Abstract
The biochemical effects in Carcinus maenas and contamination levels in seawater and sediments of Bizerta Lagoon (northeast of Tunisia) were investigated. The levels of metals and hydrocarbons were higher in seawater and sediments in Menzel Bourguiba and Cimentery in February and July than in the other sampling sites. Differences among sites for glutathione S-transferase, catalase, acetylcholinesterase activities, and the content of lipid peroxidation and metallothioneins in two important organs which accumulated contaminants (the gills and the digestive gland) of the C. maenas were found and possibly related to differences in metal and hydrocarbon levels. The seasonal variation of biomarkers was possibly associated with chemical contamination and also with the high fluctuation of physico-chemical characteristics of the sites. The integrated biomarker response values found in the five sites is in good agreement with hydrocarbon and trace metal concentrations detected in the water and sediments of the stressful places where crabs are living.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The marine littoral ecosystem and estuaries are threatened by increasing levels of various pollutants originating from human activities and urban, agricultural, and industrial discharges. A considerable number of chemicals have already been released into the environment and persist in sediment, water, and biota. This situation endangers the health of organisms inhabiting this ecosystem (Pereira et al. 2009).
Marine ecosystem pollution can be assessed by using bioindicators. Assuming that the first contact between organisms and chemical occurs at a cellular level, the cellular responses to toxicants appear early to environmental stressors and alert before severe and irreversible damages appear in the ecosystems. Some biochemical parameters are used as biomarkers based on a previous and deep knowledge of pollutant effects and organism’s defense systems (López-Barea 1995).
It is widely recognized that the use of a battery of biomarkers may be useful to evaluate the various responses to mixtures of pollutants in organisms under stress and may allow better evaluation of environmental risks (Viarengo et al. 2007). Biomarker responses may also be more meaningfully linked to environmental contamination and provide additional information about their bioavailability and toxicity. The integration of a battery of biomarkers coupled with chemical analyses of priority pollutants may provide comprehensive indications of ecosystem health (Montes-Nieto et al. 2010). A combined biochemical and chemical approach has been extensively used to assess the ecological risk of Tunisian littoral coasts (Hamza-Chaffai et al. 2003; Louiz et al. 2008; Bouraoui et al. 2009).
Bizerta Lagoon is located in Northern Tunisia and is considered as an economically important body of water due to a variety of fishing and aquaculture activities. Many pollutants have been detected in recent years in the northern Bizerta Lagoon due to the impact of industrial and agricultural production and domestic sewage from Menzel Abderrahmen and Menzel Bourguiba cities (Dellali et al. 2001; Louiz et al. 2008). In addition, this water body is subject to eutrophication phenomena during the summer period (Aissa 1991; Mahmoud et al. 2010). In this aquatic area, many studies have investigated the application of biochemical tools, but these biomarker approaches have been restricted to certain species such as the Mediterranean clam Ruditapes decussatus (Dellali et al. 2001, 2004), the mussel Mytilus galloprovincialis (Dellali et al. 2001; Khessiba et al. 2001, 2005), and Hexaplex trunculus (Roméo et al. 2006).
Despite the enormous array of data available on the biomarker responses in mollusks of Bizerta Lagoon, there is little information about the seasonal variation of contaminants and/or biochemical responses of the Mediterranean crab (Carcinus maenas) in this area. We previously found a high DNA damage and catalase activity in R. decussatus of the site contaminated by domestic sewage and associated with a pesticide rainfall period (Jebali et al. 2007). Recently, the biomonitoring study using a combined approach of biochemical and chemical analysis showed a high metal load and serious biochemical alterations in the gills and digestive gland of C. maenas collected from Bizerta Lagoon (Ghedira et al. 2011). A battery of biomarkers was measured in two important detoxification organs (the gills and the digestive gland) of the Mediterranean crab C. maenas, which is widespread in the Bizerta Lagoon.
The biomarker approach, coupled with chemical analysis, was used to examine the environmental status of several sites in the lagoon and to assess the relationships between pollutants and biological responses. Glutathione S-transferase (GST) conjugates different electrophilic compounds with glutathione during phase II of biotransformation reactions, enhancing the polarity of these compounds in order to enable their excretion, and has frequently been used as an indicator of increased phase II reactions in contaminant-exposed animals (Sáenz et al. 2010). The antioxidant enzymes such as catalase (CAT) and lipid peroxidation (TABRS) have been proposed as biomarkers of contaminant-mediated oxidative stress in a variety of marine organisms. Their induction reflects a specific response to a variety of pollutants including pesticides and metals (Banni et al. 2009; Ghedira et al. 2011). Acetylcholinesterase (AChE) inhibition in sentinel organisms has been widely used as a biomarker of exposure to organophosphate and carbamate pesticides (Lundbaye et al. 1997; Rickwood and Galloway 2004). Altered AChE activity has also been attributed to other classes of contaminants (Bonacci et al. 2008).
Metallothioneins (MTs) are low molecular weight (about 7 kDa) cysteine-rich and metal-binding proteins (Roesijadi 1992). The induction of MTs as a measure of response to metal exposure in aquatic organisms has been widely investigated in laboratory and field conditions (Reynders et al. 2008; Alhama et al. 2006; Boutet et al. 2002).
Integrated biomarker response (IBR) was computed with the obtained biomarker measurements in Mediterranean crab (C. maenas) to assess the ecological risk of the polluted area in the Bizerta Lagoon and establish a possible correlation between the distribution of pollutants and their biological effects.
In this study, metals and hydrocarbons were analyzed in water and sediments collected from different sites of Bizerta Lagoon (Tunisia) and Kuriat Island. The selected biochemical biomarkers were measured in the gills and digestive gland of the Mediterranean crab (C. maenas) collected from the same sites. An IBR index and a star plot were developed for interpretation of those biomarker responses to assess the biological health status of crabs.
Materials and methods
Study area
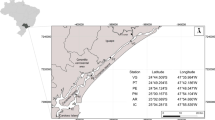
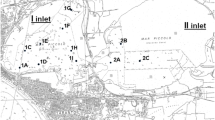
The sampling sites were located in Bizerta Lagoon (37°8′ N, 9°46′ E and 37°16′ N, 9°56′ E) (Fig. 1). They were chosen because of their differences in environmental conditions and potential contamination (Trabelsi and Driss 2005). Menzel Bourguiba (S2) (37°08′ N, 9°48′ E) is characterized by high metallurgy activities with the presence of the factory of El Fouledh (Louiz et al. 2008); Menzel Jemil (S3) (37°13′ N, 9°55′ E), which is located in the aquaculture part of the lagoon, is exposed in summer to eutrophication process and is characterized by the release of the shellfish and an industrial contamination (Louiz et al. 2008); Menzel Abdelrahmen (S4) (37°13′ N, 9°51′ E) which is located between the port and the old rejection of National Organization of Water purification (ONAS) is characterized by urban effluent (Mahmoud et al. 2010); Cimentery (S5) ( 37°15′ N, 9°50′ E) is located between the platform and the cement plant and is influenced by urban discharges. Organisms captured were collected from Kuriat Island (S1) (35°47′ N, 11°01′ E), located in the central east of Tunisia; this site is characterized by no apparent contamination sources and thus considered in our field work as a reference site (Jebali et al. 2011).
Crab and sediments sampling
Intermoult C. maenas specimens (75 per station) were collected at four different sites from Bizerta Lagoon and from Kuriat Island in February and July 2010. The biometric measurements of the collected crabs at the study sites are presented in Table 1. Twelve crabs of each site were immediately dissected in situ, and gills and digestive glands were removed and washed briefly in ice-cold homogenizing buffer and then transported to the laboratory. Samples were stored at −80 °C until biochemical analysis.
Three samples of surface sediments (up to 10 cm in depth), collected in the same place where the crabs were sampled, were mixed, transported to the laboratory, and stored for 3 days at 4 °C until chemical analysis.
