Abstract
The impacts of the Batts drain on two chronically exposed fish (O. niloticus and C. gariepinus) were assessed using multiple biomarkers. Concentrations of metals in water and sediments (Cu, Zn, Fe, Cd, Pb, and Al) showed significant elevations near the Batts discharges (site 2) compared to the reference site (site 1). The liver and gills of fish collected from site 2 showed marked elevations in the catalase, superoxide dismutase, glutathione peroxidase, and thiobarbituric acid reactive substance levels. In addition, significant reductions in glutathione-reduced contents were also recorded. Tissue and species-specific antioxidant responses were associated with excessive generations of reactive oxygen species, which were visualized fluorescently. Various histological alterations were observed in the gills and livers of both species. These alterations varied between compensatory responses (ex: epithelial thickening and lifting) and irreversible damage (ex: necrotic degeneration). Based on the level of lipid peroxidation and the frequency of histopathological modifications, O. niloticus demonstrated greater resistance to the same level of pollution than C. gariepinus. Using integrated biomarkers to evaluate the real impacts of untreated discharges of the Batts drain is applied for the first time on the selected fish species at the studied sites.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
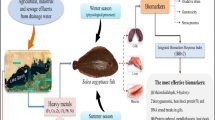
Metal pollution of aquatic bodies, especially closed lakes, is a global environmental problem. Despite the existing environmental conservation laws, the unregulated waste disposal associated with ineffective water management has resulted in the deterioration of many Egyptian lakes (Abdel-Khalek et al. 2020). Lake Qaroun is one of the most affected lakes by anthropogenic activities. It is located in the Fayoum depression on the edge of Egypt’s western desert, about 80 km from Cairo’s southwest, and covers about 226 km2. It is a closed basin with a 40 km length, 5.7 km width, and 4.2 average depth. The Batts is a significant drain that pours its drainage into the lake. This drain collects agricultural discharges (the main cause of metal pollution) from the Fayoum province’s eastern and northeastern regions and directs them to lake Qaroun (Zaghloul et al. 2011). Therefore, lake Qaroun and its biota are suffering from the high pollution loads that come from the Batts drain. A massive amount of metals has accumulated in the aquatic ecosystem as a result of the massive release of untreated discharges, causing several toxicological problems in the exposed organisms (Naz et al. 2021). Aqueous metals are among the most destructive contaminants due to their high bioaccumulation efficiency, persistence, and ability to interact with numerous biological components (Rajeshkumar et al. 2018; Sauliutė et al. 2020). Metal ions can excessively induce reactive oxygen species (ROS) generation through different metal-related reactions. For example, transition metal ions can change the valence state; therefore, they support the Fenton reactions by producing hydroxyl radicals (Temiz and Kargın 2022). Hence, redox-active metals (for example, Cu and Fe) might disrupt essential non-enzymatic and enzymatic antioxidant components and mediate the overproduction of ROS (Hermenean et al. 2015). Excessive ROS production with a defective scavenger capacity of the antioxidant defense system may cause oxidative stress and high lipid peroxidation levels (Massoud et al. 2021). Catalase (CAT), superoxide dismutase (SOD), glutathione peroxidases (GPx), and reduced glutathione (GSH) are part of the cellular antioxidant defense system against the adverse effects of ROS. Relying on the classical chemical analyses of different aquatic pollutants in the environmental components only (ex: water, sediment) cannot determine the real toxicological impacts of these pollutants on various organisms. Therefore, the cellular antioxidant battery and the level of ROS production are recommended as valuable biomarkers during environmental studies (Turan et al. 2020). Moreover, the cell membranes (lipid-rich cellular components) are vulnerable to being targeted by ROS, so thiobarbituric acid reactive substances (TBARS; one of the breakdown products of the lipid peroxidation process) can be used as a good indicator for oxidative damage (Hedayati et al. 2021). Using integrated biomarkers is an effective tool for monitoring environmental stresses and reveals critical information concerning the toxicological effects on chronically exposed living organisms that chemical approaches cannot detect (Greani et al. 2017). Among the most indicative biomarkers, histopathological changes can provide early alarms for chronic stressors on various tissues and the overall health status of fish (Abdel-Khalek et al. 2016). For example, Oreochromis niloticus (Nile tilapia) and Clarias gariepinus (Catfish) are suitable animal models for ecotoxicological field studies due to their high sensitivity and resistance to various aquatic contaminants (Turan et al. 2020; Abu Shnaf et al. 2021). Therefore, the present research aimed to (1) impact analysis of the Batts drain discharges on the health profile of O. niloticus and C. gariepinus involving an integrative approach of oxidative stress and histological biomarkers and (2) compare the different responses of two fish species facing the same pollution level. Applying those integrated biomarkers for the first time at the studied sites can assess the real impacts of the Batts drain on the selected fish species, in addition identify the most susceptible and resistant fish species based on their health profile.
