion of lamellae) observed with other severe alterations (lamellae alteration and fibroma) were more likely to represent a progressive loss of gills’ biological functions (respiration, osmotic, and ionic regulations) rather than protective mechanisms and conceivably could lead to dysfunctional or even nonfunctional gills. Histopathological damage in aquatic organisms may decrease individual fitness through disturbing the homeostasis and proper functioning of vital biological processes (e.g., detoxification, endocrine functioning, respiration, osmoregulation, nutrient absorption). Thereby, these histopathological responses are highly ecologically relevant (Au 2004). Krishnakumar et al. (1990) reported that toxic effects of metals observed at the tissue level were in agreement with those observed at the organismic level. Indeed, these authors showed that filtration rate, scope for growth, and growth efficiency of metals exposed mussels decreased significantly as a consequence of tissue alterations. Studies in P. viridis exposed to Cu indicated that impaired clearance rates were likely caused by structural damage of the cilia (Nicholson 2003). It is likely that histopathological damage to the gill interferes with feeding and ultimately growth; thus, they may have a potentially ecological impact on population (Nicholson and Lam 2005). Histopathological lesions of gills have been reported previously in clam R. philippinarum and crab Carcinus maenas exposed to a mixture of metals (Martín-Díaz et al. 2008; Ben Khedher et al. 2014). Our results are similar to those found in R. decussatus collected from contaminated areas in Southern Portuguese coast (Costa et al. 2013) and in R. philippinarum exposed in laboratory to different metals (Cd, Cu, and Zn) (Martín-Díaz et al. 2005).
Conclusions
The present study is the first that investigated both the chemical and the multimarker approaches for Tunis lagoon biomonitoring. Our work demonstrated a crucial contamination of surface sediments by heavy metals with a spatial variation. The clams R. decussatus sampled from the Tunis lagoon accumulated high metal levels. The battery of biomarker responses measured in gill tissues allowed the discrimination among sites and was correlated with metal contaminations. A marked inhibition of AChE activity and a higher induction of MDA level, CAT, and GST activities were observed in clams sampled from site Z2. Moreover, results revealed an overexpression of genes involved in mitochondrial metabolism (cox1 and 16S rRNA), antioxidant defense (sod), protein reparation (hsp70), and detoxification (mt) in gills of clams collected from the Tunis lagoon. An occurrence of various histopathological alterations in gills confirmed a metal contamination impact on clams. The multivariate analysis for the overall data revealed that clams from site Z2 were the most affected by metal contamination, whereas in sites Z1 and Z3 clams were less impacted. Overall, this study demonstrated the potential of transcriptional, biochemical, and histopathological responses as early-warning biomarkers in monitoring programs and recommends their development to complement traditional chemical analyses.
References
Accornero A, Gnerre R, Manfra L (2008) Sediment concentrations of trace metals in the Berre lagoon (France): an assessment of contamination. Arch Environ Contam Toxicol 54:372–385
Achard-Joris M, Gonzalez P, Marie V, Baudrimont M, Bourdineaud JP (2006) Cytochrome c oxydase subunit I gene is up-regulated by cadmium in freshwater and marine bivalves. Biometals 19:237–244