Chemical analysis
Water physico-chemical parameters
Water quality was assessed at each crab sampling time. The physico-chemical quality of the Bizerta Lagoon waters was monitored in situ over 2 months (February and July). Salinity (g/L), temperature (°C), dissolved oxygen (mg/L), and pH were measured at the five sampling sites with a Multi 350i Multimeter.
Metals analysis
Seawater samples were filtered at the time of collection through a 0.45-μm membrane and acidified for the determination of dissolved elements. All water samples were analyzed by inductively coupled plasma/atomic emission spectrometry (ICP/AES) using OPTIMA 3300 RL according to the standard NF EN ISO 11885. A total of ten elements were tested.
For the preparation of sediment samples, 0.5 g of crushed and sieved sediment was weighed; 2 mL of nitric acid and 6 mL of hydrochloric acid were added; the tubes were placed for 35 min in the oven at 105 °C; the obtained solution was placed in tubes of 50 mL and completed with distilled water. The samples were prepared according to the standard NF X31-147 (1996) and analyzed by ICP/AES using OPTIMA 3300 RL according to the standard NF EN ISO 11885.
Hydrocarbons analysis
For water and sediments, the samples were analyzed by gas chromatography–mass spectrometry (GC–MS) using Agilent Technologies 7683B Series Injector, according to the standard NF EN ISO 93772.
Extraction
The analytical procedure used for polycyclic aromatic hydrocarbons (PAHs) was a modification of the method described by Kelly et al. (2000). Briefly, the extraction of samples is according to the following steps: For sediment, 20 g of crude sediment was weighed and placed into an Erlenmeyer flask; 50 mL of hexane (solvent extraction) was added; the Erlenmeyer flask was shaken vigorously in a dimensional shaker for 30 min. The resulting solution was filtered by adding anhydrous sodium sulfate into a beaker.
For the extraction of seawater sample, 50 mL hexane was added and sealed in the sample bottle (filled to about 80 %); the sample bottle was shaken vigorously in a dimensional shaker for 30 min and transferred to another container using a funnel in order to separate the two phases by recovering the aqueous phase in the original bottle; the obtained solution was dried with anhydrous sodium sulfate into a flask.
Extraction yield
The determination of extraction efficiency for each series was extracted by doping a liter of distilled water with an aliquot of the standard quality control solution (preparation of standard mixture consisting of two different types of mineral oil (type A: diesel fuel without additives, showing discrete peaks on the chromatogram; type B: lubricating oil, without additives, with a boiling range greater than that of type A and with unresolved signals in the chromatogram)) in the extractant (hexane) and then adding acetone before dilution in water to boost quality control.
Purification
This step aims to remove the impurities and polar compounds that can exist in the extract by using the following steps: using an eluent of non-polar solvent, condition the cartridge with six to ten times the total volume of the cartridge with a non-polar solvent, usually the solvent of the sample; load the sample into the cartridge, elute unwanted compounds with a non-polar solvent, elute the first compound of interest with a polar solvent, elute the rest of the compounds of interest with a more polar solvent gradually, and finally leave the old cartridge after finding all of the compounds.
Concentration
Concentrate the organic phase recovered from the sample to a volume of about 1 ml in a hood fume specific for evaporation and completely free from any source of hydrocarbons, and then transfer the sample in the vials using a syringe and finally accurately measure the volume of the extract.
Measurement
Control system performance is done on a monthly basis by injecting the standard mixture solution of n-alkanes. The C40/C20 ratio is at least equal to 0.8. Calculating the ratio C40/C20 is done on a control chart and includes measuring of samples, calibration of solutions, and the blank test solution by GC–MS.
Quality control
For the purpose of analytical quality control during the digestion and analysis of samples, internationally certified reference material and blanks were included in the set of samples to check the precision and accuracy of the analysis. We used certified reference marine sediment AGLAE 08 M40.1 (General Association of Analytical Laboratories of the Environment, France). Analytical results indicate a good agreement between the certified and measured values. Calibration and working standard solutions were made by stepwise dilution of the stock solution. Standards were made using certified solutions acidified with Suprapur quality HNO3 to the same pH as the samples. Sample preparation blanks were analyzed to document that the samples were not contaminated with metals during the digestion procedure. The measured trace metal concentrations of the certified reference material were within the certified range.
For quality assurance, the precision and accuracy of determinations were checked by recovery tests and repeated analyses of the subsamples of the standards. Adopted analytical procedures indicated that 96–100 % recoveries of the spiked metals and certified materials were obtained from the tested samples, which are in agreement with certified values. Signal drift was monitored through analyzing calibration standards and a reagent blank with every ten samples, which typically changed by 3–5 % throughout an analytical run in every instance. All reagents used were of analytical grade or Suprapur quality. Milli-Q water (Millipore, Bedford, MA, USA) was used to prepare standard solutions, dilutions, and blanks throughout the experiments. All glassware used were treated with a sulfo-nitric solution overnight and washed with Milli-Q water before use.
For hydrocarbons, the reference material is the result of a standard mixture consisting of two different types of mineral oil (type A: diesel fuel without additives, showing discrete peaks in the chromatogram; type B: lubricating oil without additives, with a boiling range higher than that of type A and with unresolved signals on the chromatogram) with the extraction solvent (Stock solution: n-tetracontane and n-decane in a volume of the extracting agent (hexane)).
Biochemical analysis
In order to satisfy all analyses, biochemical parameters were analyzed based on the individual samples of two tissues.
Cell-free extract preparations
All steps for cell-free extract preparation were carried out at 4 °C. Organs (gills and digestive gland) were homogenized in ice-cold phosphate buffer (100 mmol/L; pH 7.5; 1 mmol/L EDTA; 1 mmol/L reduced glutathione; GSH) at a rate of 3 mL/g (buffer volume/tissue weight). Homogenates were then centrifuged at 9,000 × g for 15 min. The supernatant of each sample was stored at –20 °C, for no longer than a week, until enzyme activity determination. Total protein content in the supernatant (S9) was measured following the Bradford method (Bradford 1976), at 595 nm, using bovine serum albumin as standard protein.
Catalase activity
Catalase (CAT) activity was determined by the method of Claiborne (1985) which involves measuring the rate of enzymatic decomposition of H2O2 determined as absorbance decrease at 240 nm. The assay mixture consisted of 780 μL of sodium phosphate buffer (0.1 M, pH 7.5 and 25 °C), 200-μL solution of 0.5 mM H2O2, and 20 μL of cytosolic fraction. The results were expressed as micromoles H2O2 consumed per minute per milligram protein.
Glutathione S-transferase activity
GST activity was assayed by the method described by Habig et al. (1974) using 1-chloro-2,4-dinitrobenzene as substrate and glutathione (1 and 4 mM final concentration, respectively) in 100 mM sodium phosphate buffer, pH 7.5. All GST activity assays were made under conditions of linearity with respect to incubation time. The results were expressed as nanomoles produced per minute per milligram protein.
Lipid peroxidation level
Lipid peroxidation was estimated in terms of thiobarbituric acid reactive species (TBARS) with the use of 1,1,3,3-treaethyloxypropan as a standard. The reaction was determined at 532 nm using a thiobarbituric acid reagent by the method described by Buege and Aust (1978). TBARS content was expressed as nanomoles of TBARS per milligram of protein.
Acethylcholinesterase activity
Acetylcholinesterase activity (AChE) was measured at room temperature in duplicate according to the colorimetric method of Ellman et al. (1961). In a typical assay, 1,050 μL of 0.1 mol/L phosphate buffer, 50 μL of 0.008 mol/L dithiobisnitrobenzoate, 50 μL of supernatant (S9), and 50 μL of 0.045 mol/L acetylthiocholine substrate were successively added. The enzymatic reaction rate was quantified spectrophotometrically at 412 nm against a blank without substrate for each activity measurement. In order to subtract the spontaneous hydrolysis of substrate, a second blank was performed without a sample. Enzyme activity was recorded over 5 min after addition of the substrate concentration. AChE activity was expressed as specific activity (nanomole of substrate hydrolysed per minute per milligram of protein).