Materials and methods
Sampling sites
The sampling sites were as follows: Site 1 (which represents the reference site) was on the River Nile, south of Giza governorate, Egypt. This site was chosen because of its distance from any source of pollution and effluent discharge. GPS: 30° 00′ 02.034″ N and 31° 12′ 55.7532″ E. Site 2 (at the inlet of the Batts drain): The majority of human activities are nearby the eastern section of lake Qaroun and the largest number of agricultural drains extends from the Batts drain. The Batts drain discharges its contents directly into lake Qaroun; therefore, site 2 was selected close to the discharge point of this drain. GPS: 29° 28′58.98″ N and 30° 49′08.02″ E. (Fig. 1).
Water, sediment, and fish collection
Water sampling was performed using standard procedures by APHA (2017). Water samples (n = 4 per site) were collected from the studied sites in glass containers. For metal analysis, concentrated hydrochloric acid was added to decrease the pH to < 2 of the obtained samples to prevent microbial reactions. Metals in sediment are inert, but they can slowly leak into the water column in response to various events that could endanger ecosystems. To safeguard the aquatic environment, accurate evaluation of metal contamination in sediments near water is required to assess and identify the impact of natural and/or anthropogenic resources. The sediment samples (n = 4 per site) were collected in polyvinyl chloride (PVC) bottles from each site at 20 cm depth, then locked and stored at 4 °C as detailed by Cabrera et al. (1992).
The two selected fish species, Oreochromis niloticus and Clarias gariepinus, were caught during the summer season of 2019 (period of maximum evaporation rate and maximum pollutant concentrations), with expert fishermen’s help. The 36 adult male fish of both species (9 fish/species/site) with mean body weights of 195.51 ± 7.46 and 353.67 ± 24.4 g and average total lengths of 19.95 ± 0.36 and 37.91 ± 1.02 cm for O. niloticus and C. gariepinus, respectively, were obtained from the studied sites. Fish were transferred in large plastic containers with excellent aeration conditions to the ecology laboratory of the Zoology Department, Faculty of Science, Cairo University, using portable oxygen pumps. The dissection of fish was done after decapitation, and the gills and hepatic tissues were isolated for further studies. The Institutional Committee on Animal Care and Use (IACUC) of Cairo University, Faculty of Science, Cairo, Egypt, reviewed and approved this study with the accreditation number CU-I-F-39–19.
Metal analysis
The concentrations of Cu, Zn, Fe, Pb, Cd, and Al metals were determined in water and sediment using Inductively Coupled Argon Plasma, ICAP 6500 Duo Thermo Scientific, England. An amount of 1000 mg/L multi-element certified standard solution (Merck, Germany) was used as a stock solution for instrument standardization. The detection limits are shown in Table 1.
Acid digestion of sediment samples was conducted according to the procedure mentioned by Neugebauer et al. (2000). The samples were dried at 80 °C in an oven for 8 h to dry out entirely. The samples were then digested with concentrated hydrochloric acid. The mixtures were carefully stirred and placed on a heat plate; then, the temperature was gradually increased to 100 °C until the appearance of a clear solution. The resulting solutions were transported to a 25-mL volumetric flask and diluted to a known volume with de-ionized water. The studied metal concentrations were expressed as mg/L in water and as mg/kg dry weight in sediments.
Quality assurance and quality control procedures
To correct background absorption, blank samples (prepared in the same way as samples but without the sample) were prepared along with each sample set. The analysis procedure was validated using standard reference material (Lake Superior fish 1946 NIST, National Institute of Standards and Technology, USA), with metal recovery ranges ranging from 94 to 105%. A standard solution with a known concentration of each metal was used during the measuring process to verify the accuracy of the measurement.
