Abstract
Bilberries (Vaccinium myrtillus L.) and black currants (Ribes nigrum L.), dark blue berries rich in anthocyanins, were processed with an aid of commercial pectinolytic enzyme preparations, and the effect of processing on berry anthocyanins was investigated. The enzyme preparations were dosed based on their polygalacturonase activity from 1 to 100 nkat/g of berry mash. The juice yields were determined by weighing, and anthocyanin analyses were performed with HPLC. The bilberry and black currant juice yields increased significantly in enzyme-aided treatments with comparison to control, even with the lowest (1 nkat/g) polygalacturonase dosage. The anthocyanin yield increased by up to 83% for bilberries and up to 58% for black currants in enzyme-aided treatments as compared to control. The results showed that higher polygalacturonase dosage was needed for black currant to achieve the maximal juice and anthocyanin yields than for bilberries. The stability and the profile of extracted anthocyanins were greatly affected by the glycosidase side activities present in the enzyme preparations, which were able to hydrolyze certain anthocyanins to the corresponding aglycones. In addition, the data indicate that anthocyanidin rutinosides were more easily extracted than those of glucosides, which prevailed over the arabinosides and galactosides. Thus, prior to processing it is important to know the intact anthocyanin structures of the raw material, and the activity profile of the enzyme preparation to obtain optimal anthocyanin extractability and enzyme dosage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anthocyanins, secondary metabolites of plants, are water-soluble glycosides and acylglycosides of anthocyanidins, which are polyhydroxyl and polymethoxyl derivatives of 2-phenylbenzopyrylium (flavylium cation) (Fig. 1) [1]. Anthocyanins belong to the class of flavonoids, which posses a typical A-ring benzoyl and B-ring hydroxycinnamoyl structure. Over 4,000 flavonoids have been identified in plants, out of which approximately 600 naturally occurring anthocyanin structures have been determined [2]. Anthocyanins are mostly responsible for the red and blue colours of fruits and flowers, and therefore they can be used as food colourants. In addition, anthocyanins possess a wide range of biological activities, which suggest that they could have beneficial effects on human health. Their antioxidant and free radical scavenging, antimutagenic, anti-inflammatory, and anticarcinogenic properties have been extensively reviewed [3–6].
Berries are rich in many health promoting substances, such as vitamin C, soluble fibre, and flavonoids. Bilberries (Vaccinium myrtillus L.) and black currants (Ribes nigrum L.) are usually consumed as fresh berries, jams, purees, nectars and juices. Currently also berry wines and dried berry products are available, and the interest in using berry based ingredients in various foods is increasing. Bilberries and black currants are rich in anthocyanins, which have been identified and quantified in previous studies [7–10]. Arabinosides, galactosides and glucosides of cyanidin, delphinidin, malvidin, peonidin and petunidin are found in bilberry, whereas mainly four anthocyanins are present in black currant, representing glucosides and rutinosides of delphinidin and cyanidin.
Pectinolytic enzymes are commonly used in industrial berry processing to facilitate juice extraction. With these enzymes the cell wall network is disrupted and subsequently the juice yield is enhanced [11, 12]. Concomitantly with the juice yield, the extractability of phenolic components is increased [13, 14]. We have previously shown that the use of a commercial pectinolytic enzyme preparation in berry juice processing may also have a negative impact on the yield of extracted anthocyanins [13]. The objective of the current study was to investigate the effects of enzyme-assisted processing on the yield and anthocyanin profile of bilberry and black currant juices, with the aim to minimize the enzyme dosage and negative effects, and to maximize the extractability of anthocyanins.
Materials and methods
Samples
Bilberries and black currants were obtained from Kiantama Ltd. (Suomussalmi, Finland). Berries were harvested in 2002, and kept frozen (−20 °C) until processing.
