Abstract
Treatment with cell wall-degrading enzymes is an important step during juice production to enhance juice yield and the amount of value-adding compounds like polyphenols. Enzymatic side activities may lead to unintended alterations of the polyphenol profile. We determined the effects of enzyme treatment on juice yield and content and profile of anthocyanins using four commercial pectinolytic and two cellulolytic enzymes during bilberry juice production. While enzyme dosage at commercial level (0.5 nkat/g) caused only small increases in juice yield but considerably higher anthocyanin yields, significant changes in the anthocyanin profile could be observed, which were related to the glycoside type as well as to the aglycone. Application of excessive enzyme dosage (10 nkat/g) significantly improved both juice yield and total anthocyanin content. Extractability of anthocyanins seems to be more relevant to profile changes during juice processing when usual enzyme dosages are applied, whereas excessive dosages lead to changes caused by enzymatic side activities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Berries containing high amounts of phenolic compounds like anthocyanins, phenolic acids and flavonols are growing in popularity because of the value-adding effect of these substances [1]. In particular, bilberries have been reported to be a rich source of polyphenols, especially anthocyanins [1–3]. Due to their appealing color and sweet taste [4], they are not only eaten fresh, but also processed into juices and jams [5]. Anthocyanins constitute one of the most important groups of polyphenols in bilberries [6]. The aglycones delphinidin (dp), cyanidin (cy), petunidin (pt), peonidin (pn) and malvidin (mv) occur as 3-O-glycosides with attached glucose (glu), galactose (gal) and arabinose (ara) moieties, respectively. The resulting profile includes 15 different anthocyanins. This complex fingerprint has frequently been used for authentication of bilberry products because they represent high-valued crops and adulteration is a common problem [7–11]. Variations of the anthocyanin profile are not exclusively the result of fraudulent practices, but also occur inevitably due to technological influences during the production process [12–14]. One of the main processing steps which may greatly affect the anthocyanin profile is the enzymatic treatment. Application of enzymes has several implications during fruit juice processing. Enzymes reduce the viscosity and thus increase juice yields and expedite production [15, 16]. They cause disruption of the cell wall network, enhancing the extractability of polyphenols, which are predominantly associated with the fruit peel [17, 18]. Pectinases, cellulases, hemicellulases and amylases have been reported to be suitable for enzymatic treatment and the release of polyphenols [18]. Two important representatives of pectinases are polygalacturonases, which hydrolyze α-1,4-glycosidic linkages of the polygalacturonic acid backbone, and pectin lyases, which lead to the cleavage of α-1,4-glycosidic bonds with the introduction of an unsaturated moiety at the non-reducing end [19]. Both enzymes show endo- and exo-activities leading to random or sequential degradation, respectively [19]. Cellulases include three different hydrolytic enzymes, that are, an endo-(l,4)-β-d-glucanase activity that randomly cleaves β-linkages of amorphous cellulose, an exo-(l,4)-β-d-glucanase activity, which releases sequentially cellobiose from either the reducing or the non-reducing end of cellulose, and a β-glucosidase activity that releases glucose from cellobiose [20]. However, the most frequently applied preparations in commercial berry processing are pectinolytic enzymes [15]. The great variety of commercial enzyme preparations is required because of the complex diversity of plant cell walls, which are composed of cellulose, hemicellulose and pectin as their main polysaccharides [21–23]. The structure of pectin is very diverse as it includes acidic polymers like homogalacturonans and rhamnogalacturonans with neutral polymers like arabinans, galactans and arabinogalactans attached [21]. Besides the numerous positive effects of enzymatic treatment, adverse effects may also be observed. A considerable loss of anthocyanins during fruit processing has been reported, which was attributed to side activities of the enzyme preparations [24–27]. β-Glucosidase, β-galactosidase or α-l-arabinosidase side activities have been discussed in this context [18]. However, a pure β-glucosidase had only little influence on the anthocyanins of elderberries, indicating that an anthocyanin-specific β-glucosidase seems to be responsible for the observed degradation [28]. Moreover, the application of enzymes may lead to secondary effects. Since pectin has been reported to interact with anthocyanins [29], it is reasonable that interaction of pectin degradation products with anthocyanins might also occur.
Accordingly, the application of technological processes such as enzymatic treatment may lead to various contrary effects. On the one side, they preserve or enhance the amount of value-adding compounds in the final products by facilitating their extractability. On the other side, they may also cause considerable losses of these compounds due to the side effects mentioned above.
The objective of this study was to characterize the changes in the anthocyanin profile caused by enzymatic side activities during bilberry juice production in order to obtain a better understanding of the underlying mechanisms. Since most published studies demonstrate the effects of extremely high enzyme dosages, the difference between the effect of commercially common dosages and excessive dosages was of particular interest as there is only little information about profile changes at industrially relevant dosages.
Materials and methods
Samples
Bilberries (Vaccinium myrtillus L.) were purchased in July 2014 from Stahl GmbH (Bodenwöhr-Blechhammer, Germany) and stored at −20 °C until further use.