Antioxidant biomarkers
Tissue homogenates for gills and livers were prepared (0.5 g tissue in 2.5 mL of cold specific buffer for each antioxidant biomarker) and centrifuged at 1073 g for 10 min in a cooling centrifuge (4 °C). All antioxidant biomarkers were measured using biodiagnostic kits from Biodiagnostic Dokki, Giza, Egypt. The activities of CAT (CA 25 17) enzymes (U/g proteins) were estimated based on terminating the reaction between CAT and the specified H2O2 volume (after a certain time) with a catalase inhibitor as detailed by Aebi (1984). The specific buffer used in the homogenization was 50 mM potassium phosphate, pH 7.4, 1 mM EDTA, and 1 mL/L Triton X100. As described by Nishikimi et al. (1972), SOD (SD 25 21) can inhibit the phenazine methosulphate–mediated reduction of nitroblue tetrazolium dye. Therefore, SOD activities (U/g proteins) were measured according to the inhibition rate of the previous reaction. The SOD homogenizing solution was 100 mM potassium phosphate, pH 7.0, containing 2 mM EDTA. In the presence of glutathione reductase, the GPx enzyme (GP 2524) can convert organic peroxide to oxidized glutathione, which is then recycled to its reduced state. Based on the reducing properties of the GPx enzyme, the activities of GPx (U/g proteins) were colorimetrically determined as shown by Paglia and Valentine (1967). The buffer constituents were 50 mM phosphate buffer, pH 7.0, containing 5 mM EDTA and 1 mM 2-mercaptoethanol. Utilizing the ability of GSH (GR 25 11) to reduce 5, 5′-dithiobis 2-nitrobenzoic acid into a yellow-colored compound, GSH could be assessed (mmol/g protein) colorimetrically at 405 nm as provided by Beutler et al. (1963). The homogenizing buffer components were 50 mM potassium phosphate, pH 7.5, and 1 mM EDTA. The concentration of TBARS (a byproduct of lipid peroxidation) was measured (nmole/g tissue) according to the method of Ohkawa et al. (1979), in which the TBARS (MD 25 29) reacts with thiobarbituric acid, generating a colored end-product. The color intensity at 534 nm was equivalent to TBARS concentrations. The homogenizing buffer was 50 mM potassium phosphate, pH 7.5.
Visualization of generated ROS
As Wang and Joseph (1999) and Siddiqui et al. (2010) described, ROS generation was determined in the gills and livers of both selected species collected from the studied sites. The method relies on the passive passing of 2,7-dichlorofluorescin diacetate (DCFH-DA), which interacts with ROS to produce the highly fluorescent chemical dichlorofluorescein compound (DCF). After homogenizing the studied tissue samples, DCFH-DA (20 mM) was added to the cell suspension, and the samples were kept in the dark for 30 min before being visualized and photographed with a fluorescence microscope (Optika B 353LD2 LED trinocular fluorescence microscope) at excitation and emission wavelengths of 485 nm and 528 nm, respectively, and × 20 magnification. For ROS quantification, each cell in each image was analyzed using Image J software (version 1.50, USA) and the data are represented as corrected total cell fluorescence (CTCF). CTCF = integrated density − (area of selected cell × mean fluorescence of background).
Histopathological alterations
The gills and liver tissues were isolated and washed in a physiological saline solution (0.9% NaCl) to remove excess blood or any debris. The washed tissues were conserved in a 10% formalin solution. According to Bernet et al. (1999), the preserved tissues were processed in graded series of ethanol, paraffin sectioned at 4 μm, and then stained using Hematoxylin and Eosin and finally photographed by light microscopy. Nine specimens/tissue/species/site were used in the histological study, and the percentage of common alterations was calculated (n = 18; 2 slides of different 9 fish). After the examination of all recorded alterations in all slides (18/tissue/species/site), the percentage of appearance for each alteration was calculated as shown:
Statistical analysis
All data were expressed as mean ± SE. The raw data were normally distributed as determined by the Shapiro–Wilk and Kolmogorov–Smirnov tests, as well as homogenous as determined by Levene’s test. Data were statistically analyzed (P < 0.05) with Student’s t-test, ANOVA test, and Duncan’s test to evaluate differences among different fish and sites. The statistical analysis was conducted using Statistical Processor Systems Support, SPSS software, version 25.0, IBM, Chicago, IL, USA.
Results
Concentrations of metals in water and sediments
The concentrations of metals in water and sediments collected from the studied sites are presented in Table 2. A significant (P < 0.05) increase in all measured metals was observed in samples from Site 2 (except for Cu concentration in water) compared to the reference site. Metal concentrations in water and sediment samples from site 1 were within the permissible concentrations based on international and national guideline values for metal concentrations in aquatic bodies (MacDonald et al. 2000; MWRI 2013). The concentration of all metals in water samples at site 2 exceeded the guideline values except for copper metal. The concentrations of metals in sediment samples of site 2 were less than the guideline values except for Cu and Cd metals.