Arini A, Daffe G, Gonzalez P, Feurtet-Mazel A, Baudrimont M (2014) What are the outcomes of an industrial remediation on a metal-impacted hydrosystem? A 2-year field biomonitoring of the filter-feeding bivalve Corbicula fluminea. Chemosphere 108:214–224
Au DW (2004) The application of histo-cytopathological biomarkers in marine pollution monitoring: a review. Mar Pollut Bull 48:817–834
Banni M, Bouraoui Z, Ghedira J, Clearandeau C, Jebali J, Boussetta H (2009) Seasonal variation of oxidative stress biomarkers in clams Ruditapes decussatus sampled from Tunisian coastal areas. Environ Monit Assess 155:119–128
Banni M, Negri A, Dagnino A, Jebali J, Ameur S, Boussetta H (2010) Acute effects of benzo[a]pyrene on digestive gland enzymatic biomarkers and DNA damage on mussel Mytilus galloprovincialis. Ecotoxicol Environ Saf 73:842–848
Banni M, Attig H, Sforzini S, Oliveri C, Mignone F, Boussetta H, Viarengo A (2014) Transcriptomic responses to heat stress and nickel in the mussel Mytilus galloprovincialis. Aquat Toxicol 148:104–112
Bebianno MJ, Géret F, Hoarau P, Serafim MA, Coelho MR, Gnassia-Barelli M, Roméo M (2004) Biomarkers in Ruditapes decussatus: a potential bioindicator species. Biomarkers 9:305–330
Ben Khedher S, Jebali J, Kamel N, Banni M, Rameh M, Jrad A, Boussetta H (2013) Biochemical effects in crabs (Carcinus maenas) and contamination levels in the Bizerta Lagoon: an integrated approach in biomonitoring of marine complex pollution. Environ Sci Pollut Res 20:2616–2631
Ben Khedher S, Jebali J, Houas Z, Nawéli H, Jrad A, Banni M, Boussetta H (2014) Metals bioaccumulation and histopathological biomarkers in Carcinus maenas crab from Bizerta lagoon, Tunisia. Environ Sci Pollut Res 21:4343–4357
Bendell-Young L, Harvey HH (1991) Metal concentrations chromids in relation to the geochemical characteristics of surficial sediments. Arch Environ Contam Toxicol 21:202–211
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram of protein utilizing the principal of protein-dye binding. Anal Biochem 72:248–254
Bryan GW, Langton WJ (1992) Bioavailability, accumulation and effects of heavy metals in sediments with special reference to United Kingdom estuaries: a review. Environ Pollut 76:89–131
Buege JA, Aust SD (1978) Microsomal lipid peroxidation. Method Enzymol 52:302–310
Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT (2009) The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem 55:611–622
Cambier S, Gonzalez P, Durrieu G, Maury-Brachet R, Boudou A, Bourdineaud JP (2010) Serial analysis of gene expression in the skeletal muscles of zebrafish fed with a methylmercury-contaminated diet. Environ Sci Technol 44:469–475
Cheggour M, Chafik A, Fisher NS, Benbrahim S (2005) Metal concentrations in sediments and clams in four Moroccan estuaries. Mar Environ Res 59:119–137
Claiborne A (1985) Catalase activity. In: Greenwald RA (ed) CRC handbook of methods for oxygen radical research. CRC Press Inc., Boca Raton, pp 283–284
Costa PM, Carreira S, Costa MH, Caeiro S (2013) Development of histopathological indices in a commercial marine bivalve (Ruditapes decussatus) to determine environmental quality. Aquat Toxicol 126:442–454
Cravo A, Pereira C, Gomes T, Cardoso C, Serafim A, Almeida C, Rocha T, Lopes B, Company R, Medeiros A, Norberto R, Pereira R, Araújo O, Bebianno MJ (2012) A multibiomarker approach in the clam Ruditapes decussates to assess the impact of pollution in the Ria Farmosa lagoon, South Coast of Portugal. Mar Environ Res 75:23–24
De la Torre FR, Ferrari L, Salibian A (2005) Biomarkers of a native fish species (Cnesterodon decemmaculatus) application to the water toxicity assessment of a peri-urban polluted river of Argentina. Chemosphere 59:577–583