Juice processing
For inactivation of polyphenol oxidase (PPO), 500 g batches of berries were placed in plastic bags, thawed overnight at 8 °C and blanched afterward for 5 min at 80 °C in a shaking water bath. Subsequently, they were cooled in an ice bath and pooled again. In 200 g batches, they were stored at −20 °C until juice processing. After mashing the berries with a stomacher for 6 s, the enzyme preparations shown in Table 1 were added at two dosage levels (0.5 and 10 nkat/g). Incubation was performed in a shaking water bath (60 rpm, 50 °C) for 120 min. Samples were placed in a hot water bath at 95 °C for 2 min to inactivate the enzymes and subsequently cooled at 5 °C in an ice bath. Juice pressing was performed in 3 cycles with a Para Press (Paul Arauner GmbH and Co. KG, Kitzingen, Germany) by applying a pressure of 3 bar for 10 s each time. Juice and pomace were stored at −20 °C for further analysis.
Chemicals and standards
Ultrapure water was obtained from a Synergy purification system (Millipore, Molsheim, France). HPLC-grade acetonitrile, acetic acid and potassium sodium tartrate tetrahydrate were from VWR (Mannheim, Germany). Formic acid (≥98 %), 3,5-dinitrosalicylic acid and d(+)-glucose monohydrate were purchased from Sigma-Aldrich (St. Louis, USA). Methanol was from Th. Geyer (Renningen, Germany), and regenerated cellulose filters (Chromafil RC-20/15 MS) were supplied by Macherey–Nagel (Düren, Germany). Malvidin 3-O-glucoside (≥87 %) was obtained from Phytoplan (Heidelberg, Germany). Pectin (Pectin Classic AU–L 024/10) was purchased from Herbstreith & Fox GmbH (Werder, Germany), d(+)-galacturonic acid from Fluka Analytical (Steinheim, Germany), and cellobiose and sulfuric acid from AppliChem GmbH (Darmstadt, Germany).
Extraction and quantification of anthocyanins
Fresh berries and pomace samples were extracted according to a method described by Koponen et al. [30] with minor modifications. The sample (30 g) was homogenized with 100 mL extraction solvent A (water/methanol/hydrochloric acid, 78/20/2, v/v/v) using an Ultra Turrax T18 basic (IKA-Werke GmbH & CO. KG, Staufen, Germany) for 2 min. Subsequently, 15 mL of solvent A was added to 3 g homogenate, and after 1 min of shaking, the sample was centrifuged for 10 min at 3941g (Zentrifuge, Thermo Fisher Scientific, Schwerte, Germany). After 2 repetitions, the pellet was extracted with extraction solvent B (water/methanol/hydrochloric acid, 49/50/1, v/v/v). The combined supernatants were made up to 50 mL with water. Anthocyanin quantification was performed as reported by Heffels et al. [31]. Juices were injected directly after membrane filtration. Anthocyanins were detected at 520 nm and quantified as mv-3-glu equivalents by external calibration (range 1.18–471.37 mg/L). The total anthocyanin content was calculated as the sum of individual anthocyanins.
Polygalacturonase activity
For the quantification of reducing sugars released from pectin, 450 µL of 0.1 % pectin suspension in sodium citrate buffer (pH 4) and 50 µL of 10 % aqueous enzyme solution were incubated for 5 min at 50 °C in a shaking water bath. The reaction was terminated by the addition of 750 µL of dinitrosalicylic acid (DNS) reagent containing 10 g/L 3,5-dinitrosalicylic acid, 30 g/L potassium sodium tartrate tetrahydrate and 16 g/L sodium hydroxide in water. After boiling for 5 min, cooling in iced water and centrifugation for 5 min at 10,947g, the absorption was measured at 540 nm (Genesys 6, Thermo Fisher Scientific, Schwerte, Germany). Polygalacturonase activity was expressed as d(+)-galacturonic acid equivalents released during the enzymatic reaction [28, 32–35].
β-Glucosidase activity
The amount of glucose released due to enzymatic treatment of cellobiose was quantified in order to determine the β-glucosidase activity. A reaction mixture containing 100 µL of 10 % aqueous enzyme solution and 900 µL of cellobiose solution (4 g/L) in sodium citrate buffer (pH 4) was incubated for 30 min at 50 °C in a shaking water bath. Enzyme activity was stopped by placing the reaction mixture in a hot water bath (95 °C) for 5 min and afterward in an iced water bath for 5 min. After 5 min of centrifugation at 10,947g, the mixtures were kept at −20 °C until quantification of glucose via HPLC [36]. Quantification was conducted using a Shimadzu LC-6A pump, a Waters 717plus autosampler (injecting 10 μL), a Waters column thermostat Jetstream 2 plus (at 60 °C) and a Waters 2414 refraction index detector. The column was a Nucleogel ION 300OA (Macherey–Nagel, Düren, Germany). Sulfuric acid (2.5 mM) was delivered at an isocratic flow of 0.3 mL/min for 60 min.
Statistics
For statistical analysis and principal component analysis (PCA), the XLSTAT software was used. An ANOVA was performed to determine significant differences. The level of significance was defined as p ≤ 0.05.
