Abstract
Combined effects of concentration of lactose (5 g/L), NaCl (20 g/L) and aero/anaerobiosis on production of tyramine by Enterococcus durans CCDM 53 were subjected to a study. The influence of the above factors and temperature of cultivation (10 ± 1 °C) was monitored under conditions applied in real technological processes of cheese production; the enterococci act as non-starter lactic acid bacteria. Production of tyramine by E. durans CCDM 53 was mainly influenced by both concentration of NaCl in cultivation medium and presence/absence of oxygen in the environment. The highest production of tyramine occurred during cultivation under anaerobic conditions in the presence of the highest (20 g/L) applied concentration of NaCl and lactose (5 g/L). In the media with equal concentrations of NaCl and lactose, the concentrations of tyramine grew higher under anaerobic conditions than in aerobic environment. Regarding cultivation media with various levels of NaCl and lactose, higher production of tyramine was always found in the anaerobic environment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bacteria of the Enterococcus genus are commonly present in various environments. They are often isolated from fermented foods, especially from dairy or meat products and olives. Their presence is often connected with the cheeses produced from sheep’s or goat’s milk, where they form constituents of so-called non-starter lactic acid bacteria (NSLAB). Enterococci (either as starters or NSLAB) play a very important role during ripening of cheeses mainly due to their metabolic activities, which influence the final sensory characteristics of cheeses [1–3]. Although enterococci have widely been applied in production of fermented foods and their application as food preservatives is being considered [4, 5], they are not “generally recognized as safe” (GRAS) at present. Their role is still highly controversial mainly due to some of their properties such as ability to produce virulent factors, their resistance to many antibiotics and also production of biogenic amines [1, 2, 6].
Together with the other lactic acid bacteria and enterobacteria, enterococci can be considered highly important producers of biogenic amines, mainly of tyramine and histamine [3, 7–9]. Biogenic amines (BA) are predominantly produced by decarboxylase activity of microorganisms present in foodstuff. Production of BA is a strain-specific property, thus the different strains of the same species can produce different BA [10–13].
Enterococci can survive pasteurization during milk processing [8], and they are even able to multiply in intestinal environment [14]. Regarding the capacity of these bacteria to produce BA in the above mentioned sites, it is necessary to study conditions under which they are able to form BA. Research on kinetics of BA production implemented under different experimental conditions can be very helpful for finding preventive measures to eliminate or reduce their formation and for appropriate increase in food safety.
The kinetics of decarboxylation reactions is influenced by a wide complex of factors. Reaction temperature, pH of cultivation medium, presence/absence of oxygen, concentration of NaCl, and growth phase of cells as well as availability of carbon sources, presence of growth factors and some others belong to the most important ones [13, 15–19].
The aim of the present study was to evaluate selected factors, which can influence formation of tyramine by E. durans, previously reported as its producer [11]. Because enterococci are often isolated from cheeses, minimal levels of the factors (additions of lactose, NaCl and cultivation in aerobic/anaerobic environments) were selected to follow real conditions that are commonly applied in technological processes of cheese production.
Materials and methods
Bacterial strain and culture conditions
Effects of additions of lactose and/or NaCl (both from Lach-Ner, Neratovice, Czech Republic) and cultivation under aerobic/anaerobic conditions on production of tyramine during production of cheeses were tested using the E. durans CCDM 53 (formerly Lactococcus lactis subsp. lactis) strain. It was obtained from the Cultures Collection of Dairy Microorganisms Laktoflora (MILCOM, Prague, Czech Republic). Cultivation of E. durans was performed in M17 broth (Oxoid, Basingstoke, UK) with addition of 2 g/L tyrosine (Sigma-Aldrich, St. Louis, USA) at 10 ± 1 °C in the period of 15 days. The pH of cultivation media ranged between 6.70 and 6.75 in all in all the experiments (CyberScan pH510, Eutech).
The research was focused on the following problems: (1) the effect of lactose additions (0.0, 2.5, 5.0, 7.5 and 10 g/L); (2) the effect of NaCl additions (0.0, 10 and 20 g/L); and (3) the influence of aerobic or anaerobic environments (totally 30 combinations of experimental factors).