Metallothioneins content)
Metallothioneins (MTs) content was evaluated in triplicate in gills and digestive gland homogenates according to the spectrophotometric method described by Viarengo et al. (1997) based on cystein titration and using reduced glutathione as standard.
The protein concentrations of samples were measured using Bradford’s method (Bradford 1976) with bovine serum albumin as the standard.
Statistical analysis
The results were expressed as means ± standard deviation (SD; n = 6). SPSS software (version 17.0) was used for statistical analysis. The biochemical data were first tested for normality and homogeneity of variance to meet statistical demands. One-way analysis of variance and Duncan’s test were used to analyze raw biomarker data for comparison of responses between sites. All differences were considered as significant at p < 0.05. Different letters a, b, c, and d indicated significant differences between groups. Asterisks (single, double, and triple) indicate significant differences between seasons at p < 0.05, p < 0.01, and p < 0.001, respectively.
Correlation tests were also carried out between organic and inorganic contaminants and biomarkers and physico-chemical parameters. These comparisons were performed by applying the Pearson coefficient (SPSS Soft. 17.0) to determine the strength of the relation between two variables.
Integrated biomarkers response determination
A method for combining all the measured biomarker responses into one general “stress index” termed “integrated biomarker response” (IBR; Beliaeff and Burgeot 2002) was applied to evaluate the integrated impact of toxicants from different monitoring sites. The procedure described below was used (for each biomarker):
-
1.
Calculation of mean and SD for each station.
-
2.
Standardization of data for each station: \( \left. {{{{X_i^{\prime}\left( {{X_i} - \overline x } \right)}} \left/ {S} \right.}} \right) \), where X i ′ is the standardized value of the biomarker, X i is the mean value of a biomarker from each station, \( \overline x \) is the mean of the biomarker calculated for all the stations, and S is the standard deviation calculated for the station-specific values of each biomarker.
-
3.
Using standardized data, addition of the value obtained for each station X i ′ to the absolute (non-negative) value of the minimum value B = X i ′ + │X min│ in the data: B = X i ′ + │X min│, where B = X i ′ + │X min│ is the minimum value for all stations and for each biomarker and B is the score of each biomarker and for each station. Result: adjusts the lowest value in the set to 0 (for all the biomarkers treated this way).
-
4.
Calculation of star plot areas by multiplication of the obtained value of each biomarker (B i ) with the value of the next biomarker, arranged as a set.
-
5.
Summing up of all values. The corresponding IBR value is \( \left\{ {\left[ {{{{\left( {{B_{{1}}} \times {B_{{2}}}} \right)}} \left/ {{2}} \right.}} \right] + \left[ {{{{\left( {{B_{{2}}} \times {B_{{3}}}} \right)}} \left/ {{2}} \right.}} \right] + \cdots + \left[ {{{{\left( {{B_n}_{{ - {1}}} \times {B_n}} \right)}} \left/ {{2}} \right.}} \right]} \right\} \) and B n are two scores of two successive biomarkers. Result: IBR (average of different arrangements of biomarkers in the set).
Results
Physico-chemical characteristics of seawater
The physico-chemical parameters of water at the sampling sites in February and July are listed in Table 2. The values of temperature, salinity, pH, and dissolved oxygen remained relatively constant throughout each sampling period, and there were no significant differences between the sites. Interestingly, the analysis of temporal variations showed high fluctuations of physico-chemical parameters. Thus, the temperature, salinity, and pH increased, whereas the dissolved oxygen decreased in July at all sampling sites. A significant negative correlation was recorded between the dissolved oxygen and the other physico-chemical parameters (temperature, r = −0.966, p < 0.01; salinity, r = −0.947, p < 0.01; and pH, r = −0.883, p < 0.01).
Chemical concentration in seawater and sediments
The summarized results of the chemical analysis are shown in Table 2. The chemical characterization of the seawater and sediment samples indicates that S2 and S5 are the most contaminated sites of Bizerta Lagoon in February and July; they contain mixtures of contaminants (metals and hydrocarbons). The levels of Fe, Pb, Mn, Ni, Zn, and Cr were higher in seawater and sediments in S2 and S5 in February and July than in the other sampling sites. Moreover, the hydrocarbons were found at high level in sediments of S2 and S5 in February and less in July.
Biomarker responses
Five biochemical biomarkers assayed in the digestive gland of crabs from Bizerta Lagoon sites were significantly different between sites and time. The levels of CAT activity and TBARS level for gills and digestive glands differed among sites and between times (Fig. 2). In February, gills CAT was decreased in all the sites of Bizerta Lagoon when compared to S1. A similar profile was observed in the digestive gland. The highly decreased gills CAT activity was about 48.58 % at S5 and about 38.78 and 42.48 % in the digestive gland of crabs at S3 and S5, respectively (Fig. 2). Further CAT decrease was observed in July and in tissues of crabs. We estimated 40.47, 35.51, and 43.33 % in gills and 45.58, 43.89, and 44.86 % in the digestive gland of crabs collected from S2, S3, and S5, respectively. In relation to temporal variation, a high CAT activity was observed in both organs of crabs from polluted sites, but the enhancement was significant only for gills CAT of crabs from S2 and S3.
Responses of catalase (CAT), glutathione S-transferase (GST), acetylcholinesterase (AChE) activities, and metallothionein (MTs) and lipid peroxidation (TBARS) levels in the gills and digestive gland of crabs collected from the five sites in February and July. All results are expressed as mean ± SD; n = 6. Letters a, b, c, and d indicate significant differences between sites. *p < 0.05, **p < 0.01, and ***p < 0.001 indicate significant differences between seasons, respectively
A significant negative correlation was recorded between the CAT activity and AChE in gills and digestive gland at r = −0.704, p < 0.05 and r = −0.777, p < 0.01, respectively.
Concerning TBARS level, it was significantly increased at S2 and S5 in gills and digestive gland of crabs sampled in both times (February and July) in comparison to those at S1. The increase of gill TBARS level was 27.85 and 35.46 % at S2 and 30.41 and 29.78 % at S5. In the digestive gland, the increase was about 87.82 and 16.28 % at S2 and 30.80 and 40.36 % at S5. Significant temporal variations in TBARS levels were observed in both organs of crabs from reference and polluted sites. Crabs collected in February showed a higher TBARS level than those of crabs collected in July.
Interestingly, a significant negative correlation between TBARS level and MTs was detected in gills (r = −0.897, p < 0.01). The seasonal variations of TBARS level in both organs (gills and digestive gland) show significant negative correlations with physico-chemical parameters. The increase of salinity and seawater in July was negatively associated with gill (r = −0.692, p < 0.05; pH r = −0.706, p < 0.05) and also digestive gland TBARS levels (salinity, r = −0.683, p < 0.05; pH, r = −0.717, p < 0.05).
The GST activity increased in both tissues of crabs at S2 and S5 in February when compared to the crabs from S1. The induction was 1.36- and 1.27-fold in gills and 1.27- and 1.21-fold in digestive gland of crabs at S2 and S5 compared to those of S1. In July, higher levels were found in the gills of the S2 and S5 crabs than in those of S1. In digestive gland, the GST activity was higher in February than in July; however, in gills, the enhancement of activity was significant only in crabs from S1, S2, and S5.
The temporal variation of digestive gland GST activity shows a significant negative correlation between GST and some physico-chemical parameters (temperature, r = −0.883, p < 0.01; salinity, r = −0.921, p < 0.01; pH, r = −0.925, p < 0.01) and significant positive correlation with dissolved oxygen (r = 0.852, p < 0.01).
Significant correlations were recorded between the GST activity in gills and other biochemical biomarkers (TBARS, r = 0.909, p < 0.01; MTs, r = −0.871, p < 0.01; AChE, r = 0.697, p < 0.05). Indeed a significant positive correlation was recorded between the GST activity in digestive gland and TBARS (r = 0.865, p < 0.01).