Results and discussion
Enzyme activity
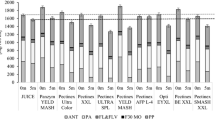
The polygalacturonase activity of all enzyme preparations investigated ranged from 1304 to 4593 nkat/mL. Table 2 shows the polygalacturonase activities ranked in an ascending order. All enzyme preparations, which are labeled as polygalacturonases by the manufacturers, showed medium to high polygalacturonase activities. A comparison with literature data is difficult because of differing enzyme preparations, differing assays, or the usage of uncommon units to describe enzyme activity. While a polygalacturonase activity of 1230 nkat/mL was reported for Pectinex BE XXL [37], our analysis revealed an activity of 3254 nkat/mL. Similar differences are reported in the literature. Although applying the same assay, Pectinex BE 3-L revealed an activity of 4900 nkal/mL in a study of Buchert et al. [26] and an activity of 11,950 nkat/mL in a study of Puuponen-Pimiä et al. [37].
The β-glucosidase activity ranged from 15 to 920 nkat/mL. As shown in Table 2, the difference between the β-glucosidase activities of the enzyme preparations investigated is more pronounced than those between their polygalacturonase activities. Moreover, the β-glucosidase activity is considerably lower than the polygalacturonase activity, reflecting that the β-glucosidase should be regarded as a side activity in the investigated enzyme preparations. It was reported that glycosidases are generally associated with pectinolytic enzymes [28, 38]. Whereas the high β-glucosidase activity of Vegazym HC is reasonable because the group of cellulases encompasses cellobiases [20, 39], the considerably lower β-glucosidase activity of Rohament CL is remarkable. Although both enzymes are labeled cellulases, differing β-glucosidase activities can be explained by the microbial origin of the enzymes, which are derived from different microbial strains (Trichoderma longibrachiatum vs. T. reseei). Therefore, the three cellulase main activities endo-l,4-β-d-glucanase, exo-l,4-β-d-glucanase and β-glucosidase may be differently expressed for both enzyme preparations.
For the production of a juice with improved polyphenol contents, a high polygalacturonase activity is necessary to degrade the cell wall, which in turn leads to an increased release of polyphenols and galacturonic acid [26]. Polygalacturonase and β-glucosidase activities of the enzymes investigated show a high correlation of 0.685, suggesting that enzymes with a high polygalacturonase activity also have a marked β-glucosidase activity. This needs to be considered in juice production because a pronounced β-glucosidase activity might lead to unintended degradation of anthocyanins [26, 28].
Effect of enzyme-assisted processing of bilberries on juice yield and anthocyanin content
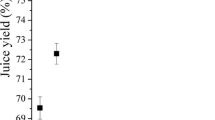
Bilberries were processed applying six commercial preparations which represent the enzyme activities of polygalacturonase, pectin lyase and cellulase. All enzymes were used at two dosage levels (0.5 and 10 nkat/g) based on their polygalacturonase activity. The lower level of 0.5 nkat/g represents the dosage recommended by the manufacturer, whereas the higher level of 10 nkat/g can be considered an excessive dosage similar to those reported in the literature [26, 37, 40]. In order to characterize the resulting juices, both juice yield (Fig. 1) and total anthocyanin content (Fig. 2) were determined.
Enzyme dosages of 0.5 nkat/g had only little effect on the juice yield compared with a reference juice produced without the addition of any enzyme (Fig. 1). The largest increase in juice yield (5 %) was achieved by the application of VinoUFC and RPTE100. However, the various enzyme preparations did not lead to significantly different juice yields at a dosage of 0.5 nkat/g.
A higher dosage of 10 nkat/g resulted in significantly higher juice yields compared with the control juice for all enzyme preparations except for VHC. Polygalacturonases and pectin lyase PBEXXL led to an increase of about 10 %, and pectin lyase RPTE100 increased the juice yield about 17 %. The cellulase VHC did not affect the juice yield, but application of RCL increased the yield by 10 %. These findings are in accordance with Koponen et al. [27] who observed a significantly increased bilberry juice yield applying similar enzyme preparations at a level of 1 nkat/g without further increases at 10 and 100 nkat/g. Buchert et al. [26] reported similar increasing bilberry juice yields at a dosage of 1000 nkat/g, noting that the maximum yield was already obtained at dosages below 50 nkat/g. Similar increases in juice yield due to maceration with pectinolytic enzymes were reported for other fruits like black currant [40–42], elder berry [43] and plum [44]. However, the dosages used significantly exceed the commonly applied levels.