The corresponding cultivation medium (5 mL of M17 with additions of 2 g/L tyrosine and respective amounts of lactose and/or NaCl) was inoculated with overnight culture (25 μL) of the tested strain (~106 CFU/mL). Half the samples were cultivated in aerobic and the other ones in anaerobic environment. Anaerobic environment was realized by covering the cultivation medium with sterile paraffin oil (1 mL; PLIVA-Lachema Diagnostika, Brno, Czech Republic). The sample collections were done on daily basis up to 7th day and then on 10th, 12th and 15th day of cultivation using randomized selection of two test tubes for each strain and each combination of the factors. The experimental design was implemented in triplicate. After cultivation, on the above days, measurements of pH and cell counts were done in the M17 media with different concentrations of lactose and salt and with aerobic or anaerobic conditions.
Growth parameters
Bacteria were counted on M17 agar plates (30 ± 1 °C; 2 days) on the days when the samples for determination of tyramine were collected. The dependence of the logarithm of the relative population size (y = ln (N t /N 0); where N t is total number of CFU/mL at t time and N 0 is total number of CFU/mL at the beginning of cultivation) on the time of culturing t (h) is described by means of the three-parameter Gompertz model:
where μ m is the maximum specific growth rate (h−1); λ is the lag time (h); A is the asymptote [A = ln(N ∞ /N 0)] defined as the maximum value of the logarithm of the relative population size reached [20]. To calculate μ, λ and A parameters, non-linear regression analysis (Marquardt–Levenburg method) was used for μ > 0, λ > 0 and A > 0.
Modeling of tyramine production
Production of tyramine in cultivation medium after bacteria incubation and subsequent cell removal (centrifugation at 10,000×g, 30 min; filtration by 0.45 μm membrane filter, Millipore) was monitored by ion-exchange chromatography (Automatic Amino Acids Analyzer AAA400, Ingos Prague, Czech Republic). The parameters for chromatographic separation were adjusted according to Buňková et al. [11]. Each fraction of the supernatant was analyzed thrice using the standard addition method. Gompertz models (modification acc. [20]) were applied for modeling of tyramine production by the E. durans strain. Relationship between concentration of tyramine y (mg/L) and reaction time t (h) was expressed as:
where μ BA is the maximum tyramine production rate (mg tyramine/L/h); λ BA is the delay period (the time that had passed before the tyramine production was firstly detected; h); A BA is the asymptote defined as the maximum tyramine production (mg/L). The Marquardt–Levenburg method was also used for μ BA > 0, λ BA > 0 and A BA > 0. For each level of each factor tested, the dependence of the tyramine content (y) on time (t) was calculated (using Gompertz model) six times (2 replicate tubes in three experiments). For a graphic illustration, the μ BA and λ BA values were converted from hours to days.
The absolute rate of tyramine production (mg/L day) can be related to the absolute growth rate (CFU/mL day) by a constant yield factor for tyramine formation (Y TYR/CFU, mg of tyramine/CFU) according to the Eq. 3 [16]:
where TYR t and TYR0 (mg/L) are the concentrations of tyramine at t and 0 times, respectively; and N t and N 0 are total numbers of CFU/mL at t and 0 times, respectively. The Marquardt–Levenburg method was applied for Y TYR/CFU > 0. The parameter Y TYR/CFU was expressed in mg × 109/CFU. Kruskall–Wallis and Mann–Whitney tests were used for comparison of the Gompertz parameters determined from growth curves and the tyramine production curves.
Results
Correlation coefficients (r) of (1) relation between the logarithm of the relative population size and cultivation time, (2) dependence of the tyramine concentration on the time of cultivation and also (3) models calculating yield factors (Y TYR/CFU) showed values above 0.81 (p < 0.05).