Chemicals, enzymes and enzyme activities
Acetonitrile (HPLC grade, >99.8%), methanol (HPLC grade, >99.8%), formic acid (pro analysi, 98–100%), and hydrochloric acid (HCl) were purchased from VWR International Ltd. (Espoo, Finland). Anthocyanin mixture of 3-O-beta-glucosides of delphinidin, cyanidin, petunidin, peonidin, pelargonidin and malvidin was obtained from Polyphenols Laboratories AS (Sandnes, Norway). Four commercial enzyme preparations were used. Econase CE was obtained from AB Enzymes (Rajamäki, Finland), Biopectinase CCM from Quest International Ireland Ltd. (Carrigaline, Ireland), Pectinex Smash XXL from Novozymes (Bagsvaerd, Denmark), and Pectinex BE-3L from Novozymes (Dittinger, Switzerland). Hydroxyethylcellulose and citrus pectin were obtained from Fluka Chemie (Buchs, Switzerland), locust bean gum, p-nitrophenyl-alpha-L-arabinofuranoside and p-nitrophenyl-beta-L-arabinofuranoside from Sigma-Aldrich Chemie (Steinheim, Germany), birchwood glucuronoxylan from Roth (Karlsruhe, Germany), beta-glucan from Megazyme (Wicklow, Ireland), polygalacturonic acid from Koch-Light Laboratories (Suffolk, England), p-nitrophenyl-beta-D-glucopyranoside from Calbiochem (San Diego, CA), and o-nitrophenyl-beta-d-galactopyranoside from Merck (Darmstadt, Germany). The activity profiles of the enzyme preparations at pH 3.5 were determined as described previously [13].
Juice processing
The details of a laboratory-scale juice production have been presented earlier [13], and are only briefly described here. Prior to incubation with enzyme preparations, bilberries and black currants were thawed and mashed. A 50 g sample of the berry mash was weighed, and 5.0 mL of water–enzyme solution was added. The enzyme preparations were dosed at 1, 10 and 100 nkat/g of mash (Econase CE, Biopectinase CCM and Pectinex BE 3-L) or 1, 10 and 20 nkat/g of mash (Pectinex Smash XXL) based on their polygalacturonase (PG) activity representing enzyme preparation volumes from 0.03 to 92 mL/kg berry mash. After the processing juice yield was determined by weighing the pressed juice, which was compared to the initial sample weight. The treatments were carried out in four replicates. Control treatment was carried out correspondingly but omitting the enzymes. The samples were frozen and stored at −20 °C until anthocyanin analysis.
Analysis of anthocyanins
Frozen juices were thawed, and a 4.7 mL sample was weighed for the analysis. After adjusting the pH below 1 by adding 50 μL of HCl (c(HCl) = 12 mol/L), the sample volume was adjusted to 5 mL with methanol. Prior to HPLC analysis, the samples were filtered through a 0.45 μm GH polypropylene syringe filter (Pall Life Sciences, Ann Arbor, MI). For the anthocyanins present in unprocessed berries the details of the extraction procedure have been described elsewhere [7].
A 10 μL injection of the filtrates was separated on a 250 × 4.6 mm i.d., 5 μm LiChroCart Purospher Star RP-18e column (Merck, Darmstadt, Germany) with a 4 × 4 mm i.d. guard column using a HP 1100 series HPLC (Waldbronn Analytical Division, Waldbronn, Germany) equipped with a quaternary pump, an autosampler and a diode array detector linked to a HP ChemStation data handling system. The analysis of anthocyanins was performed using 8.5% (v/v) aqueous formic acid as eluent A and HPLC grade acetonitrile:methanol (85:15, v/v) as eluent B. The flow rates of the mobile phase were 0.85 mL/min for 0–12 min and 0.7 mL/min for 13–90 min. The gradient used was as follows: 0–2 min, 4–6% B; 2–4 min, 6–8% B; 4–13 min, 8–9% B; 13–20 min, 9–10% B; 20–40 min, 10–11% B; 40–53 min, 11% B; 53–65 min, 11–19% B; 65–81 min 19–35% B; 81–84 min, 35–80% B, followed by an isocratic elution for 4 min and then returning to the initial conditions for 6 min before the next injection. Anthocyanins were detected at 520 nm. Reference compounds, retention times and UV/Vis spectra provided by HPLC–DAD were used to verify the identity of anthocyanins published in previous studies [7–10]. Quantification of anthocyanins was carried out using representative anthocyanidin 3-O-glucosides as external standards and was based on the method described earlier by Koponen and co-workers [5]. The concentrations are expressed in mg/kg of juice, for the weight of the aglycone.