Differences of digestive gland and gills MTs in biomarker levels among Bizerta Lagoon sites were found. In February as in July, the MTs levels in both tissues and the maximum of induction were observed in the digestive gland of crabs at S2 (66.68 and 65.06 % in February and July, respectively) and S5 (67.28 and 75.49 % in February and July, respectively). These differences in MTs levels between sites were well noted in the digestive gland (S2 + S5 > S3 + S4 > S1) and less in gills (S5 > S2 > S3 + S4 + S1) of crabs sampled at different sites.
Concerning temporal variation, the MTs levels in the digestive gland and gills of crabs were greater in July than in February. The enhancement in MTs levels was observed in most of the sampling sites and in both organs of crabs from the reference and polluted sites.
Significant negative correlations between MTs and AChE were recorded in the gills and digestive gland (r = −0.656, p < 0.05; r = −0.820, p < 0.01), respectively.
AChE activity changes significantly in the crabs from the five sites. In both tissues and in February as in July, AChE was significantly reduced in crabs from all the sampling sites when compared to S1. Moreover, a critical AChE reduction was observed in both tissues of crabs from S2 and S5 in both seasons. The lowest AChE activity was found in the digestive gland of crabs collected in July at S5, S2, S3, and S4, representing 94.54, 94.13, 77.28, and 65.74 % decrease, respectively, when compared to S1.
In relation to temporal variations, we observed different patterns in AChE activity of crabs from reference and polluted sites. In gills, a significant AChE activity was observed between February and July in crabs from S1 and polluted sites. In digestive gland, similar temporal patterns in AChE activity was observed in crabs from polluted sites; a significant AChE activity was higher in February than in July, whereas in those from S1 a non-significant AChE activity was observed between February and July.
Positive correlations were recorded between the AChE in gills and chemical parameters in water (Cd, r = 0.925, p < 0.01; Cu, r = 0.821, p < 0.01; Mn, r = 0.895, p < 0.01; Ni, r = 0.846, p < 0.01) and in sediment (Cd, r = 0.688, p < 0.05; Fe, r = 0.653, p < 0.05; Pb, r = 0.912, p < 0.01; Mn, r = 0.685, p < 0.05; Ni, r = 0.688, p < 0.05; hydrocarbons, r = 0.730, p < 0.05).
Relationships between abiotic factors, contaminants, and biomarkers
The multiple Pearson correlation test allowed to find significant negative correlations between the dissolved oxygen and the other physico-chemical parameters (temperature, r = −0.966, p < 0.01; salinity, r = −0.947, p < 0.01; pH, r = −0.883, p < 0.01).
Concerning the possible links of environmental data to crab biomarkers, positive correlations were recorded between the gills AChE and chemical parameters in water (Cd, r = 0.925, p < 0.01; Cu, r = 0.821, p < 0.01; Mn, r = 0.895, p < 0.01; Ni, r = 0.846, p < 0.01) and in sediment (Cd, r = 0.688, p < 0.05; Fe, r = 0.653, p < 0.05; Pb, r = 0.912, p < 0.01; Mn, r = 0.685, p < 0.05; Ni, r = 0.688, p < 0.05; hydrocarbons, r = 0.730, p < 0.05).
Gills lipid peroxidation level (TBARS) was correlated with contaminants in water (Cd, r = 0.685, p < 0.05; Mn, r = 0.670, p < 0.05; Ni, r = 0.800, p < 0.01) and in sediment (Ni, r = 0.644, p < 0.05; Pb, r = 0.754, p < 0.05). In digestive gland, the lipid peroxidation level was associated with contaminants in water (Co, r = 0.821, p < 0.01; Cr, r = 0.716, p < 0.05; Ni, r = 0.725, p < 0.05).
MTs are cellular proteins rich in thiols groups highly inducible by metals. Thus, a positive correlation between metals and MTs induction in gills and digestive gland was found (data not shown). The temporal variation of MTs level was negatively associated with metals. Gills MTs level were correlated with metals in seawater (Cd, r = −0.807, p < 0.01; Ni, r = −0.720, p < 0.05) and in sediment (Cd, r = −0.761, p < 0.05; Ni, r = −0.820, p < 0.01; Pb, r = −0.718, p < 0.05; Co, r = −0.688, p < 0.05) and with sediment hydrocarbons (r = −0.770, p < 0.01). In digestive gland, the MTs level was also associated with metals in seawater (Cd, r = −0.742, p < 0.05; Cu, r = −0.830, p < 0.01) and with sediment Pb (r = −0.730, p < 0.05).
This result is not surprising because the enhancement of MTs level was associated with a decrease of metals in the environment and the MTs increase may be explained by the involvement of other factors such as pH of seawater (pH, r = 0.645, p < 0.05) or salinity (salinity, r = 0.603, p < 0.065).
The temporal variation of digestive gland GST activity shows a significant negative correlation between GST and some physico-chemical parameters (temperature, r = −0.883, p < 0.01; salinity, r = −0.921, p < 0.01; pH, r = −0.925, p < 0.01) and a significant positive correlation with dissolved oxygen (r = 0.852, p < 0.01).
The effects of stressful conditions may induce toxicity through different sub-cellular pathways. Thus, a link between altered biochemical parameters may be found. Interestingly, significant positive correlations between TBARS level and MTs were found in gills in February (r = 0.900, p < 0.05) and in July (r = 0.950, p < 0.05) and in digestive gland (r = 0.887, p < 0.05) in July. The temporal variations of TBARS level in both organs (gills and digestive gland) show significant negative correlations with physico-chemical parameters. The increase of salinity and seawater in July was negatively associated with gills (r = −0.692, p < 0.05; pH, r = −0.706, p < 0.05) and also digestive gland TBARS levels (salinity, r = −0.683, p < 0.05; pH, r = −0.717, p < 0.05).
Gills GST activity was significantly correlated with MTs in February (r = 0.936, p < 0.05) and in July (r = 0.984, p < 0.01). The temporal variations of gills GST activity were significantly correlated with other biochemical biomarkers (TBARS, r = 0.909, p < 0.01; MTs, r = −0.871, p < 0.01; AChE, r = 0.697, p < 0.05). In digestive gland, GST activity was related to TBARS and MTs in February (TBARS, r = 0.887, p < 0.05; MTs, r = −0.977, p < 0.01). Indeed a significant positive correlation was recorded between the GST activity in digestive gland and MDA (r = 0.865, p < 0.01).
Gills AChE was negatively linked to MTs in February (r = −0.969, p < 0.01). Indeed the temporal variation of AChE activity was associated with MTs in gills (r = −0.656, p < 0.05) and in digestive gland (r = −0.820, p < 0.01).
Integrated biomarker response
In order to diagnose and compare the overall physiological state of crabs studied at the Bizerta Lagoon and S1, the IBR index was applied to the measured five biomarkers. IBR in C. maenas collected from different sites was evaluated and is represented as star plot (Fig. 3). In general, IBR values show a large range of variation at different monitoring sites. The IBR values tended to increase in contaminated sites as an indicator of environmental stress. Moreover, IBR response depends on sampling sites, analyzed tissue, and season. Thus, in February, IBR values are given the following order in gills S2 > S5 > S3 > S4 > S1 and S5 > S2 > S4 > S3 > S1 in digestive gland. In July, distinct patterns of star plots were obtained in gills and digestive gland. IBR values are given the following order in gills S2 > S5 > S4 > S1 > S3 and S2 > S3 > S4 > S5 > S1 in digestive gland.
Considering each site individually (Fig. 4), the biomarker with great contribution in S2 and S5 (the most impacted sites) is MTs. Globally, deteriorating crab health indicates the presence of metals and other compounds of prooxidant nature such as hydrocarbons (Table 2).
Discussion
The routine physico-chemical parameters of water did not change significantly at different stations in Bizerta Lagoon. However, high seasonal fluctuations of physico-chemical parameters were observed in the current study and previously reported in the field studies (Mahmoud et al. 2010; Banni et al. 2011). The high seasonal physico-chemical fluctuations are among the ecological characteristics of Bizerta Lagoon. Thus, the low salinity, temperature, pH, and high oxygenation values measured in winter can be explained by the large freshwater discharge (20 Mm3/year) from Ichkeul Lake through the Tinja River (Harzallah 2002; Mahmoud et al. 2010). Salinity was higher in the polluted sites in summer. Our results are in accordance with those found by Amado et al. (2006) in the Patos lagoon (Brazil) where the salinity was higher in summer in “Coroa do Boi” (polluted site).