Enzymatic and non-enzymatic antioxidant biomarkers
The studied enzymatic and non-enzymatic antioxidant biomarkers in the liver of O. niloticus and C. gariepinus collected from the studied sites are represented in Table 3. Compared to the reference fish, the gills of both studied species showed elevated enzymatic activities of CAT, SOD, and GPx in addition to increased TBARS concentrations. Sharp decreases in GSH levels were also recorded in both species collected from the polluted site (the Batts). The maximum lipid peroxidation rate (as indicated by TBARS level) is recorded in C. gariepinus of site 2. As shown in Table 4, there are significant elevations in CAT and GPx (maximally in O. niloticus at site 2) and SOD (maximally in C. gariepinus at site 2) compared to the reference fish, while GSH levels were sharply decreased in both fish species of site 2. The enzymatic and non-enzymatic antioxidant biomarkers in the liver showed the same trend as in gills but with lower TBARS concentrations indicating more effective antioxidant responses and less lipid peroxidation rate.
Visualization of generated ROS in gills and livers of studied species
In Fig. 2, the results of ROS generation in gills and livers are represented. The microscopic fluorescence images revealed excessive production of intracellular ROS (indicated by the high intensity of fluorescence) in the gills and livers of both studied species inhabiting site 2.
The photographic images of ROS generation in the liver and gills of O. niloticus and C. gariepinus collected from the studied sites. Graph (A) shows a significant (P < 0.05) induction in ROS exemplified as corrected total cell fluorescence (CTCF) in the liver and gills of the studied fish (columns with the same letter in for each tissue are not significantly different (P < 0.05); otherwise, they do)
Histopathological alterations
Figure 3 shows the histopathological alterations in the gill tissues of both species under study. The structural deformities in the studied tissues were widely observed in specimens collected from site 2. Several alterations such as epithelial lifting, fusion in secondary lamellae, epithelial thickening, hyperplasia, and cartilaginous deformation were recorded in gills. The observed histopathological changes in the liver (Fig. 4) were disorganized hepatocytes, infiltration of red blood cells, vacuolization in hepatocytes, necrotic damage, congestion in hepatopancreatic tissue, and congestion in hepatocytes. Based on the frequency of deformities as shown in Table 5, the tissues collected from site 1 showed normal histological structures and regular cellular arrangements with less observable structural damage compared to the tissues from site 2.
The histopathological alterations in the livers of O. niloticus and C. gariepinus collected from the studied sites. HP, hepatopancreatic tissue; INF, infiltration of red blood cells; V, vacuolation; HC, hepatocytes; ND, necrotic damages; C, congestion; DH, disarranged hepatocytes. Scale bar = 100 μm
Discussion
The dense anthropogenic activities nearby lake Qaroun and the massive effluents through the Batts drain have badly affected the optimal inhabiting conditions of many fish species. These untreated discharges not only alter the chemical equilibrium of the water but also deteriorate the health status of the chronically exposed fish. Lake Qaroun was designated as one of the hotspots of metal contamination in El-Fayoum province (Abdel-Khalek et al. 2020). Metal bioaccumulation has been linked to the emission of agricultural and industrial effluents, as well as waste dumping in aquatic media, which can harm the aquatic environment. The majority of studied metals in water and sediment samples at site 2 exceeded the safe values recommended by the Ministry of Water Resources and Irrigation (Egypt) for non-freshwater bodies (MWRI 2013). This site is impacted by the Batts drain (the largest drain at lake Qaroun), which receives agricultural drainage water from the eastern side of El-Fayoum valley, as well as several anthropogenic activities. These findings were in line with those of Khalil et al. (2017), who found that the eastern section of lake Qaroun had higher metal pollution levels than the western side due to the impact of the Batts drainage and numerous anthropogenic activities in this area. Individual metal toxicity assessments may not provide a realistic environmental scenario because science metals in combination exhibit additive toxicity when compared to their individual effects (Javed et al. 2017). Thus, studying internal toxicological responses of suitable bio-indicators provides more accurate interactive hazardous effects compared to external metal speciation analysis. In this context, the present study evaluated the impact of untreated water discharges as a continuous source of metal pollution on multiple biomarker responses. Exposure to high metal concentrations promotes ROS overproduction such as superoxide anion radical, hydroxyl radical, and hydrogen peroxide (H2O2) through several mechanisms such as Fenton- and Haber–Weiss-type reactions (Saglam et al. 2014). The generated ROS leads to significant mobility of antioxidant components to mitigate the harmful effects of these free radicals. Because the liver is the primary pollutant