A hydrolysis of bilberry anthocyanins was performed to verify the presence of anthocyanidins in the processed juices. The control bilberry juice was treated with HCl (final concentration in solution c(HCl) = 0.84 mol/L) at 85 °C for 90 min to hydrolyze the anthocyanidin glycosides to the corresponding aglycones. The identification was based on the retention times and the data obtained from a spiked juice sample.
Statistical analysis
Differences between non-enzymatic (control) and enzyme treatments were tested using one-way analysis of variances (ANOVA) with Bonferroni post hoc test. All data were processed by SPSS 14.0 for Windows (SPSS inc., Chicago, IL). Differences at p < 0.05 were considered statistically significant.
Results and discussion
Activity profile of enzyme preparations
The four enzyme preparations used were dosed based on their PG activity, which resulted in the presence of variable amounts of other enzyme activities in the treatments. The activity profile of the enzyme preparations is shown in Table 1, where all the values are expressed relative to the PG activity of 10 nkat. Polygalacturonase, beta-glucanase and xylanase were the main activities in Biopectinase CCM and Pectinex BE 3-L preparations and pectin lyase (PL) in Pectinex Smash XXL preparation. Endoglucanase, beta-glucanase and xylanase dominated in Econase CE. In comparison to other preparations, Pectinex Smash XXL contained both PL and PG activity. Thus the calculated pectin depolymerizing activity, i.e. the sum of PG and PL activities, for example with 10 nkat/g PG dosage was 99 nkat/g, which was substantially higher as compared to the other preparations used at the same PG dosage. Additionally, alpha-arabinosidase, beta-arabinosidase, beta-galactosidase and beta-glucosidase side activities were present in Econase CE, Biopectinase CCM and Pectinex BE 3-L preparations, whereas Pectinex Smash XXL did not contain any of these activities. Based on the enzyme dosages used in the present study, the highest activity for beta-galactosidase was found in Pectinex BE 3-L treatments, for alpha-arabinosidase and beta-glucosidase in Econase CE treatments and for beta-arabinosidase in Pectinex BE 3-L and Econase CE treatments.
Juice yields
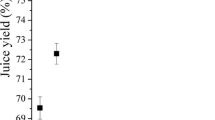
The use of enzyme preparation in the processing of bilberries increased juice yields significantly (p < 0.001) in all the experiments compared to the control treatment (no enzyme added) (Fig. 2a). All enzyme preparations produced juice yields of over 72%, with the exception of Econase CE (cellulase). This is apparently due to lack of some necessary berry cell wall degrading activities in Econase CE, or alternatively the reason is the suboptimal performance of this Trichoderma derived enzyme preparation at acidic pH (pH of the berry mash was 3.0 ± 0.2). Within the enzyme-aided treatments, neither the origin of PG activity (i.e. the preparation used) nor the PG dosage had any significant (p > 0.05) effect on the juice yield, with the exception of the Biopectinase CCM treatment with the lowest PG dosage and all the Econase CE treatments. In our previous report [13] we noted that bilberry processing with an extensive enzyme dosage (PG dosage 1,000 nkat/g berry mash) increased the juice yield by 16–19%. The present data shows that this level of increase is already achieved with lower enzyme dosages. Thus we conclude that a very moderate PG dosage (1 nkat/g berry mash or possibly lower) is effective to improve the bilberry juice yield.