The pollution of hydrocarbons and trace metals at S2 and S5 is the most serious problem compared with that at the other sites. It has been demonstrated that at least five different contamination sources to Bizerta Lagoon were identified: metallurgy activities, industrial contamination, urban effluent, releases of cements, and agricultural activities (Yoshida et al. 2002a, b).
Moreover, Menzel Bourguiba (S2), a heavily metallurgic industrialized area, presents sediment contamination by heavy metals at toxic levels (Yoshida et al. 2002a, b) and PAHs (Trabelsi and Driss 2005; Louiz et al. 2007, 2008).
The contamination of the environment causes the generation of reactive oxygen species (ROS) (Lima et al. 2007) that induce or inhibit the activity of some biological composites called biomarkers (e.g., GSH, CAT…). Crabs are susceptible to the effects of ROS and have developed effective antioxidant defenses, including antioxidant substances (vitamin E, reduced glutathione, and carotenoids) and enzymes (CAT, GR, GPx) (Pereira et al. 2009). Oxidative stress occurs when the rate of ROS generation exceeds the antioxidant defense system. Its deleterious effects include oxidation of proteins, DNA, and carbohydrate components, as well as peroxidation of unsaturated lipids in cell membranes (Almeida et al. 2007). CAT is known to protect the cell by reducing H2O2 to H2O. CAT responds to a wide range of contaminants capable of ROS production such as PAHs, polychlorinated biphenyls (PCBs), heavy metals, and pesticides by increase or decrease of enzyme activities. Thus, CAT responses were observed in many field studies using bivalve or crustaceans as bioindicators (Regoli and Principato 1995; Jebali et al. 2007; Vioque-Fernández et al. 2009). The results revealed that CAT activity was high in gills and digestive gland of the control specimen collected at S1 compared to those contaminated of Bizerta Lagoon (S2, S3, S4 and S5). Such trends in CAT activities can be found in other native organisms at polluted sites according to the levels and duration of pollutant exposure. The catalase repression is similar to those reported by Ghedira et al. (2011) in gills and digestive gland of C. maenas collected from Bizerta Lagoon as a contaminated area. Similar results were also found by Mahmoud et al. (2010) in gills and digestive gland in the mollusk Fulvia fragilis collected from two sites in Bizerta Lagoon.
Decreased CAT activities in crabs at polluted sites have been found in addition to a reduced capability of neutralizing ROS and an increased susceptibility to oxidative stress (Montes-Nieto et al. 2007, 2010).
Moreover, the antioxidant enzyme CAT was significantly low in the digestive gland of C. maenas collected from a polluted area (Domingo Rubio, Spain) in summer (Montes-Nieto et al. 2010). Decreased CAT activity has also been reported in mice dwelling at sites contaminated by toxic metals (Montes-Nieto et al. 2007). CAT activity was also shown as a sensitive biomarker to pollutants in other studies since the digestive gland of crayfish Procambarus clarkii from a polluted area showed lower activity than the reference animals (Vioque-Fernández et al. 2009). Pan and Zhang (2006) showed that antioxidant enzymes, including CAT, decrease with time or with exposure to high metal levels.
The biological importance of CAT is more evident from other studies due to the fact that H2O2 is the main cellular precursor of the hydroxyl radical, which is a highly reactive and toxic form of ROS. Enzymes of the antioxidant defense system can be induced by the enhanced production of ROS as a protection mechanism against oxidative stress or inhibited when deficiency of the system occurs (Wang et al. 2012). In fact, Pereira et al. (2010) showed an induction in CAT activity in feral fish Liza aurata collected from Óbidos lagoon (Portugal) in summer. The induction of CAT activity has been also described in fish liver exposed to contaminants under field conditions (Orbea et al. 2005). Wang et al. (2012) showed an increase of CAT activity after transplantation of the clams Ruditapes philippinarum for 7 days in situ along Maluan Bay (China). Vlahogianni et al. (2007) found an increase in antioxidant enzyme activity in transplanted species with increasing metal xenobiotics. Regoli et al. (2004) showed an increase in CAT activity during the first 2 weeks of mussel transplantation at an industrialized harbor of NW Italy followed by a progressive decrease.
The high inhibition of CAT activity may have a critical consequence in crabs. This is with agreement with the high levels of lipid peroxidation (TBARS) observed in gills and digestive gland of crab at sites S2 and S5. Lipid peroxidation alters the normal structural and functional properties of the cell, ultimately leading to cytotoxicity by dismantling the membrane structure in association with various adaptive reactions and changes in physiological status (Almeida et al. 2007). The higher lipid peroxidation observed in both organs of crabs from S2 and S5 in February than in July may be related to the discharges of urban and industrial wastes and runoff, leading to the chemical contamination of the lagoon by various toxic compounds such as metals and hydrocarbons (Mahmoud et al. 2010).
Increased levels of lipid peroxidation have been observed in crab upon exposure to different stressful field conditions.
Thus, lipid peroxidation inductions were evident in both organs of crabs from sites receiving metal and hydrocarbon pollutant inputs, particularly S2 and S5. Furthermore, the positive correlations between analyzed metals such as Cd, Ni, and Pb and lipid peroxidation may explain the high TBARS levels in S2 and S5, a clear oxidative stress indication. In addition, high lipid peroxidation was also associated with MTs induction in crabs from the most impacted sites (S2 and S5). However, induced MTs could not eliminate the toxic metals due to the high levels observed in seawater and sediments at sites S2 and S5. Further studies focusing on metal and hydrocarbon accumulations in target organs (gills and digestive gland) are in progress to elucidate the biochemical effects and histopathological lesions.
Montes-Nieto et al. (2010) also showed that the pattern of lipid peroxidation was higher in crabs from metal-contaminated sites than those from the reference sites. Our results confirm those of Mahmoud et al. (2010) in the mollusk F. fragilis collected from Bizerta Lagoon. Furthermore, the seasonal fluctuations of lipid peroxidation (TBARs), neutral lipid, and lipofuscin accumulations were significant in the clam R. decussatus from Bizerta Lagoon, and a higher induction was noted in autumn and summer (Banni et al. 2009).
GST activity is a biomarker of defense (Michel et al. 1998; Hajime et al. 2005). It is obviously a defense against oxidative damage and peroxidative products of DNA and lipids (George 1994). GST, as phase II enzymes, is induced by a variety of natural and synthetic compounds (Tlili et al. 2010). It was highly induced by pollutants at S2 and S5. This result confirms those in mammals (Ruiz-Laguna et al. 2001) and in bivalves (Romero-Ruiz et al. 2006; Funes et al. 2006) exposed to high metal concentrations, but at S3 and S4 crabs displayed lower GST at problem sites compared to S1 reference site, although in general with no significant differences. These results are similar to those found in crayfish P. clarkii (Vioque-Fernández et al. 2009). In winter, we showed higher activities of GST. Crabs (C. maenas) from the Óbidos lagoon also exhibited higher activities of GST in winter at the same site, which were related to a high availability of metals in that period (Pereira et al. 2010).
The high GST activity in C. maenas from the most polluted sites (S2 and S5) may be related to the hydrocarbons found in sediments in both sampling times as released by nearby industries. GST response to toxic chemicals is induced by organic contaminants as part of the phase II biotransformation pathway (Tsangaris et al. 2010). In addition, the high GST activity was correlated with lipid peroxidation and MTs induction. This induction in GST activity could indicate a defense of crabs against oxidative stress damage produced by adverse environmental contaminants such as metals and hydrocarbons. Other field studies showed that the induction of the GST activity occurred in the presence of hydrocarbons in sediments (Lu et al. 2009) and trace metals (AS, Cd, Cr, Fe, Hg, Mn, and Pb) Martin-Diàz et al. (2008). Similar results reported in C. maenas from a eutrophic and metal-contaminated coastal system show high GST activity (Pereira et al. 2009). Wang et al. (2011) showed that, with Cd contamination, R. philippinarum increased the activity of GSTs.