Although enzyme dosages of 0.5 nkat/g did not increase the juice yield significantly, a pronounced effect on the total anthocyanin content of the corresponding juices was observed. Polygalacturonases and pectin lyase PBEXXL increased the amount of anthocyanins between 23.0 and 29.3 % compared to the control juice (Fig. 2). Pectin lyase RPTE100 and cellulase RCL increased the anthocyanin content by about 10.1–13.9 %, whereas VHC had no significant impact on the anthocyanins content compared to the control juice. Applying 10 nkat/g of the enzyme preparations resulted in an even more pronounced increase in total anthocyanins. At the higher dosage, polygalacturonases and pectin lyase PBEXXL showed the largest effects in comparison with the control juice with increases of 28.3–41.9 %, whereas pectin lyase RPTE100 and the cellulases tended to lead to smaller increases of 10.4–25.3 %. Juices elaborated with enzymes generally showed higher anthocyanin yields than the control juice. Similar increases in anthocyanin and flavonol yields during the production of bilberry juice [27, 37], elderberry juice [43] and black currant juice [42] and concentrate [45] were reported previously, when various polygalacturonases and pectin lyases above dosages used in industrial production were applied. Cellulases were reported to enhance cell wall degradation of black currant pomace, thereby facilitating the release of anthocyanins [46]. Yet, there are reports about decreased anthocyanin contents during blueberry [14, 47], lingonberry [13] and black currant [44] juice processing. Glycosidase side activities of the enzyme preparations applied and prolonged treatments were supposed to be responsible for the anthocyanin losses [26, 27].
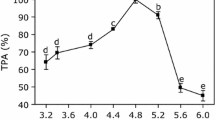
As shown in Fig. 3, anthocyanins present in bilberry were almost entirely recovered in the respective juice and pomace. The summarized recovery ranged from 81 to 94.9 % and from 84.5 to 96.2 % for 0.5 nkat/g and 10 nkat/g, respectively. Loss of anthocyanins due to physical removal of the anthocyanin-rich skins during pressing is the main reason for the lack of anthocyanins in comparison with the fresh berry [44], which showed an anthocyanin content of 4.2 mg/g fresh weight. The applied enzyme dosage had no significant effect on the total anthocyanin recovery. But apparently the recovery into the juice was slightly higher after treatment with 10 nkat/g.
The present study revealed no decreases in the anthocyanin yield compared with the control juice although enzymes with considerable β-glucosidase activities were applied. It can therefore be assumed that the β-glucosidase activity is not directly responsible for anthocyanin degradation. Furthermore, the enhanced release of anthocyanins due to enzymatic treatment may outweigh an anthocyanin loss. It has to be considered that besides immediate interactions between enzyme and anthocyanins, secondary effects may occur as well. It is conceivable that besides pectin also pectin degradation products interact with anthocyanins resulting in a loss of individual anthocyanins. All discussed effects will lead to an alteration of the anthocyanin profile during juice processing.
Principal component analysis
In order to reveal the underlying mechanisms of anthocyanin profile changes during juice processing due to enzymatic treatment, a principal component analysis was performed. This technique enables the reduction of numerous variables to a few principal components while preserving as much variance of experimental data as possible. Thereby, the influences of different enzyme preparations regarding the individual anthocyanins become more apparent. The analysis included the anthocyanin profile of fresh bilberry fruits, the juices produced thereof and the corresponding pomaces (see Tables 1 and 2 of the Supporting Information). The latter were included because they contain the remaining anthocyanins, which were not extracted into the juices, and therefore complete the anthocyanin profile.
The statistical model obtained for the lower enzyme dosage of 0.5 nkat/g describes 91.9 % (F1: 76.0 %, F2: 15.9 %) of the total variance of the anthocyanin profile. As shown in Fig. 4, the fresh fruit, the juices, and the pomaces are well separated into three distinct groups by PCA. While the fresh fruit is characterized by the highest portions of cy-arabinoside and galactosides of cy, dp and pt, the juices show increased portions of mv- and pn-glycosides. Pomaces show the highest portions of dp-glycosides as well as pt-galactoside and pt-arabinoside. This distribution shows the basic change in composition of the anthocyanin profile occurring in bilberry fruits during juice production. While the genuine anthocyanin profile differs a lot from the profile of corresponding juices and pomaces, the distribution of the latter two is less pronounced. Thus, profile differences between juices produced with different enzyme preparations seem to be small. This is supported by the position of the control sample.
As the control juice is prepared without any pectinolytic enzyme, the changes in the anthocyanin profile compared with the fresh fruit must be attributed to non-enzymatic effects. Anthocyanins show an uneven extractability [31], and it has been reported that thermal treatments like blanching, boiling and steaming affect the anthocyanin content of red cabbage [48], black raspberry [49], blueberry [50, 51], strawberry and blackberry [52] products. Thus, the narrow allocation of juices to the control juice leads to the assumption that the anthocyanin profiles are affected by differences in extractability and thermal stability rather than by enzymatic degradation. Nevertheless, there are differences in the anthocyanin profiles, which can be attributed to the applied enzymes.
Both juices processed using polygalacturonases (R10L and VinoUFC) are negatively correlated with F2, whereas all other enzyme-treated juices as well as the control juice are positively correlated. Polygalacturonases lead to changes of the anthocyanin profile that are different from those caused by the application of pectin lyases or cellulases. Juices made using R10L and VinoUFC correlate negatively with F2, and all factor loadings of galactosides show a positive correlation with F2. This indicates the reduction of the galactoside portions in these juices compared to juices produced with pectin lyases and cellulases. It can be assumed that the polygalacturonase activity causes cleavage of the anthocyanin galactosides. Viljanen et al. [13] observed similar effects applying a pectinase at 100 nkat/g on lingonberries. They found the main anthocyanin cy-3-gal to be very sensitive to pectinase treatment because approximately 60 % was degraded, whereas cy-3-glu and cy-3-ara were not affected by enzymatic treatment.