The juice yield obtained in the enzymatic processing of black currants ranged from 51 to 65%, and increased significantly (p < 0.05) (with the exception of two treatments with low enzyme dosages) as compared to control treatment (47%) (Fig. 2b). These yields are somewhat lower than those reported by Landbo and Meyer [14], but significant variations in juice yields have usually been obtained depending on pressing device. Compared to bilberry treatments, a higher enzyme dosage was needed to obtain the maximal effect. The black currant juice yields tended to increase with increased enzyme dosage, as previously noted by Bagger-Jørgensen and Meyer [15]. However, the moderate PG dosage (10 nkat/g) was as effective as the highest PG dosage in Econase CE and Pectinex Smash XXL treatments, whereas in Biopectinase CCM and Pectinex BE 3-L treatments the highest PG dosage was the most effective one. Moreover, the level of juice yield increase remained the same, even if black currants were treated with PG dosage of 1,000 nkat/g [13]. Black currant cell walls are known to be thicker and contain more pectins than those of bilberry [16]. Therefore pectinases had a more pronounced role in aiding the juice extractability in black currants as compared to bilberries. The juice yields obtained in laboratory-scale processing cannot be transferred directly into industrial processing scale. The industrial juice production contains other processing steps (fining, pasteurization, concentration) which affect the final juice yield. However, the final yield is always dependent on the enzymatic incubation step, which is mimicked by our laboratory-scale processing. Thus, the effect of different enzyme preparations and dosages is most likely the same for industrial processing, although the yields may differ from those presented here.
Effect of enzymatic processing on concentration of anthocyanins
The anthocyanin content in unprocessed bilberries used for juice processing was 7,640 mg/kg consisting of 15 individual compounds representing anthocyanidin glucosides (33%), arabinosides (30%), and galactosides (37%). After the processing, the total anthocyanin concentration (TAC), including both glycosidic and aglyconic forms of anthocyanidins, found in control juice was 3,520 mg/kg and varied from 2,970 to 5,140 mg/kg in enzyme treated juices (Table 2). Althought no free anthocyanidins (aglycones) were detected in unprocessed bilberries, these compounds were observed in processed juices. In most of the experiments the TAC found in bilberry juices was not significantly increased by enzymatic processing.
The anthocyanin content in unprocessed black currants used for processing was 3,170 mg/kg consisting of glucosides (21%) and rutinosides (79%) of delphinidin and cyanidin. After the processing, the TAC in control juice was 2,790 mg/kg and varied in enzyme treated juices from 2,870 to 3,330 mg/kg (Table 2). The use of moderate or high enzyme dosages during black currant processing increased significantly the TAC of juices as compared to control treatment. Interestingly, the processing of black currant resulted in more frequent differences between control and enzyme treated juices than in the case of bilberries (Table 2).
For both berries, the highest TAC was observed in juices treated with the highest dosage of Pectinex Smash XXL. This may be explained by the fact that the total pectin depolymerizing activity in Pectinex Smash XXL (as mentioned above) was much higher than that in other preparations.
Effect of enzymatic processing on yield of anthocyanins
The TAC alone, however, is insufficient to evaluate the effectiveness of enzymatic processing. Thus, the total anthocyanin yield (TAY), including both glycosidic and aglyconic forms of anthocyanidins, was used to describe the effectiveness of enzymatic processing on berry anthocyanins. The TAY was calculated according to the following equation:
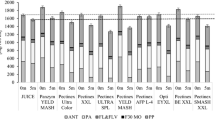
The TAY in enzymatically processed bilberry juices was significantly higher than in control juice, with the exception of two treatments (Econase CE and Biopectinase CCM) with the lowest enzyme dosage (Fig. 3). The highest TAYs were clearly obtained by Pectinex Smash XXL, the preparation without any side activities. The TAYs in the enzyme-aided treatments were significantly affected by the used PG (or pectin depolymerizing) dosage. With the exception of Pectinex BE 3-L treated juices, the lowest TAYs were always observed in treatments with the lowest PG dosage, and significantly differed from treatments with the highest PG dosage. Although the highest TAYs were achieved with the highest PG dosage, they were not significantly higher than those in juices treated with moderate PG dosage (10 nkat/g). The TAYs obtained with PG dosage of 1,000 nkat/g [13] are at the same level or even lower than those observed in this study suggesting that an optimal PG or pectin depolymerizing dosage is between 10 and 100 nkat/g.