detoxification/biotransformation tissue and the gills are external tissues that are constantly exposed to water, both tissues are used to assess oxidative stress and fish ability to scavenge excess ROS (Ogunwole et al. 2021). The complementary reactions of SOD/CAT enzymes are classified as the earliest defense mechanisms against free radicals. The role of both enzymes is to convert superoxide radicals to oxygen (O2) and H2O2, and finally into inert water molecules (Yousefi et al. 2021). The activation of both co-working enzymes indicates a massive generation of ROS and hence excessive production of H2O2. These results are in agreement with Turan et al. (2020) who demonstrated a positive relationship between CAT-SOD activations in C. gariepinus and aqueous metal exposure from the polluted Orontes River. Kumar et al. (2021) suggested CAT and SOD as trustworthy biomarkers for biomonitoring of metal pollution in marine ecosystems using thirty different fish species. GPx is an important enzyme for detoxifying H2O2 and organic peroxides (Temiz and Kargın 2022). The enhancement of SOD and CAT responses was combined with a significant elevation in the GPx activities, revealing that the SOD-CAT defense mechanism could not scavenge the excessive ROS and that the participation of GPx was necessary. Continuous and chronic exposure to metal pollution increases the conjugation rate between metals and GSH (through its thiolate sulfur atom), forming GS–metal complexes (Javed et al. 2016). The strong affinity of SH residues in GSH for metals explains the recorded reduction in GSH content (abundant thiol molecule) of fish collected from the polluted site. The induction of several enzymatic and non-enzymatic antioxidant defense components was unable to avoid lipid peroxidation as signposted by elevated TBARS levels in the liver and gills of site 2. The ineffective antioxidant capacities after chronic exposure to mixed metal pollution were in agreement with Arojojoye et al. (2018), who reported altered antioxidant responses in the liver and muscle of Clarias gariepinus collected from the Igbokoda River in South-Western Nigeria. Based on the overall induction of antioxidant components and the level of TBARS (a byproduct of lipid peroxidation), O. niloticus showed more significant potential and flexibility to resist the continual flux of superoxide radicals compared to C. gariepinus facing the same pollution level. ROS, when present at a proper physiological level, has key functions in regular cell functions, such as antigen fighting, regulating various intercellular signals, and allowing appropriate maturation in reproductive systems (Sinenko et al. 2021). However, when ROS are particularly abundant and exceed the antioxidant coping capacities, oxidative stress can lead to cellular dysfunction via peroxidation of lipids, damage to proteins, and DNA (Ibrahim et al. 2021). Oxidative stress is linked to several pathologies, including histopathological damage, because of its harmful influence on cells (Alchalabi et al. 2016). The most common histopathological alterations in gills were epithelial thickening of primary lamellae, epithelial lifting at the bases and tips of secondary lamellae, severe hyperplasia with ballooning swelling in secondary lamellae, and secondary lamellar fusion. The adaptive anatomical changes such as gill hyperplasia and detached lamellar epithelium may increase the depth of the epithelial cell layer, providing an effective barrier between the exterior and interior environments (Marinović et al. 2021). Furthermore, the reported telangiectasis (ballooning swelling) might be used as ion storage to accumulate metals from water and enhance the adhesion between lamellae to develop an anatomical barrier against external pollutants (Santos et al. 2021). If several telangiectatic lamellae are present, the respiratory function may be compromised, and if the fish is further stressed, a severe reduction in oxygen concentration may occur (Marinović et al. 2021). Blood flow increases in newly established conditions of reduced oxygen concentration, increasing the level of circulatory disturbances. The most pronounced circulatory alteration, according to Rašković et al. (2013), was hyperemia, which represents an increased blood supply due to a disruption in gas exchange and telangiectasis which represents reversible swollen blood vessels in the secondary gill lamellae. The fusion of lamellae may reduce the surface area exposed to the contaminants of the Batts effluents. The vacuolization of hepatocytes was reported in O. niloticus exposed to metal pollution as a mark of abnormal accumulation of fat in the cytoplasm, protein breakdown, and metabolic malfunction (Massoud et al. 2021). The appearance of hepatic venules occupied by red blood cells indicates a consequence of the loss of cellular membrane integrity. These changes are often linked to a chronic and progressive necrotic state (Mahboob et al. 2020). The severe histological damage in gills and liver tissues indicates the direct injurious impacts of metals that are in continuous contact with those tissues during ion exchanges and the detoxification processes. Furthermore, oxidative stress and massive ROS accumulation have been linked to a variety of cellular and histological changes in fish (Javed et al. 2017).