In the present study, the application of polygalacturonases at low dosages only led to decreased portions of cy-3-gal indicated by its highly positive correlation with F2 and decreased portions of dp-3-gal and pt-3-gal indicated by their highly positive correlation with F1. However, pn-3-gal and mv-3-gal showed increased portions as they correlate highly negatively with F1. This inhomogeneous distribution of galactosidic factor loadings contradicts the above-mentioned assumption of an exclusively glycoside-related enzymatic degradation. Previous studies on blueberries demonstrated the alteration of the anthocyanin profile during maceration to be dependent on the aglycone [14, 50].
Skrede et al. [14] ranked the anthocyanins in the following order of decreasing stability during blueberry juice production: mv > cy > pn > pt > dp. Likewise, Lee et al. [50] found increased malvidin portions accompanied by decreasing delphinidin and petunidin portions during blueberry juice processing. These findings are in line with the present results. At a dosage of 0.5 nkat/g, all juices show decreased portions of dp- and pt-glycosides and increased proportions of mv- and pn-glycosides irrespective of their sugar moiety. Both effects occurred during juice processing at an enzyme dosage of 0.5 nkat/g, and thus, it cannot be differentiated whether changes in anthocyanin profile are based on aglycone- or glycoside-depending effects.
Juices obtained with the aid of the cellulases RCL and VHC and the pectin lyase RPTE100 are quite different from those produced using polygalacturonases. They show higher ratios of cy-3-gal as well as mv- and pn-3-gal because they are associated with higher values for F2 and lower values for F1. The distinct position along F2 indicates a reduced portion of glucosides, as pt-3-glu and dp-3-glu are negatively associated with F2. Yet, a clear decrease in all glucoside portions cannot be derived, due to the highly negative correlation of the glucosides of pn, mv and cy with F1, while the juices show almost no separation along F1. Hence, a clear association of the cellulases with the degradation of the respective glucosides cannot be deduced either.
The juice produced with the aid of the pectin lyase PBEXXL was expected to be allocated close to the juices produced using the other pectin lyase. However, it takes an intermediate position between polygalacturonases and pectin lyases. Thus, the main activity of this lyase is accompanied by a strong polygalacturonase side activity. Puupponen-Pimiä et al. [37] reported similar strong side activities of PBEXXL.
The pomaces can well be distinguished from their corresponding juices along the axis of F1. This separation predominantly reflects an inhomogeneous release of anthocyanin from the fruit during juice processing, resulting in considerably different anthocyanin profiles of juices and the remaining pomaces.
Dosages of 10 nkat/g are significantly above those typically applied in commercial processes. As reported in numerous previous studies, the influence of enzymatic treatment on the anthocyanin profile applying high dosages is more distinct. The PCA shown in Fig. 5 represents 93.6 % of the total variance of the original data. The principal component F1 accounts for 58.8 % and F2 accounts for 34.7 %. Similar to the results obtained at lower dosage, the fresh fruit, juices and pomaces are well separated into three groups. The variance of the juices is considerably more pronounced, implying a greater influence of the enzyme preparations on the anthocyanin profiles. A distinct separation of groups along the axis of F1 can be made, which separates the juices from the corresponding pomaces and the fresh fruit. The fruit and pomaces are associated with positive values for F1, whereas the juices are negatively associated with F1.
Juices prepared with the aid of polygalacturonases align with positive values for F2, whereas juices obtained with cellulase treatment are correlated negatively with F2. Although all juices are negatively correlated with F1, the extent of negative correlation is less for cellulases than for polygalacturonases. This implies a stronger alteration of the profile due to polygalacturonases activity compared with the fresh fruit. Considering the loadings of variables, similar trends like those for lower dosage can be observed, yet to a greater extent.
All galactosides show reduced portions in juices produced with polygalacturonases because the loadings of galactosides are associated with positive values for F1 and negative values for F2. Only mv-3-gal is correlated negatively with both F1 and F2. This supports the above-mentioned assumption that the profile alteration due to the application of enzymes is glycoside related.
The glucosides are highly correlated with negative values for F1 except for dp-3-glu, which shows a highly positive correlation with F2. Because juices produced using cellulases are associated with higher values for F1 and lower values for F2 compared with juices obtained with the aid of polygalacturonases, they contain higher proportions of galactosides and lower proportions of glucosides. A degrading effect of cellulases on the glucosidic bonds is conceivable and further substantiates the above-mentioned hypothesis of a glycoside-related degradation. Nevertheless, it has to be stressed that effects for cellulases are less pronounced than for polygalacturonases.