Based on the UV/Vis spectra, retention times and literature data [7–10] the anthocyanin pool found in bilberry and processed juices consisted of five anthocyanidin structures, which were linked with three different sugar moieties. Both glycosides and aglycones were detected in processed bilberry juices, and their profiles are presented in Table 3 and Fig. 4a, b. The presence of aglycones was confirmed by acid hydrolysis. The control bilberry juice was treated with HCl to hydrolyze the anthocyanidin glycosides to the corresponding aglycones (Fig. 4c). The retention times of five anthocyanidins present in the hydrolysate were the same as in processed juices. Furthermore, Pectinex BE 3-L treated juice was spiked with the hydrolysate resulting in co-elution of five aglycones present in both processed juice and hydrolysate (Fig. 4d).
HPLC chromatograms at 520 nm of anthocyanins and anthocyanidins in control (a), Pectinex BE 3-L treated (100 nkat/g) (b), hydrolyzed control (c) and spiked Pectinex BE 3-L treated (d) bilberry juice. Peak identification: 1 delphinidin 3-galactoside; 2 delphinidin 3-glucoside; 3 cyanidin 3-galactoside; 4 delphinidin 3-arabinoside; 5 cyanidin 3-glucoside; 6 petunidin 3-galactoside; 7 cyanidin 3-arabinoside; 8 petunidin 3-glucoside; 9 peonidin 3-galactoside; 10 petunidin 3-arabinoside; 11 delphinidin (aglycone); 12 peonidin 3-glucoside; 13 malvidin 3-galactoside; 14 peonidin 3-arabinoside; 15 malvidin 3-glucoside; 16 malvidin 3-arabinoside; 17 cyanidin (aglycone); 18 petunidin (aglycone); 19 peonidin (aglycone); 20 malvidin (aglycone)
The anthocyanidin galactoside yields were higher in all Pectinex Smash XXL treated juices and juices treated with 10 nkat/g of Econase CE and Biopectinase CCM as compared to control treatment. The anthocyanidin galactoside yields were not increased by the highest dosages of Econase CE and Biopectinase CCM. Moreover, in juices treated with Pectinex BE 3-L the anthocyanidin galactoside yield was unchanged with the lowest dosage and decreased 50–93% by higher enzyme dosages (10 and 100 nkat/g) as compared to control. These observations are highly linked to the beta-galactosidase side activity present in the pectinolytic enzyme preparations. Wightman and Wrolstad [17] demonstrated that the beta-galactosidase dosage of 2 nkat/g of berry mash can effectively hydrolyze the cyanidin 3-galactosides during cranberry juice processing. The present results support this fact. We may conclude that in most of the experiments the beta-galactosidase dosage of 2 nkat/g was high enough to significantly hydrolyze the galactose-substituted anthocyanins to their aglycone and sugar moieties.
The yield of anthocyanidin glucosides and arabinosides was significantly higher in most of the enzymatic treatments as compared to control. With some exceptions, the highest yields of anthocyanidin glucosides and arabinosides were achieved with the highest PG dosage and the lowest yields with the lowest (1 nkat/g) dosage.