Conclusion
These integrated biomarkers were applied for the first time to the selected fish species at the studied sites. Persistent exposure to untreated discharges from the Batts drain had potential health concerns as indicated by antioxidant system disruption and histological alterations in two economically important fish species, O. niloticus and C. gariepinus. The oxidative stress and structural damage in the liver and gills were attributed to the direct metallic injurious effects and excessive generation of ROS. O. niloticus showed better tolerance to the same level of pollution than C. gariepinus based on the level of lipid peroxidation and the frequency of histopathological changes. Continuous monitoring of the effluent quality near the Batts drain is needed to improve the health status of fish and increase their ability to survive and reproduce.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
References
Abdel-Khalek AA, Hamed A, Marie MA (2016) The accumulation potency of bulk and nano zinc metal and their impacts on the hematological and histological perturbations of Oreochromis niloticus. Water Air Soil Pollut 227:206. https://doi.org/10.1007/s11270-016-2908-x
Abdel-Khalek AA, Zayed HS, Elsayad SM, Zaghloul KH (2020) Assessment of metal pollution impacts on Tilapia zillii and Mugil cephalus inhabiting Qaroun and Wadi El-Rayan Lakes, Egypt, using integrated biomarkers. Environ Sci Pollut Res 27:26773–26785. https://doi.org/10.1007/s11356-020-09095-3
Abu Shnaf ASM, Abd El‐Aziz SH, Ata AM (2021) Cyto‐histopathological and protein polymorphism alterations in five populations of Nile tilapia ( Oreochromis niloticus ) as biomonitor for water heavy metal pollution. J Fish Biol 1–11 https://doi.org/10.1111/jfb.14798
Aebi H (1984) Catalase in Vitro. Meth Enzymol 105:121–126. https://doi.org/10.1016/S0076-6879(84)05016-3
Alchalabi ASH, Rahim H, Aklilu E, Al-Sultan II, Aziz A, Malek MF, Ronald SH, Khan MA (2016) Histopathological changes associated with oxidative stress induced by electromagnetic waves in rats’ ovarian and uterine tissue. Asian Pac J Reprod 5(4):301–310. https://doi.org/10.1016/j.apjr.2016.06.008
APHA (2017) American Water Works Association. Standard methods for the examination of water and wastewater 23rd edition. American Public Health Association, New York
Arojojoye OA, Oyagbemi AA, Afolabi JM (2018) Toxicological assessment of heavy metal bioaccumulation and oxidative stress biomarkers in Clarias gariepinus from Igbokoda River of South Western Nigeria. Bull Environ Contam Toxicol 100(6):765–771. https://doi.org/10.1007/s00128-018-2341-5
Bernet D, Schmidt H, Meier W, Burkhardt-Holm P, Wahli T (1999) Histopathology in fish: proposal for a protocol to assess aquatic pollution. J Fish Dis 22:25–34. https://doi.org/10.1046/j.1365-2761.1999.00134.x
Beutler E, Duron O, Kelly MB (1963) Improved method for the determination of blood glutathione. J Lab Clin Med 61:882–888
Cabrera F, Conde B, Flores V (1992) Heavy metals in the surface sediments of the tidal river Tinto (SW Spain). Fresenius Environ Bull 1:400–405
Greani S, Lourkisti R, Berti L, Marchand B, Giannettini J, Santini J, Quilichini Y (2017) Effect of chronic arsenic exposure under environmental conditions on bioaccumulation, oxidative stress, and antioxidant enzymatic defenses in wild trout Salmo trutta (Pisces, Teleostei). Ecotoxicology 26(7):930–941. https://doi.org/10.1007/s10646-017-1822-3
Hedayati SA, Sheikh Veisi R, Hosseini Shekarabi SP, Shahbazi Naserabad S, Bagheri D, Ghafarifarsani H (2021) Effect of dietary lactobacillus casei on physiometabolic responses and liver histopathology in common carp (Cyprinus carpio) after exposure to iron oxide nanoparticles. Biol Trace Elem Res. https://doi.org/10.1007/s12011-021-02906-9
Hermenean A, Damache G, Albu P, Ardelean A, Ardelean G, Puiu Ardelean D, Horge M, Nagy T, Braun M, Zsuga M, Kéki S, Costache M, Dinischiotu A (2015) Histopatological alterations and oxidative stress in liver and kidney of Leuciscus cephalus following exposure to heavy metals in the Tur River, North Western Romania. Ecotoxicol Environ Safe 119:198–205. https://doi.org/10.1016/j.ecoenv.2015.05.029