The differences between the two pectin lyases PBEXXL and RPTE100 are even more apparent at a dosage level of 10 nkat/g compared to the differences which have been observed at a dosage of 0.5 nkat/g. While the juice produced using RPTE100 shows changes in the profile similar to those of the juices prepared with the aid of cellulases, the application of PBEXXL leads to an intermediate allocation between polygalacturonases and cellulases. This kind of profile changes confirms the strong polygalacturonase side activity of the pectin lyase PBEXXL. Interestingly, the non-treated control juice is associated with the juices after treatment with cellulases and the pectin lyase RPTE100. As the control juice indicates the changes in the anthocyanin profile due to non-enzymatic effects, the distance to all other juices represents the degree of the enzymatic influences. This implies that the effects due to polygalacturonases and the pectin lyase PBEXXL are considerably higher compared with those of the other enzymes.
The above-discussed trends can also be observed for the corresponding pomaces. The control pomace is closely related to the pomaces obtained after treatment with cellulases and the pectin lyase RPTE100. They are characterized by reduced glucoside proportions compared with the pomaces resulting from treatment with polygalacturonase. The latter, in turn, show decreased proportions of galactosides. The pomace obtained after treatment with pectin lyase PBEXXL is allocated between the pomaces of polygalacturonase-treated berries and those after application of pectin lyase RPTE100 and cellulases, similar to the distribution in the group of juices.
Authentication aspects
The addition of pectinolytic enzymes and cellulases during mash treatment at industrially common dosages (0.5 nkat/g) considerably influences the anthocyanin profile of bilberry juices. The degree of these variations needs to be considered because these profiles are frequently used for authenticity control. As it was discussed above, also non-enzymatic effects or secondary effects are relevant. The changes in anthocyanin profiles of closely related Vaccinium species caused by the different extractability of the anthocyanins were recently demonstrated [31]. The natural differences in anthocyanin profiles exceed the variations caused by industrial juice processing and therefore do not affect authenticity studies. In contrast, excessive enzyme dosages result in more pronounced differences, which may be relevant to authentication.
Conclusion
The anthocyanin profile of bilberries is altered during juice processing. The type and extent of alteration is affected by the enzyme activity and dosage. At a dosage level of 0.5 nkat/g commonly used in industrial processing, polygalacturonases and cellulases show a preferred degradation of galactosides and glucosides, respectively. At the same time, all juices, including the non-treated control juice, show a decreased proportion of dp- and pt-glycosides. Thus, no conclusion can be made whether juice processing alters the anthocyanin profile depending on the aglycone or the glycoside moiety. However, the slight differences between profiles of the control juice and juices produced with the aid of enzymes suggest that non-enzymatic effects predominate. Extractability and thermal stability of anthocyanins are more relevant to changes in anthocyanins during juice processing at an industrially common dosage level of enzymes than the action of the enzyme itself. The well-reported hypothesis of glycoside-dependent anthocyanin degradation during juice processing is supported by the results of the application of enzymes exceeding common dosages (10 nkat/g). In this case, alterations regarding the anthocyanin profiles are more pronounced. Juices obtained with the aid of polygalacturonases and cellulases show clearly decreased galactoside and glucoside amounts, respectively. However, it should be noted that these effects only occur by overdosing pectinolytic enzymes and cellulases, as shown in the present study as well as in the literature. The anthocyanin profile is altered during juice production due to various effects. The positive influence on the extractability of anthocyanins seems to exceed the degrading effects of the added enzyme. However, the degradation of cell walls might lead to secondary effects which influence the anthocyanin profile. The reported interactions of phenolic compounds with cell wall polysaccharides may also occur with fragments of the latter. The complex variety of cell wall degradation products and their effects on polyphenols necessitate a prudent application of enzymes regarding dosage and main activity during the processing of different fruits.