Interestingly, the results indicate that the glycoside moieties are not equally accessible to the action of the glycosidases. Higher activities of beta-glucosidase and beta-arabinosidase were needed to achieve a similar hydrolyzing effect as with beta-galactosidase. Some evidence for the action of beta-glucosidase and beta-arabinosidase was observed with the dosages of 5 nkat/g (in Econase CE treatment) and 2 nkat/g (in Pectinex BE 3-L treatment), respectively, but these effects were rather mild. The results presented by Mandalari and co-workers [18] indicate that the effectiveness of glycosidases is not only dependent on the activity, but also on both the structure of flavonoid substrate and the microbial origin of enzyme preparation. In addition to glycosidase activities in enzyme preparation, the anthocyanins may be degraded by an action of endogenous enzymes, for example polyphenol oxidase (PPO) activity together with chlorogenic acid present in berries as suggested by Kader and co-workers [19].
As a result of the degradation of anthocyanins, the anthocyanidin (aglycone) yield increased significantly in bilberry juices, and was clearly dependent on the used glycosidase dosage. This increase is unwanted, because the processing and storage stability of aglycones is expected to be lower than that of anthocyanins. The degradation of red-blue pigments results in brown-coloured juices with poor quality. A low level of the aglycones was also detected in control juice. This indicates that not only the added enzymes, but also the other processing steps and conditions can induce minor hydrolysis of anthocyanins. In addition, the hydrolysis in control treatment may be due to endogenic hydrolases, which can be liberated from the cell wall matrix during processing.
The distribution of different anthocyanidin aglycones was not affected by enzymatic processing. The relative quantities of different anthocyanidins (including both glycosidic and aglyconic forms) in unprocessed berries and all the processed juices were: 37–40% (delphinidins), 28–30% (cyanidins), 14–16% (malvidins), 12–14% (petunidins) and 4–6% (peonidins). In contrast to the study of Skrede and co-workers [20], our results indicate that the different aglycone structures possess similar stability during the juice production. The instability of an intact anthocyanin molecule is a cause of the action of glycosidases, and therefore dependent on the structure of sugar rather than aglycone moiety.
The enzymatic processing significantly increased the TAY in most of the black currant juices as compared to control juice (Fig. 5). As in the case of bilberry processing (above), the used PG dosage had an effect on the TAY observed in black currant processing. There were significant differences in TAY between treatments with the highest and the lowest enzyme dosages, but not with the highest and moderate (10 nkat/g) dosage. In terms of the highest TAY, no significant differences between the enzyme preparations were noted. Our previously published results [13] reveal that the TAYs obtained from treatments with PG dosage of 1,000 nkat/g are slightly higher than those from the present treatments. This indicates that an optimal PG or pectin depolymerizing dosage for black currant processing is probably near to 100 nkat/g.
The yields of four individual anthocyanins, as well as the TAY detected in enzymatically processed black currant juices increased in a dosage-dependent manner. The relative quantity of delphinidin 3-glucoside (12–15%), delphinidin 3-rutinoside (45–47%), cyanidin 3-glucoside (5–6%) and cyanidin 3-rutinoside (34–36%) remained the same in unprocessed berries and all the processed juices (Fig. 6). This finding is supported by a previous study [14], where black currants were processed using ten different pectolytic enzymes. Moreover, no free anthocyanidins were detected in processed black currant juices. This supports the fact that rutinosidase and rhamnosidase activities were not present and the beta-glucosidase activity in the enzyme preparations studied was unable to hydrolyze the black currant anthocyanins to the corresponding aglycones. This finding is very interesting, because the beta-glucosidase activity in the present experiments was up to 5 nkat/g, a level which in the case of beta-galactosidase was high enough to hydrolyze the anthocyanidin galactosides in bilberry processing. This indicates that the beta-glucosidase in these preparations is not as effective as beta-galactosidase and/or the structure of the anthocyanidin glucosides restricts the glucosidase action and/or there are some inhibitors for beta-glucosidase in black currant.