Dedeh A, Ciutat A, Tran D, Bourdineaud JP (2014) DNA alterations triggered by environmentally relevant polymetallic concentrations in marine clams Ruditapes philippinarum and polychaete worms Hediste diversicolor. Arch Environ Contam Toxicol 67:651–658
Dedeh A, Ciutat A, Treguer-Delapierre M, Bourdineaud JP (2015) Impact of gold nanoparticles on zebrafish exposed to a spiked sediment. Nanotoxicology 9:71–80
Dellali M, Romeo M, Gnassia-Barelli M, Aïssa P (2004) A multivariate data analysis of the clam Ruditapes decussatus as sentinel organism of the Bizerta lagoon (Tunisia). Water Air Soil Pollut 156:131–144
Demayo A, Taylor MC, Taylor KW, Hodson PV, Hammond PB (1982) Toxic effects of lead and lead compounds on human health, aquatic life, wildlife plants, and livestock. CRC Crit Rev Environ Control 12:257–305
Ellman GL, Courtney KO, Andres V, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Ennouri R, Chouba L, Magni P, Kraiem MM (2010) Spatial distribution of trace metals (Cd, Pb, Hg, Cu, Zn, Fe, and Mn) and oligo-elements (Mg, Ca, Na and K) in surface sediments of the Gulf of Tunis (Northern Tunisia). Environ Monit Assess 163:229–239
Fang Y, Yang H, Wang T, Liu B, Zhao H, Chen M (2010) Metallothionein and superoxide dismutase responses to sublethal cadmium exposure in the clam Mactra veneriformis. Comp Biochem Physiol C 151:325–333
Fu J, Wang H, Billah SM, Yu H, Zhang X (2014) Heavy metals in seawater, sediments, and biota from the coastal area of Yancheng City, China. Environ Toxicol Chem 33:1697–1704
Fulton MH, Key PB (2001) Acetylcholinesterase inhibition in estuarine fish and invertebrates as an indicator of organophosphorus insecticide exposure and effects. Environ Toxicol Chem 20:37–45
Geret F, Bebianno MJ (2004) Does zinc produce reactive oxygen species in Ruditapes decussatus? Ecotoxicol Environ Saf 57:399–409
Geret F, Serafim A, Bebianno MJ (2003) Antioxidant enzyme activities, metallothioneins and lipid peroxidation as biomarkers in Ruditapes decussates. Ecotoxicology 12:417–426
Ghate HV, Mulherkar L (1979) Histological changes in the gills of two freshwater prawn species exposed to copper sulphate. Indian J Exp Biol 17:838–840
Gonzalez P, Dominique Y, Massabuau JC, Boudou A, Bourdineaud JP (2005) Comparative effects of dietary methylmercury on gene expression in liver, skeletal muscle, and brain of the zebrafish (Danio rerio). Environ Sci Technol 39:3972–3980
Gonzalez P, Baudrimont M, Boudou A, Bourdineaud JP (2006) Comparative effects of direct cadmium contamination on gene expression in gills, liver, skeletal muscles and brain of the zebrafish (Danio rerio). Biometals 19:225–235
Habig W, Pabst M, Jakoby W (1974) Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Hamida L, Medhiouband MN, Cochard JC, Romdhane, Le Pennec M (2004) Étude comparative du cycle de reproduction de la palourde Ruditapes decussatus en milieu naturel (sud Tunisie) et contrôlé (écloserie). Cah Biol Mar 45:291–303
Harbridge W, Pilkey OH, Whaling P, Swetland P (1976) Sedimentation in the lake of Tunis: a lagoon strongly influenced by man. Environ Geol 1:215–225
Hellal MEA, Hellal F, El Khemissi Z, Jebali R, Dachraoui M (2011) Trace metals in algae and sediments from the north-eastern Tunisian lagoons. Bull Environ Contam Toxicol 86:194–198
Janssen RPT, Peijnenburg WJGM, Posthuma L, Van Den Hoop MAGT (1997) Equilibrium partitioning of heavy metals in Dutch soils. I. Relationship between metal partition coefficients and soil characteristics. Environ Toxicol Chem 16:2470–2478