References
Kähkönen MP, Heinämäki J, Ollilainen V, Heinonen M (2003) Berry anthocyanins: isolation, identification and antioxidant activities. J Sci Food Agric 83:1403–1411
Häkkinen SH, Kärenlampi SO, Heinonen IM, Mykkänen HM, Törrönen AR (1999) Content of the flavonols quercetin, myricetin, and kaempferol in 25 edible berries. J Agric Food Chem 47:2274–2279
Müller D, Schantz M, Richling E (2012) High performance liquid chromatography analysis of anthocyanins in bilberries (Vaccinium myrtillus L.), blueberries (Vaccinium corymbosum L.), and corresponding juices. J Food Sci 77:340–345
Laaksonen O, Sandell M, Kallio H (2010) Chemical factors contributing to orosensory profiles of bilberry (Vaccinium myrtillus) fractions. Eur Food Res Technol 231:271–285
Howard LR, Castrodale C, Brownmiller C, Mauromoustakos A (2010) Jam processing and storage effects on blueberry polyphenolics and antioxidant capacity. J Agric Food Chem 58:4022–4029
Može S, Polak T, Gašperlin L, Koron D, Vanzo A, Poklar Ulrih N, Abram V (2011) Phenolics in Slovenian bilberries (Vaccinium myrtillus L.) and blueberries (Vaccinium corymbosum L.). J Agric Food Chem 59:6998–7004
Fügel R, Carle R, Schieber A (2005) Quality and authenticity control of fruit purées, fruit preparations and jams—a review. Trends Food Sci Technol 16:433–441
Penman KG, Halstead CW, Matthias A, de Voss JJ, Stuthe JMU, Bone KM, Lehmann RP (2006) Bilberry adulteration using the food dye amaranth. J Agric Food Chem 54:7378–7382
Filip M, Vlassa M, Copaciu F, Coman V (2012) Identification of anthocyanins and anthocyanidins from berry fruits by chromatographic and spectroscopic techniques to establish the juice authenticity from market. J Planar Chromatogr Mod TLC 25:534–541
Primetta AK, Jaakola L, Ayaz FA, Inceer H, Riihinen KR (2013) Anthocyanin fingerprinting for authenticity studies of bilberry (Vaccinium myrtillus L.). Food Control 30:662–667
Gardana C, Ciappellano S, Marinoni L, Fachechi C, Simonetti P (2014) Bilberry adulteration: identification and chemical profiling of anthocyanins by different analytical methods. J Agric Food Chem 62:10998–11004
González-Neves G, Favre G, Piccardo D, Gil G (2015) Anthocyanin profile of young red wines of Tannat, Syrah and Merlot made using maceration enzymes and cold soak. Int J Food Sci Technol 51:260–267
Viljanen K, Heiniö R, Juvonen R, Kössö T, Puupponen-Pimiä R (2014) Relation of sensory perception with chemical composition of bioprocessed lingonberry. Food Chem 157:148–156
Skrede G, Wrolstad RE, Durst RW (2000) Changes in anthocyanins and polyphenolics during juice processing of highbush blueberries (Vaccinium corymbosum L.). J Food Sci 65:357–364
Grassin C, Coutel Y (2009) In: Whitehurst RJ, van Oort M (eds) Enzymes in food technology, 1st edn. Wiley, Oxford
Sandri IG, Lorenzoni CMT, Fontana RC, da Silveira MM (2013) Use of pectinases produced by a new strain of Aspergillus niger for the enzymatic treatment of apple and blueberry juice. LWT Food Sci Technol 51:469–475
Acosta-Estrada BA, Gutiérrez-Uribe JA, Serna-Saldívar SO (2014) Bound phenolics in foods, a review. Food Chem 152:46–55
Landbo A, Meyer AS (2001) Enzyme-assisted extraction of antioxidative phenols from black currant juice press residues (Ribes nigrum). J Agric Food Chem 49:3169–3177
Kashyap D, Vohra P, Chopra S, Tewari R (2001) Applications of pectinases in the commercial sector: a review. Bioresour Technol 77:215–227
Bhat MK, Bhat S (1997) Cellulose degrading enzymes and their potential industrial applications. Biotechnol Adv 15:583–620
Albersheim P, Darvill AG, O’Neill MA, Schols HA, Voragen Alphons G J (1996) An hypothesis: the same six polysaccharides are components of the primary cell walls of all higher plants. Prog Biotechnol 14:47–55
Thakur BR, Singh RK, Handa AK (1997) Chemistry and uses of pectin—a review. Crit Rev Food Sci Nutr 37:47–73
Yadav S, Yadav PK, Yadav D, Yadav KD (2009) Pectin lyase: a review. Process Biochem 44:1–10
Huang HT (1955) Fruit color destruction, decolorization of anthocyanins by fungal enzymes. J Agric Food Chem 3:141–146
Wightman JD, Wrolstad RE (1996) β-Glucosidase activity in juice-processing enzymes based on anthocyanin analysis. J Food Sci 61:544–548
Buchert J, Koponen JM, Suutarinen M, Mustranta A, Lille M, Törrönen R, Poutanen K (2005) Effect of enzyme-aided pressing on anthocyanin yield and profiles in bilberry and blackcurrant juices. J Sci Food Agric 85:2548–2556
Koponen JM, Buchert J, Poutanen KS, Torronen AR, Törrönen AR (2008) Effect of pectinolytic juice production on the extractability and fate of bilberry and black currant anthocyanins. Eur Food Res Technol 227:485–494
Pricelius S, Murkovic M, Souter P, Guebitz GM (2009) Substrate specificities of glycosidases from Aspergillus species pectinase preparations on elderberry anthocyanins. J Agric Food Chem 57:1006–1012
Fernandes A, Brás NF, Mateus N, de Freitas V (2014) Understanding the molecular mechanism of anthocyanin binding to pectin. Langmuir 30:8516–8527