As shown in this paper, the difference between control and enzyme treatments is more evident in the TAY than in the TAC. This demonstrates that the liberation of anthocyanins is highly dependent on the degradation of berry cell wall matrix. However, the liberation of different anthocyanidin glycosides into the juice was not similar. The anthocyanidin glucosides present in bilberries were extracted more easily than those of galactosides and arabinosides. The yield of anthocyanidin galactosides and arabinosides was less than 36% in treatments with the moderate pectin depolymerizising dosage (10 nkat/g), whereas the yield of glucosides was already more than 40% in treatments with the lowest enzyme dosage of 1 nkat/g. Moreover, the extraction of rutinosides was more abundant than that of glucosides in black currant processing resulting in the highest yield for rutinosides as compared to other glycosides. This suggests that some anthocyanins are more tightly bound to the berry matrix than others, or they have differences in solubility. These factors are still poorly known and remain to be investigated. We conclude that the sugar moiety influences the extractability of berry anthocyanins, and to achieve the same yield for anthocyanidin galactosides and arabinosides as that for glucosides and rutinosides, higher PG dosages have to be used in juice production.
The total anthocyanin yield from unprocessed berries was 28–51% for bilberry and 42–66% for black currant. This fact indicates that theoretically up to 70% of berry anthocyanins are still in press residues, although the processing steps will certainly cause some losses of these compounds. Previous studies [21, 22] have demonstrated that blueberry (Vaccinium corymbosum cv. Rubel) and black currant (Ribes nigrum var. Ben Lomond) press residues are a good source of anthocyanins and other polyphenols, and may have nutritional benefits and the potential to be natural colourants. Efficient technologies to disrupt the residual cell wall material in the press residues are, however, needed. It can be anticipated that the press residues are highly concentrated with rather resistant polymer structures, into which the anthocyanins and other phenolics are imbedded.
Conclusion
In conclusion, the juice yield and the extractability of anthocyanins present in bilberries and black currant increased by the use of enzymatic processing. For bilberries, the use of the lowest enzyme dosages (PG dosage of 1 nkat/g) already delivered a significant juice yield increase as compared to control, and was not significantly improved with the higher dosages (PG dosages from 10 to 100 nkat/g). However, the extractability of anthocyanins into the juice was significantly improved with the higher dosages, but no clear improvement was observed with the highest enzyme dosages compared to the moderate PG dosage (10 nkat/g). For black currant, the highest juice and anthocyanin yields were achieved with the highest enzyme dosages used. This indicates that the pectin depolymerizising dosage near to 100 nkat/g might be needed to produce the optimal anthocyanin and juice yields in black currant processing.
The hydrolysis of anthocyanins may occur during the juice processing, if the enzyme preparations possess high monoglycosidase activities. This hydrolyzing effect, however, is dependent on both the anthocyanin structures present in berry/fruit and the activity profile of enzyme preparation. Moreover, the action of specific enzymes may be affected by several other factors, such as the processing time and temperature, pH, the origin of enzyme, and other substances (inhibitors) present in berries. For industrial point of view the aglycones are more unstable than the glycosidic forms, and this may result in an unwanted change in the juice colour. The colour quality of juices is also influenced by non-enzymatic factors, which may degrade the anthocyanins and aglycones. Processing and storage conditions (time, temperature, light, pH, clarification, level of oxygen), as well as the level of sugar and ascorbic acid, other phenolics and highly reactive compounds present in juice may affect the colour stability [23–25]. These aspects related to the storage stability of the colour in the enzymatically processed juices remain to be studied.
Thus, we note that for the presented enzyme-aided berry juice processing conditions, the pectin depolymerizing dosage should be at least 10 nkat/g of berry mash, and the monoglycosidase side activity should not exceed the dosage of 2 nkat/g of berry mash. In this way, the extractability and the stability of anthocyanins can be enhanced during the processing resulting in the berry juices with increased level of health-promoting compounds.