Ibrahim ATA, Banaee M, Sureda A (2021) Genotoxicity, oxidative stress, and biochemical biomarkers of exposure to green synthesized cadmium nanoparticles in Oreochromis niloticus (L). Comp Biochem Physiol C Toxicol Pharmacol 242:108942. https://doi.org/10.1016/j.cbpc.2020.108942
Javed M, Ahmad I, Usmani N, Ahmad M (2016) Studies on biomarkers of oxidative stress and associated genotoxicity and histopathology in Channa punctatus from heavy metal polluted canal. Chemosphere 151:210–219. https://doi.org/10.1016/j.chemosphere.2016.02.080
Javed M, Ahmad MI, Usmani N, Ahmad M (2017) Multiple biomarker responses (serum biochemistry, oxidative stress, genotoxicity and histopathology) in Channa punctatus exposed to heavy metal loaded waste water. Sci Rep 7(1):1–11. https://doi.org/10.1038/s41598-017-01749-6
Khalil MT, Fishar MR, Shakir SH, Amer AS, Nassif MG (2017) Impact of drainage water on macrobenthos structure of Lake Qaroun, El-Fayoum. Egypt Egypt Aquat Biol Fish 21(2):17–32. https://doi.org/10.21608/EJABF.2017.3289
Kumar N, Bhushan S, Gupta SK, Kumar P, Chandan NK, Singh DK, Kumar P (2021) Metal determination and biochemical status of marine fishes facilitate the biomonitoring of marine pollution. Mar Pollut Bull 170:112682. https://doi.org/10.1016/j.marpolbul.2021.112682
Lushchak VI (2011) Environmentally induced oxidative stress in aquatic animals. Aquat Toxicol 101(1):13–30. https://doi.org/10.1016/j.aquatox.2010.10.006
MacDonald DD, Ingersoll CG, Berger TA (2000) Development and evaluation of consensus-based sediment quality guidelines for freshwater ecosystems. Arch Environ Contam Toxicol 39:20–31. https://doi.org/10.1007/s002440010075
Mahboob S, Al-Ghanim KA, Al-Balawi HF, Al-Misned F, Ahmed Z (2020) Toxicological effects of heavy metals on histological alterations in various organs in Nile tilapia (Oreochromis niloticus) from freshwater reservoir. J King Saud Univ Sci 32(1):970–973. https://doi.org/10.1016/j.jksus.2019.07.004
Marinović Z, Miljanović B, Urbányi B, Lujić J (2021) Gill histopathology as a biomarker for discriminating seasonal variations in water quality. Appl Sci 11(20):9504. https://doi.org/10.3390/app11209504
Massoud E, El-Kott A, Morsy K, Abdel-Khalek AA (2021) Assessment of hepatotoxicity induced by aluminum oxide nanoparticles in Oreochromis niloticus using integrated biomarkers: exposure and recovery. Bull Environ Contam Toxicol 106:970–977. https://doi.org/10.1007/s00128-021-03190-y
MWRI (Ministry of Water Resources and Irrigation) (2013) National Water Resources Plan/Planning Sector. Country Strategy, Egypt. Ministerial Decree No. 92 of 2013 amending the ministerial decree No. 8 of 1982 on the executive regulations of law No. 48 of 1982 concerning the protection of the Nile River and water channels from pollution
Naz S, Hussain R, Ullah Q, Chatha AMM, Shaheen A, Khan RU (2021) Toxic effect of some heavy metals on hematology and histopathology of major carp (Catla catla). Environ Sci Pollut Res 28(6):6533–6539. https://doi.org/10.1007/s11356-020-10980-0
Neugebauer EA, Sans Cartier GL, Wakeford BJ (2000) Methods for the determination of metals in wildlife tissues using various atomic absorption spectrophotometry techniques. Technical Report Series No. 337E. Canadian Wildlife Service, Headquarters, Hull. http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.578.2937&rep=rep1&type=pdf
Nishikimi M, Appaji N, Yagi K (1972) The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophys Res Common 46:849–854. https://doi.org/10.1016/S0006-291X(72)80218-3
Ogunwole GA, Abiya SE, Amaeze NH, Eze CT (2021) Antioxidant markers in gills, liver and muscle tissue of the African Sharptooth Catfish (Clarias gariepinus) exposed to subchronic levels of Ibuprofen and Dibutyl phthalate. Sci Afr 12:e00816. https://doi.org/10.1016/j.sciaf.2021.e00816
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358. https://doi.org/10.1016/0003-2697(79)90738-3
Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med 70:158–169