Koponen JM, Happonen AM, Auriola S, Kontkanen H, Buchert J, Poutanen KS, Törrönen AR (2008) Characterization and fate of black currant and bilberry flavonols in enzyme-aided processing. J Agric Food Chem 56:3136–3144
Heffels P, Weber F, Schieber A (2015) Influence of accelerated solvent extraction and ultrasound-assisted extraction on the anthocyanin profile of different Vaccinium species in the context of statistical models for authentication. J Agric Food Chem 63:7532–7538
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
Bailey MJ, Biely P, Poutanen K (1992) Interlaboratory testing of methods for assay of xylanase activity. J Biotechnol 23:257–270
Negrulescu A, Patrulea V, Mincea MM, Ionascu C, Vlad-Oros BA, Ostafe V (2012) Adapting the reducing sugars method with dinitrosalicylic acid to microtiter plates and microwave heating. J Braz Chem Soc 23:2176–2182
Wood IP, Elliston A, Ryden P, Bancroft I, Roberts IN, Waldron KW (2012) Rapid quantification of reducing sugars in biomass hydrolysates: improving the speed and precision of the dinitrosalicylic acid assay. Biomass Bioenergy 44:117–121
Sadana JC, Shewale JG, Deshpande MV (1980) High cellobiase and xylanase production by Sclerotium rolfsii UV-8 mutant in submerged culture. Appl Environ Microbiol 39:935–936
Puupponen-Pimiä R, Nohynek L, Ammann S, Oksman-Caldentey K, Buchert J (2008) Enzyme-assisted processing increases antimicrobial and antioxidant activity of bilberry. J Agric Food Chem 56:681–688
Bloor SJ (2001) Deep blue anthocyanins from blue Dianella berries. Phytochemistry 58:923–927
Bruchmann A, Fauveau C (2009) In: Whitehurst RJ, van Oort M (eds) Enzymes in food technology, 1st edn. Wiley, Oxford
Laaksonen O, Sandell M, Nordlund E, Heiniö R, Malinen H, Jaakkola M, Kallio H (2012) The effect of enzymatic treatment on blackcurrant (Ribes nigrum) juice flavour and its stability. Food Chem 130:31–41
Bagger-Jørgensen R, Meyer AS (2004) Effects of different enzymatic pre-press maceration treatments on the release of phenols into blackcurrant juice. Eur Food Res Technol 219:620–629
Landbo A, Meyer AS (2004) Effects of different enzymatic maceration treatments on enhancement of anthocyanins and other phenolics in black currant juice. Innov Food Sci Emerg Technol 5:503–513
Landbo A, Kaack K, Meyer AS (2007) Statistically designed two step response surface optimization of enzymatic prepress treatment to increase juice yield and lower turbidity of elderberry juice. Innov Food Sci Emerg Technol 8:135–142
Mieszczakowska-Frąc M, Markowski J, Zbrzeźniak M, Płocharski W (2012) Impact of enzyme on quality of blackcurrant and plum juices. LWT Food Sci Technol 49:251–256
Pap N, Pongrácz E, Jaakkola M, Tolonen T, Virtanen V, Turkki A, Horváth-Hovorka Z, Vatai G, Keiski RL (2010) The effect of pre-treatment on the anthocyanin and flavonol content of black currant juice (Ribes nigrum L.) in concentration by reverse osmosis. J Food Eng 98:429–436
Kapasakalidis PG, Rastall RA, Gordon MH (2009) Effect of a cellulase treatment on extraction of antioxidant phenols from black currant (Ribes nigrum L.) pomace. J Agric Food Chem 57:4342–4351
Brownmiller C, Howard LR, Prior RL (2008) Processing and storage effects on monomeric anthocyanins, percent polymeric color, and antioxidant capacity of processed blueberry products. J Food Sci 73:H72–H79
Volden J, Borge GIA, Bengtsson GB, Hansen M, Thygesen IE, Wicklund T (2008) Effect of thermal treatment on glucosinolates and antioxidant-related parameters in red cabbage (Brassica oleracea L. ssp. capitata f. rubra). Food Chem 109:595–605
Hager A, Howard LR, Prior RL, Brownmiller C (2008) Processing and storage effects on monomeric anthocyanins, percent polymeric color, and antioxidant capacity of processed black raspberry products. J Food Sci 73:H134–H140
Lee J, Durst RW, Wrolstad RE (2002) Impact of juice processing on blueberry anthocyanins and polyphenolics: comparison of two pretreatments. J Food Sci 67:1660–1667
Srivastava A, Akoh CC, Yi W, Fischer J, Krewer G (2007) Effect of storage conditions on the biological activity of phenolic compounds of blueberry extract packed in glass bottles. J Agric Food Chem 55:2705–2713
Patras A, Brunton NP, Da Pieve S, Butler F (2009) Impact of high pressure processing on total antioxidant activity, phenolic, ascorbic acid, anthocyanin content and colour of strawberry and blackberry purées. Innov Food Sci Emerg Technol 10:308–313
Funding
This research project was financially supported by the German Ministry of Economics and Technology (via AiF) and the FEI (Forschungskreis der Ernährungsindustrie e.V., Bonn). Project AiF 16645 N.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Compliance with ethics requirements
This article does not contain any studies with human or animal subjects.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Heffels, P., Bührle, F., Schieber, A. et al. Influence of common and excessive enzymatic treatment on juice yield and anthocyanin content and profile during bilberry (Vaccinium myrtillus L.) juice production. Eur Food Res Technol 243, 59–68 (2017). https://doi.org/10.1007/s00217-016-2722-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-016-2722-0