Abbreviations
- HCl:
-
Hydrochloric acid
- PG:
-
Polygalacturonase
- PL:
-
Pectin lyase
- TAC:
-
Total anthocyanin concentration
- TAY:
-
Total anthocyanin yield
References
Macheix JJ, Fleuriet A, Billot J (1990) Flavonoids. In: Macheix JJ, Fleuriet A, Billot J (eds) Fruit phenolics. CRC Press, Boca Raton, pp 39–80
Andersen OM, Jordheim M (2005) Anthocyanins. In: Andersen OM, Markham KR (eds) Flavonoids: chemistry, biochemistry and applications. CRC Press, Boca Raton
Clifford MN (2000) J Sci Food Agric 80:1063–1072
Kong JM, Chia LS, Goh NK, Chia TF, Brouillard R (2003) Phytochemistry 64:923–933
Hou DX (2003) Curr Mol Med 3:149–159
Prior RL (2003) Am J Clin Nutr 78(suppl):570S–578S
Koponen JM, Happonen AM, Mattila PH, Törrönen AR (2007) J Agric Food Chem 55:1612–1619
Goiffon JP, Brun M, Bourrier MJ (1991) J Chromatogr 537:101–121
Kähkönen M, Heinämäki J, Olliainen V, Heinonen M (2003) J Sci Food Agric 83:1403–1411
Määttä-Riihinen K, Kamal-Eldin A, Mattila P, González-Paramás A, Törrönen R (2004) J Agric Food Chem 52:4477–4485
Pilnik W, Voragen AG (1991) The significance of endogenous and exogenous pectic enzymes in fruit and vegetable processing. In: Fox PF (ed) Food enzymology, vol 1. Elsevier, London, pp 303–336
Voragen AG, Pilnik W, Thibault JF, Axelos MA, Renard CM (1995) Pectins. In: Stehen AM (ed) Food polysaccharides and their applications. Marcel Dekker, New York, pp 287–339
Buchert J, Koponen JM, Suutarinen M, Mustranta A, Lille M, Törrönen AR, Poutanen K (2005) J Sci Food Agric 85:2548–2556
Landbo AK, Meyer A (2004) Inn Food Sci Emerg Technol 5:303–313
Bagger-Jørgensen R, Meyer A (2004) Eur Food Res Technol 219:620–629
Hilz H, Bakx E, Schols H, Voragen A (2005) Carbohydrate Polym 59:477–488
Wightman JD, Wrolstad RE (1995) J Food Sci 60:862–867
Mandalari G, Bennett RN, Kirby AR, Lo Curto RB, Bisignano G, Waldron KW, Faulds CB (2006) J Agric Food Chem 54:8307–8313
Kader F, Haluk JP, Nicolas JP, Metche M (1998) J Agric Food Chem 46:3060–3065
Skrede G, Wrolstad RE, Durst RW (2000) J Food Sci 65:357–364
Lee J, Wrolstad R (2004) J Food Sci 69:564–573
Landbo AK, Meyer A (2001) J Agric Food Chem 49:3169–3177
Bordignon-Luiz MT, Gauche C, Gris EF, Falcão LD (2007) LWT Food Sci Technol 40:594–599
Pacheco-Palencia LA, Palo Hawken P, Talcott ST (2007) Food Res Int 40:620–628
Hubbermann EM, Heins A, Stöckmann H, Schwarz K (2006) Eur Food Res Technol 223:83–90
Acknowledgments
This study has been carried out with financial support from the Commission of the European Communities, specific RTD programme “Quality of Life and Management of Living Resources”, contract number QLRT-CT-2002-02364 “Novel enzyme-aided extraction technologies for maximized yield and functionality of bioactive components in consumer products and ingredients from by-products”, acronym MAXFUN. It does not reflect its views and in no way anticipates the Commission’s future policy in this area. Riitta Alander is thanked for the technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Koponen, J.M., Buchert, J., Poutanen, K.S. et al. Effect of pectinolytic juice production on the extractability and fate of bilberry and black currant anthocyanins. Eur Food Res Technol 227, 485–494 (2008). https://doi.org/10.1007/s00217-007-0745-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-007-0745-2