Kamel N, Burgeot T, Banni M, Chalghaf M, Devin S, Minier C, Boussetta H (2014) Effects of increasing temperatures on biomarker responses and accumulation of hazardous substances in rope mussels (Mytilus galloprovincialis) from Bizerte lagoon. Environ Sci Pollut Res 21:6108–6123
Krishnakumar PK, Asokan PK, Pillai VK (1990) Physiological and cellular responses to copper and mercury in the green mussel Perna viridis (Linnaeus). Aquat Toxicol 18:163–173
Kucuksezgin F, Kontas A, Altay O, Uluturhan E, Darilmaz E (2006) Assessment of marine pollution in Izmir Bay: nutrient, heavy metal and total hydrocarbon concentrations. Environ Int 32:41–51
Li ZH, Chen L, Wu YH, Li P, Li YF, Ni ZH (2014) Effects of mercury on oxidative stress and gene expression of potential biomarkers in larvae of the Chinese rare minnow Gobiocypris rarus. Arch Environ Contam Toxicol 67:245–251
Mallatt J (1985) Fish gill structural changes induced by toxicants and other irritants: a statistical review. Can J Fish Aquat Sci 42:630–648
Martín-Díaz ML, Blasco J, González de Canales M, Sales D, DelValls TA (2005) Bioaccumulation and toxicity of dissolved heavy metals from the Guadalquivir Estuary after the Aznalcóllar mining spill using Ruditapes philippinarum. Arch Environ Contam Toxicol 48:233–241
Martín-Díaz ML, Jiménez-Tenorio N, Sales D, Delvalls TA (2008) Accumulation and histopathological damage in the clam Ruditapes philippinarum and the crab Carcinus maenas to assess sediment toxicity in Spanish ports. Chemosphere 71:1916–1927
Matozzo V, Binelli A, Parolini M, Locatello L, Marin MG (2010) Biomarker responses and contamination levels in the clam Ruditapes philippinarum for biomonitoring the Lagoon of Venice (Italy). J Environ Monit 12:776–786
Morales-Caselles C, Martín-Díaz ML, Riba I, Sarasquete C, Del Valls TÁ (2008) Sublethal responses in caged organisms exposed to sediments affected by oil spills. Chemosphere 72:819–825
Moulis JM (2010) Cellular mechanisms of cadmium toxicity related to the homeostasis of essential metals. Biometals 23:877–896
Mzoughi N, Chouba L (2011) Distribution and partitioning of aliphatic hydrocarbons and polycyclic aromatic hydrocarbons between water, suspended particulate matter, and sediment in harbours of the West coastal of the Gulf of Tunis (Tunisia). J Environ Monit 13:689–698
Nasci C, Ros L, Nesto N, Sperni L, Passarini F, Pavoni B (2000) Biochemical and histochemical responses to environmental contaminants in clams, Tapes philippinarum, transplanted to different polluted areas of Venice Lagoon, Italy. Mar Environ Res 50:425–430
Negri A, Oliveri C, Sforzini S, Mignione F, Viarengo A, Banni M (2013) Transcriptional response of the mussel Mytilus galloprovincialis (Lam.) following exposure to heat stress and copper. PLoS One 8(6):e66802. doi:10.1371/journal.pone.0066802
Nicholson S (2003) Cardiac and branchial physiology associated with copper accumulation and detoxication in the mytilid mussel Perna viridis (L.). J Exp Mar Biol Ecol 295:157–171
Nicholson S, Lam PKS (2005) Pollution monitoring in Southeast Asia using biomarkers in the mytilid mussel Perna viridis (Mytilidae: Bivalvia). Environ Int 31:121–132
O’Connor TP (2002) National distribution of chemical concentrations in mussels and oysters in the USA. Mar Environ Res 53:117–143
Oros DR, Ross JRM (2005) Polycyclic aromatic hydrocarbons in bivalves from the San Francisco estuary: spatial distributions, temporal trends, and sources. Mar Environ Res 60:466–488
Ouertani N, Hamouda R, Belayouni H (2006) Study of the organic matter buried in recent sediments of an increasing anoxic environment surrounded by an urban area: the “Lac sud de Tunis”. Geo Eco Trop 30:21–34