Metallothioneins are considered as a metal-responsive biomarker, especially in aquatic ecosystems (Roesijadi 1994; Alhama et al. 2006; Vioque-Fernández et al. 2009). Their expression in tissues is therefore regarded as an indicator of metal contamination and widely used as a tool for biomonitoring progress (Viarengo et al. 1999; Banni et al. 2007). In crabs, the usefulness of MTs as an indicator of environmental quality was previously demonstrated (Pedersen et al. 1997; Martínez et al. 1996, 1999; Maria et al. 2009). The highly induced MTs in the most polluted sites S2 and S5 in both organs of crabs in February and July suggested a fraction of bioavailability of trace metals. Maria et al. (2009) demonstrated MTs induction in C. maenas transplanted to Olhão site (Ria Formosa lagoon) which is contaminated by metals and PAHs.
The temporal variation of MTs level was negatively correlated with some metals such as Pb in sediment (r = −0.730, p < 0.05), Cd (r = −0.742, p < 0.05), and Cu in water (r = −0.830, p < 0.01). This result is not surprising because the enhancement of MTs level was associated with a decrease of metals in the environment (seawater and sediments); the MTs increase may be explained by the involvement of other abiotic factors such as pH and salinity.
Our findings are consistent with other studies, which also showed high MTs synthesis in organisms from metal-polluted sites (Vioque-Fernández et al. 2009).
The measure of AChE inhibition is widely recognized as a biomarker of exposure to neurotoxic compounds in invertebrates, including crustaceans (Lundbaye et al. 1997; Brown et al. 2004). Other classes of compounds, such as heavy metals (Lionetto et al. 2003), may influence the AChE activity of an organism by altering the pathway of enzyme synthesis or simply by affecting the general health of the organism by reducing enzyme production. AChE activity in the crabs from S2 and S5 was dramatically more inhibited than those from the other sites. Moreover, a significant correlation between AChE and CAT was found in both organs. These findings may explain that oxidative stress alters the structure of AChE structure by oxidizing thiols.
The multi-biomarkers approach has been inspired by common procedures in human epidemiology where many responses are interpreted to diagnose a disease. A set of biochemical biomarkers would provide a more valid basis for interpretation of ecotoxicological surveys, allowing information to be summarized in the form of multivariate data set (Beliaeff and Burgeot 2002). As different biomarkers respond to different stressors, an advantage exists in using a set to assess the condition of a target species and the quality of the environment. The simple biomarker-based indices were applied to rank sampling sites and reflected contamination gradients, providing decision-making support tools to evaluate ecosystem health. As stated before, it is important to consider the magnitude and potential interaction between contaminants and biomarker responses. The results revealed that MTs have the greater contribution in both tissues of crabs from the most impacted sites (S2 and S5) and at both sampling times (February and July). This is in agreement with the high levels of metal and hydrocarbon contaminations found.
In the present work, the IBR values found in the five sites are in good agreement with the hydrocarbon and trace metal concentrations detected in water and sediments. The higher values were found in S2 and S5, stressful places for crabs to live in. Similarly, Damiens et al. (2007) found that the IBR values calculated from AChE, GST, and CAT activities and TBARS concentrations in the three successive experiments were in good agreement with copper and PCB concentrations in transplanted mussels but not with PAH concentrations.
Lu et al. (2010) used the integrated biomarker response index to combine six biomarkers (EROD, GST, GPX, GSH, SOD, and TBARS) and quantified the impact of pollution on the transplanted fishes (Carassius auratus) at polluted sites of Taihu Lake (China). IBR analyses allowed a good discrimination between different polluted sites and are in good agreement with PCBs and OCPs contents in the sediments.
Conclusion
A field study was conducted to evaluate the responses of biochemical biomarkers of crabs (C. maenas) to contamination levels in Bizerta Lagoon (Tunisia). The multiple biomarker responses measured in two distinct tissues provide discrimination between sites with different levels of contamination. The high-alteration enzymatic activities of CAT, GST, AChE, TBARs, and MTs levels in gills and digestive gland of crabs from S2 and S5 were associated with metals and hydrocarbons in sediments and seawater. The temporal variation of biomarkers was associated with chemical contamination and also with the higher fluctuation of natural abiotic parameters (dissolved oxygen, pH, salinity, and temperature).
References
Aissa, P., 1991. Ecologie des nématodes libres de la lagune de Bizerte. Dynamique et biocénotique. Thèse de Doctorat d’Etat. Faculté des Sciences, Tunis
Alhama J, Romero-Ruiz A, Lopez-Barean J (2006) Metallothionein quantification in clams by reversed-phase high-performance liquid chromatography coupled to fluorescence detection after monobromobimane derivatization. Chromatography A 1107(1–2):52–58
Almeida EA, Dias Bainy AC, Melo Loureiro AP, Martinez RR, Miyamoto S, Onuki J, Fujita BL, Machado Garcia CC, Manso PF, Eliza RG, Sigolo CA, Barbosa BC, Gracioso Martins AM, Gennari de Medeiros MH, Di Mascio P (2007) Oxidative stress in Perna perna and other bivalves as indicators of environmental stress in the Brazilian marine environment: antioxidants, lipid peroxidation and DNA damage. Comp Biochem Physiol Part A 146:588–600
Amado LL, Robaldo RB, Geracitano L, Monserrat JM, Bianchini A (2006) Biomarkers of exposure and effect in the Brazilian flounder Paralichthys orbignyanus (Teleostei: Paralichthyidae) from the Patos Lagoon estuary (Southern Brazil). Mar Pollut Bull 52:207–213
Banni M, Dondero F, Jebali J, Guerbej H, Boussetta H, Viarengo A (2007) Assessment of heavy metal contamination using real-time PCR analysis of mussel metallothionein mt10 and mt20 expression: a validation along the Tunisian coast. Biomarkers 12:369–383
Banni M, Bouraoui Z, Clerandeau C, Narbonne JF, Boussetta H (2009) Mixture toxicity assessment of cadmium and benzo[a]pyrene in the sea worm Hediste diversicolor. Chemosphere 77:902–906
Banni M, Negri A, Mignone F, Boussetta H, Viarengo A, Dondero F (2011) Gene expression rhythms in the mussel Mytilus galloprovincialis (Lam.) across an annual cycle. PLoS One 6(5):e18904. doi:10.1371/journal.pone.0018904
Beliaeff B, Burgeot T (2002) Integrated biomarker response (IBR): a useful graphical tool for ecological risk assessment. Environ Toxicol Chem 21:1316–1322
Bonacci S, Corsi I, Focardi S (2008) Cholinesterase activities in the scallop Pecten jacobaeus: characterization and effects of exposure to aquatic contaminants. Sci Total Environ 392:99–109
Bouraoui Z, Banni M, Ghedira J, Clerandeau C, Narbonne JF, Boussetta H (2009) Evaluation of enzymatic biomarkers and lipoperoxidation level in Hediste diversicolor exposed to copper and benzo[a]pyrene. Ecotoxicol Environ Saf 72:1893–1898