Rajeshkumar S, Liu Y, Zhang X, Ravikumar B, Bai G, Li X (2018) Studies on seasonal pollution of heavy metals in water, sediment, fish and oyster from the Meiliang Bay of Taihu Lake in China. Chemosphere 191:626–638. https://doi.org/10.1016/j.chemosphere.2017.10.078
Rašković B, Jarić I, Koko V, Spasić M, Dulić Z, Marković Z, Poleksić V (2013) Histopathological indicators: a useful fish health monitoring tool in common carp (Cyprinus carpio Linnaeus, 1758) culture. Cent Eur J Biol 8(10):975–985
Saglam D, Atli G, Dogan Z, Baysoy E, Gurler C, Eroglu A, Canli M (2014) Response of the antioxidant system of freshwater fish (Oreochromis niloticus) exposed to metals (Cd, Cu) in differing hardness. Turkish J Fish Aquat Sci 14(1):43–52. https://doi.org/10.4194/1303-2712-v14_1_06
Santos RMB, Monteiro SM, Cortes RMV, Pacheco FAL, Fernandes LFS (2021) Seasonal effect of land use management on gill histopathology of Barbel and Douro Nase in a Portuguese watershed. Sci Total Environ 764:142869. https://doi.org/10.1016/j.scitotenv.2020.142869
Sauliutė G, Markuckas A, Stankeviciūtė M (2020) Response patterns of biomarkers in omnivorous and carnivorous fish species exposed to multicomponent metal (Cd, Cr, Cu, Ni, Pb and Zn) mixture. Part III Ecotoxicol 29(3):258–274. https://doi.org/10.1007/s10646-020-02170-y
Siddiqui MA, Kashyap MP, Kumar V, Al-Khedhairy AA, Musarrat J, Pant AB (2010) Protective potential of trans-resveratrol against 4-hydroxynonenal induced damage in PC12 cells. Toxicol in Vitro 24(6):1592–1598. https://doi.org/10.1016/j.tiv.2010.06.008
Sinenko SA, Starkova TY, Kuzmin AA, Tomilin AN (2021) Physiological signaling functions of reactive oxygen species in stem cells: from flies to man. Front Cell Dev Biol 9:714370. https://doi.org/10.3389/fcell.2021.714370
Temiz Ö, Kargın F (2022) Toxicological impacts on antioxidant responses, stress protein, and genotoxicity parameters of aluminum oxide nanoparticles in the liver of Oreochromis niloticus. Biol Trace Elem Res 200:1339–1346. https://doi.org/10.1007/s12011-021-02723-0
Turan F, Eken M, Ozyilmaz G, Karan S, Uluca H (2020) Heavy metal bioaccumulation, oxidative stress and genotoxicity in African catfish Clarias gariepinus from Orontes river. Ecotoxicology 29(9):1522–1537. https://doi.org/10.1007/s10646-020-02253-w
Wang H, Joseph JA (1999) Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic Biol Med 27(5–6):612–616. https://doi.org/10.1016/S0891-5849(99)00107-0
Yousefi M, Farsani MN, Ghafarifarsani H, Hoseinifar SH, Van Doan H (2021) The effects of dietary supplementation of mistletoe (Viscum album) extract on the growth performance, antioxidant, and innate, immune responses of rainbow trout (Oncorhynchus mykiss). Aquaculture 536:736385. https://doi.org/10.1016/j.aquaculture.2021.736385
Zaghloul KH, Omar WA, Abdel-Khalek AA, Abo-Hegab S (2011) Ecological monitoring of Mediterranean Solea aegyptiaca transplanted into Lake Qaroun. Egypt Aust J Basic Appl Sci 5(7):851–862
Acknowledgements
The authors extend their appreciation to the Faculty of Science, Cairo University, Cairo, Egypt, for supporting the present work.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Abdel-Khalek AA and Mohamed HRH conceived and designed the research. Abdel-Khalek AA, Mohamed HRH, and Moussa MA conducted experiments. Abdel-Khalek AA and Moussa MA analyzed the data. Abdel-Khalek AA wrote the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval
This manuscript complies with the ethical rules applicable for this journal.
Consent to participate
All authors read and approved the final manuscript.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Cinta Porte
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Moussa, M.A., Mohamed, H.R.H. & Abdel-Khalek, A.A. The antioxidant defense capacities and histological alterations in the livers and gills of two fish species, Oreochromis niloticus and Clarias gariepinus, as indicative signs of the Batts drain pollution. Environ Sci Pollut Res 29, 71731–71741 (2022). https://doi.org/10.1007/s11356-022-20804-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-20804-y