Pan K, Wang WX (2012) Trace metal contamination in estuarine and coastal environments in China. Sci Total Environ 421–422:3–16
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45
Porter JS, Dyrynda PEJ, Ryland JS, Carvalho GR (2001) Morphological and genetic adaptation to a lagoon environment: a case study in the bryozoan genus Alcyonidium. Mar Biol 139:575–585
Rejomon G, Nair M, Joseph T (2010) Trace metal dynamics in fishes from the southwest coast of India. Environ Monit Assess 167:243–255
Riba I, González de Canales M, Forja JM, DelValls TA (2004) Sediment quality in the Guadalquivir estuary: sublethal effects associated with the Aznalcóllar mining spill. Mar Pollut Bull 48:153–163
Saravana Bhavan P, Geraldine P (2000) Histopathology of the hepatopancreas and gills of the prawn Macrobrachium malcolmsonii exposed to endosulfan. Aquat Toxicol 50:331–339
Schirmer K, Fischer BB, Madureira DJ, Pillai S (2010) Transcriptomics in ecotoxicology. Anal Bioanal Chem 397:917–923
Sfriso A, Argese E, Bettiol C, Facc C (2008) Tapes philippinarum seed exposure to metals in polluted areas of the Venice lagoon. Estuar Coast Shelf Sci 79:581–590
Stamler JS (1994) Redox signaling: nitrosylation and related target interactions of nitric oxide. Cell 78:931–936
Tóth L, Juhász M, Varga T, Csikkel-Szolnoki A, Nemcsók KJ (1996) Some effect of CuSO4 on carp. J Environ Sci Health B 31:627–635
Valko M, Morris H, Cronin MTD (2005) Metals, toxicity and oxidative stress. Curr Med Chem 12:1161–1208
Varotto L, Domeneghetti S, Rosani U, Manfrin C, Cajaraville MP, Raccanelli S, Pallavicini A, Venier P (2013) DNA damage and transcriptional changes in the gills of Mytilus galloprovincialis exposed to nanomolar doses of combined metal salts (Cd, Cu, Hg). PLoS One 8(1):e54602. doi:10.1371/journal.pone.0054602
Viarengo A (1989) Heavy metals in marine invertebrates: mechanisms of regulation and toxicity at the cellular level. CRC Crit Rev Aquat Sci 1:295–317
Viarengo A, Nott JA (1993) Mechanisms of heavy metals homeostasis in marine invertebrates. Comp Biochem Physiol C 104:355–372
Viarengo A, Arena N, Canesi L, Alia FA, Orunesu M (1994) Structural and biochemical alterations in the gills of copper-exposed mussels. In: Renzoni A, Mattei N, Lari L (eds) Contaminants in the environment. Lewis Publishers, Boca Raton, pp 135–144
Viarengo A, Burlando B, Ceratto N, Panfoli I (2000) Antioxidant role of metallothioneins: a comparative overview. Cell Mol Biol 46:407–417
Viarengo A, Lowe D, Bolognesi C, Fabbri E, Koehler A (2007) The use of biomarkers in biomonitoring: a 2-tier approach assessing the level of pollutant-induced stress syndrome in sentinel organisms. Comp Biochem Physiol C 146:281–300
Wetzel DL, Van Vleet ES (2004) Accumulation and distribution of petroleum hydrocarbons found in mussels (Mytilus galloprovincialis) in the canals of Venice, Italy. Mar Pollut Bull 48:927–936
Zhu XB, Xu WH, Wang XT, Huang XP, Deng LP, Kang XL, Jiang ZG, Ma XL (2005) Research on heavy metals in Ruditapes philippinarum and soda industry wastes. Chin J Oceanol Limnol 23:39–42
Acknowledgments
This study was supported by the Ministry of Scientific Research and Technology, the University of Monastir, Tunisia, the French Institute of Tunisia, and the University of Bordeaux, France.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chalghmi, H., Bourdineaud, JP., Haouas, Z. et al. Transcriptomic, Biochemical, and Histopathological Responses of the Clam Ruditapes decussatus from a Metal-Contaminated Tunis Lagoon. Arch Environ Contam Toxicol 70, 241–256 (2016). https://doi.org/10.1007/s00244-015-0185-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-015-0185-0