Boutet I, Tanguy A, Auffret M, Riso R, Moraga D (2002) Immunochemical quantification of metallothioneins in marine molluscs: characterisation of a metal exposure bioindicator. Environ Toxicol Chem 21:1009–1014
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram of protein utilizing the principal of protein-dye binding. Anal Biochem 72:248–254
Brown RJ, Galloway TS, Lowe D, Browne MA, Dissanayake A, Jones MB, Depledge MH (2004) Differential sensitivity of three marine invertebrates to copper assessed using multiple biomarkers. Aquat Toxicol 66:267–278
Buege JA, Aust SD (1978) Microsomal lipid peroxidation. Methods Enzymol (52):302–310
Claiborne A (1985) Catalase activity. In: Greenwald RA (ed) CRC handbook of methods in oxygen radical research. CRC, Florida, pp 283–284
Damiens G, Gnassia-Barelli M, Loquès F, Roméo M, Salbert V (2007) Integrated biomarker response index as a useful tool for environmental assessment evaluated using transplanted mussels. Chemosphere 66(3):574–583
Dellali M, Gnassia Barelli M, Romeob M, Aissa P (2001) The use of acetylcholinesterase activity in Ruditapes decussatus and Mytilus galloprovincialis in the biomonitoring of Bizerta Lagoon. Comp Biochem Physiol Part C 130:227–235
Dellali M, Roméo M, Gnassia-Barelli M, Aissa P (2004) A multivariate data analysis of the clam Ruditapes decussatus as sentinel organism of the Bizerta Lagoon (Tunisia). Water Air Soil Pollut 44:131–156
Ellman GL, Courtney KD, Jr Andres V, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Funes V, Alhama J, Navas JI, López-Barea J, Peinado J (2006) Ecotoxicological effects of metal pollution in two mollusc species from the Spanish South Atlantic littoral. Environ Pollut 139:214–223
George SG (1994) Enzymology and molecular biology of phase II xenobiotic-conjugating enzymes in fish. In: Malins DC, Ostrander GK (eds) Aquatic toxicology: molecular, biochemical and cellular perspectives. Lewis, Boca Raton, pp 37–85
Ghedira J, Jebali J, Banni M, Chouba L, Boussetta H, López-Barea J, Alhama J (2011) Use of oxidative stress biomarkers in Carcinus maenas to assess littoral zone contamination in Tunisia. Aquat Biol 14:87–98
Habig WH, Pabst MJ, Jakoby WB (1974) The first enzymatic step in mercapturic acid formation. Biol Chem 249(22):7130–7139
Hajime O, Ozaki K, Yoshikawa H (2005) Identification of cytochrome P450 and glutathione-S-transferase genes preferentially expressed in chemosensory organs of the swallowtail butterfly, Papilio xuthus L. Insect Biochem Mol Biol 8:837–846
Hamza-Chaffai A, Pellerin J, Amiard JC (2003) Health assessment of a marine bivalve Ruditapes decussatus from the Gulf of Gabès (Tunisia). Environ Int 28(7):609–617
Harzallah, A., 2002. Etat actuel et évolution de l’exploitation halieutique des lagunes (Bizerte). Institut National des Sciences et Technologie de la Mer, p 128
Jebali J, Banni M, de Almeida E, Boussetta H (2007) Oxidative DNA damage levels and catalase activity in the clam Ruditapes decussatus as pollution biomarkers of Tunisian marine environment. Environ Monit Assess 124:195–200
Jebali J, Ben Khedher S, Ghedira J, Kamel N, Boussetta H (2011) Integrated assessment of biochemical responses in Mediterranean crab (Carcinus maenas) collected from Monastir Bay Tunisia. Environ Sci 23(10):1714–1720
Kelly, C.A., Law, R.J., Emerson, H.S., 2000. Methods of analyzing hydrocarbons and polycyclic aromatic hydrocarbons (PAH) in marine samples. The Centre for Environment, Fisheries and Aquaculture Science (CEFAS) Science Series. Aquatic environment protection: analytical methods, no. 12. Lowestoft, England
Khessiba A, Hoarau P, Gnassia-Barelli M, Aissa P, Roméo M (2001) Biochemical response of the mussel Mytilus galloprovincialis from Bizerta (Tunisia) to chemical pollutant exposure. Arch Environ Contam Toxicol 40:222–229
Khessiba A, Roméo M, Aissa P (2005) Effects of some environmental parameters on catalase activity measured in the mussel (Mytilus galloprovincialis) exposed to lindane. Environ Pollut 133:275–281
Lima I, Moreira SM, Osten JRV, Soares AMVM, Guilhermino L (2007) Biochemical responses of the marine mussel Mytilus galloprovincialis to petrochemical environmental contamination along the north-western coast of Portugal. Chemosphere 66:1230–1242
Lionetto MG, Caricato R, Giordano ME, Pascariello MF, Marinosci L, Schettino T (2003) Integrated use of biomarkers (acetylcholinesterase and antioxidant enzyme activities) in Mytilus galloprovincialis and Mullus barbatus in an Italian coastal marine area. Mar Pollut Bull 46:324–330
López-Barea J (1995) Biomarkers in ecotoxicology: an overview. Arch Toxicol Suppl 17:57–79
Louiz I, Menif D, Ben-Attia M, Ben-Hassine OK (2007) Incidence of skeletal deformities in three species of Gobiidae from Bizerta Lagoon (Tunisia). Cybium 31:209–216
Louiz I, Kinani S, Gouzec ME, Ben-Attiab M, Menifa D, Bouchonnetd S, Porcherc JM, Ben-Hassinea OK, Aït-Aïssa S (2008) Monitoring of dioxin-like, estrogenic and anti-androgenic activities in sediments of the Bizerta Lagoon (Tunisia) by means of in vitro cell-based bioassays: contribution of low concentrations of polynuclear aromatic hydrocarbons (PAHs). Sci Total Environ 402:318–329
Lu GH, Wang C, Zhu Z (2009) The dose response relationships for EROD and GST induced by polyaromatic hydrocarbons in Carassius auratus B. Environ Contam Toxicol 82:194–199
Lu GH, Ji Y, Zhang HZ, Wu H, Qin J, Wang C (2010) Active biomonitoring of complex pollution in Taihu Lake with Carassius auratus. Chemosphere 79(5):588–594
Lundbaye AK, Curtis TM, Braven J, Depledge MH (1997) Effects of the organophosphorous pesticide, dimethoate, on cardiac and acetylcholinesterase (AChE) activity in the shore crab Carcinus maenas. Aquat Toxicol 40:23–36
Mahmoud N, Dellali M, El Bour M, Aissa P, Mahmoudi E (2010) The use of Fulvia fragilis (Mollusca: Cardiidae) in the biomonitoring of Bizerta Lagoon: a multimarkers approach. Ecol Indic 10:696–702
Maria, V.L., Santos, M.A., Bebianno., 2009. Contaminant effects in shore crabs (Carcinus maenas) from Ria Formosa Lagoon. Comparative Biochemistry and Physiology, Part C (150): 196–208
Martin-Diàz ML, Blasco J, Sales D, DelValls TA (2008) Field validation of a battery of biomarkers to assess sediment quality in Spanish ports. Environ Pollut 150:631–640
Martínez, M., Del Ramo, J., Torreblanca, A., Díaz-Mayans, J., 1996. Cadmium toxicity accumulation and metallothionein induction in Echinogammarus echinosetosus. J. Environ. Sci. Health. Part A, Environ. Science Eng. Toxic Hazard. Substance Control A31, 1605–1617
Martínez M, Del Ramo J, Torreblanca A, Díaz-Mayans J (1999) Effect of cadmium exposure on zinc levels in the brine shrimp Artemia parthenogenetica. Aquaculture 172:315–325
Michel, X., Narbonne, J.F., Mora, P., Daube` ze, M., Ribera, D., Lafaurie, M.,Budzinski, H., Garrigues, P., 1998. Indicateurs biogéochimiques de pollution des écosystèmes côtiers: expérience du groupe interface chimie-biologie des écosystèmes marins (GICBEM). In: Lagadie, L., Caquet, T. Amiard, JC, Ramade, F. (eds) Utilisation de biomarqueurs pour la surveillance de la qualité de l’environnement. Lavoisier Tec et Doc, Paris, 9–30
Montes-Nieto R, Fuentes-Almagro CA, Bonilla-Valverde D, Prieto-Alamo MJ, Jurado J, Carrascal M, Gómez-Ariza JL, López-Barea J, Pueyo C (2007) Proteomics in free-living Mus spretus to monitor terrestrial ecosystems. Proteomics 7:4376–4387
Montes-Nieto R, García-Barrera T, Gómez-Ariza JL, López-Barea J (2010) Environmental monitoring of Domingo Rubio stream (Huelva Estuary, SW Spain) by combining conventional biomarkers and proteomic analysis in Carcinus maenas. Environ Pollut 158:401–408
Orbea A, Ortiz-Zarragoitia M, Solé M, Porte C, Cajaraville M (2005) Antioxidant enzymes and peroxisome proliferation in relation to contaminant body burdens of PAHs and PCBs in bivalve molluscs, crabs and fish from the Urdaibai and Plentzia estuaries (Bay of Biscay). Aquat Toxicol 58:75–98
Pan, L., Zhang, H., 2006. Metallothionein, antioxidant enzymes and DNA strand breaks as biomarkers of Cd exposure in a marine crab, Charybdis japonica. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, (144: 1): 67-75
Pedersen SN, Lundebye AK, Depledge MH (1997) Field application of metallothionein and stress protein biomarkers in the shore crab (Carcinus maenas) exposed to trace metals. Aquat Toxicol 37:183–200
Pereira P, de Pablo H, Dulce Subida M, Vale C, Pacheco M (2009) Biochemical responses of the shore crab (Carcinus maenas) in a eutrophic and metal-contaminated coastal system (Obidos lagoon, Portugal). Ecotoxicol Environ Saf 72:1471–1480
Pereira P, de Pablo H, Pacheco M, Vale C (2010) The relevance of temporal and organ specific factors on metals accumulation and biochemical effects in feral fish (Liza aurata) under a moderate contamination scenario. Ecotoxicol Environ Saf 73:805–816
Regoli F, Principato G (1995) Glutathione, glutathione-dependent and antioxidant enzymes in mussel, Mytilus galloprovincialis, exposed to metals under field and laboratory conditions: implications for the use of biochemical biomarkers. Aquat Toxicol 31:143–164
Regoli F, Frenzilli G, Bocchetti R, Annarumma F, Scarcelli V, Fattorini D, Nigro M (2004) Time-course variations of oxyradical metabolism, DNA integrity and lysosomal stability in mussels, Mytilus galloprovincialis, during a field translocation experiment. Aquat Toxicol 68:167–178
Reynders H, Bervoets L, Gelders M, De Coen WM, Blust R (2008) Accumulation and effects of metals in caged carp and resident roach along a metal pollution gradient. Sci Total Environ 391:182–195
Rickwood, C.J., Galloway, T.S. 2004. Acetylcholinesterase inhibition as a biomarker of adverse effect: a study of Mytilus edulis exposed to the priority pollutant chlorfenvinphos. Aquatic Toxicology (67: 1): 45-56
Roesijadi G (1992) Metallothioneins in metal regulation and toxicity in aquatic animals. Aquat Toxicol 22(2):81–113
Roesijadi, G., 1994. Behavior of metallothionein-bound metals in a natural population of an estuarine mollusk. Marine Environmental Research, (38: 3):147-168
Roméo M, Gharbi-Bouraoui S, Gnassia-Barelli M, Dellali M, Aissa P (2006) Responses of Hexaplex (Murex) trunculus to selected pollutants. Sci Total Environ 359:135–144
Romero-Ruiz A, Carrascal M, Alhama J, Gómez-Ariza JL, Abian J, López-Barea J (2006) Utility of proteomics to assess pollutant response of clams from the Doñana bank of Guadalquivir Estuary (SW Spain). Proteomics 6(suppl 1):S245–S255
Ruiz-Laguna J, García-Alfonso C, Peinado J, Moreno S, Ieradi L, Cristaldi M, López-Barea J (2001) Biochemical biomarkers of pollution in Algerian mouse (Mus spretus) to assess the effects of the Aznalcóllar disaster on Doñana Park (Spain). Biomarkers 6:146–160
Sáenz LA, Seibert EL, Zanette J, Fiedler HD, Curtius AJ, Ferreira JF, Almeida EA, Freire-Marques MR, Dias-Bainy AC (2010) Biochemical biomarkers and metals in Perna perna mussels from mariculture zones of Santa Catarina, Brazil. Ecotoxicol Environ Saf 73:796–804
Tlili S, Jebali J, Banni M, Haouas Z, Mlayah A, Helal AN, Boussetta H (2010) Multimarker approach analysis in common carp Cyprinus carpio sampled from three freshwater sites. Environ Monit Assess 168:285–298
Trabelsi S, Driss MR (2005) Polycyclic aromatic hydrocarbons in superficial coastal sediments from Bizerte Lagoon, Tunisia. Mar Pollut Bull 50:344–359
Tsangaris C, Kormas K, Strogyloudi E, Hatzianestis I, Neofitou C, Andral B, Galgani F (2010) Multiple biomarkers of pollution effects in caged mussels on the Greek coastline. Comp Biochem Physiol Part C 151:369–378
Viarengo A, Ponzano E, Dondero E, Fabbri R (1997) A simple spectrophotometric method for metallothionein evaluation in marine organisms: an application to Mediterranean and Antarctic mollusks. Mar Environ Res 44:69–84
Viarengo A, Burlando B, Cavaletto M, Marchi B, Ponsano E, Blasco J (1999) Role of metallothionein against oxidative stress in the mussel Mytillus galloprovincialis. Am Physiol Soc 363:1614–1617
Viarengo A, Lowe D, Bolognesi C, Fabbri E, Koehler A (2007) The use of biomarkers in biomonitoring: a 2-tier approach assessing the level of pollutant-induced stress syndrome in sentinel organisms. Comp Biochem Physiol Part C 146:281–300
Vioque-Fernández A, Alves de Almeidab E, López-Barea J (2009) Assessment of Doñana National Park contamination in Procambarus clarkii: integration of conventional biomarkers and proteomic approaches. Sci Total Environ 407:1784–1797
Vlahogianni T, Dassenakis M, Scoullos MJ, Valavanidis A (2007) Integrated use of biomarkers (superoxide dismutase, catalase and lipid peroxidation) in mussels Mytilus galloprovincialis for assessing heavy metals’ pollution in coastal areas from the Saronikos gulf of Greece. Mar Pollut Bull 54:1361–1371
Wang ZS, Yan CZ, Pan QK, Yan YJ (2011) Concentrations of some heavy metals in water, suspended solids, and biota species from Maluan Bay, China and their environmental significance. Environ Monit Assess 175:239–249
Wang Z, Yan C, Vulpe CD, Yan Y, Chi Q (2012) Incorporation of in situ exposure and biomarkers response in clams Ruditapes philippinarum for assessment of metal pollution in coastal areas from the Maluan Bay of China. Mar Pollut Bull 64:90–98
Yoshida, M., Hamadi, K., Ghrabi, A., 2002a. Solid waste landfills and soil/sediment contamination around Bizerte Lagoon: possible pollution sources. RPP-SEPMCL Initial Report p.55-75
Yoshida M, Hamdi H, Abdulnasser I, Jedidi N (2002b) Contamination of potentially toxic elements (PTEs) in Bizerte Lagoon bottom sediments, surface sediment and sediment repository. In: Ghrabi A, Yoshida M (eds) Study on environmental pollution of Bizerte Lagoon. INRST-JICA, Tunisia, pp 13–48
Acknowledgments
This work was supported by a fund from the Ministry of Scientific Research and Technology, University of Sousse, Tunisia (Research Unit of Biochemistry and Environmental Toxicology UR 04AGR05), and “Institution de la Recherche et de l’Enseignement Supérieur Agricole, (IRESA; Tunisia)”. We are most grateful to Pr. Mohamed Ben Khedher for the extensive English revision to improve the quality of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Philippe Garrigues
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Ben-Khedher, S., Jebali, J., Kamel, N. et al. Biochemical effects in crabs (Carcinus maenas) and contamination levels in the Bizerta Lagoon: an integrated approach in biomonitoring of marine complex pollution. Environ Sci Pollut Res 20, 2616–2631 (2013). https://doi.org/10.1007/s11356-012-1156-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-012-1156-x