Among the studied factors, just presence of lactose influenced growth parameters of E. durans. We found a significant difference (p < 0.05) only between number of cells cultivated in medium with absence of lactose and in broth with presence of lactose (2.5–10 g/L; Table 1). Lactose concentration did not show any significant influence on the growth of the tested bacterial strains (p ≥ 0.05). Different concentrations of NaCl and aerobic/anaerobic conditions also exerted non-significant influence (p ≥ 0.05) on growth parameters of Gompertz model for E. durans (see Table 1).
Slow decrease in pH was observed in first 4 days of cultivation. More marked decrease in pH caused by acidification of cultivation medium was detected between 4th and 6th day. The pH of cultivation medium was practically constant until the end of cultivation (p ≥ 0.05; data not shown). Compared to the media with the lower concentrations of lactose (decrease in pH in the range 5.94–5.61 for the period of 15 days at p < 0.05), more substantial decrease in pH (in the range 5.42–5.18 in the same period) was observed in the media with the higher levels of lactose (5–10 g/L). The other tested factors had non-significant influence (p ≥ 0.05) on pH of cultivation media.
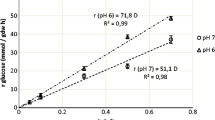
All the tested factors triggered decarboxylation of tyramine during the active growth phase of the cells. Kinetics of tyramine production reflecting the influence of evaluated external parameters is presented in Fig. 1. The highest rate of tyramine production (A BA) by E. durans (545 ± 38 mg/L) was found after 15 days of cultivation at 10 ± 1 °C and under anaerobic conditions; the cultivation medium contained 5 g/L lactose and 20 g/L NaCl (Fig. 1, part C).
The dependence of tyramine production (mg/L) by E. durans on the time of cultivation (days) under different conditions: (1) aerobic (full symbols) or anaerobic (open symbols) environment; (2) the addition of lactose (the parts a, c and e filled square open square—without lactose addition; filled triangle open triangle—2.5 g/L; filled inverted triangle open inverted triangle—5.0 g/L; the parts b, d and f filled diamond open diamond—7.5 g/L; filled circle open circle—10 g/L); and (3) the addition of NaCl (the parts a and b—without NaCl addition; the parts c and d—10 g/L; the parts e and f—20 g/L). The curves were obtained by application of Gompertz model (n = 6)
Concentration of NaCl significantly influenced tyramine production by E. durans. The maximum tyramine production (A BA) was detected in the cultivation medium with the highest concentration of NaCl (20 g/L, see Fig. 1). Similarly, the highest yield factor for tyramine formation (Y TYR/CFU; p < 0.05; Table 2) was also found in the above cultivation medium. In most cases, the maximum tyramine production rate (μ BA) and the shortest delay period (λ BA) calculated using Gompertz model were found in the same cultivation medium (Fig. 1).
Presence of lactose in cultivation medium showed a significant effect (p < 0.05) on production of tyramine by E. durans. It reached its maximum (A BA) in the presence of lactose (the highest levels were found in the M17 medium with 5 g/L of lactose), and it dropped in the medium with no lactose; the concentration of NaCl was equal in both the media. The same results were obtained regardless of availability of oxygen (Fig. 1). On the contrary, the absolute production of tyramine (yield factor for tyramine formation/Y TYR/CFU/) was higher in cultivation medium without lactose (p < 0.05; Table 2). Effect of lactose concentration was less markedly expressed especially in the case of the maximum tyramine production (A BA) and yield factor for tyramine formation (Y TYR/CFU).
Comparing the effect of oxygen presence, cultivation under anaerobic conditions in the medium with identical composition led to a higher maximum tyramine production (A BA) caused by E. durans (p < 0.05; Fig. 1). Similarly, the absolute production of tyramine (yield factor for tyramine formation/Y TYR/CFU) at the same concentration of lactose and NaCl was significantly higher (p < 0.05) under anaerobic conditions than in the environment with sufficient availability of oxygen (Table 2).
Discussion
Enterococci isolated from dairy products are commonly considered producers of BA that can be toxic for humans at higher concentrations [21, 22]. The tyramine-positive E. durans subjected to monitoring in this study can serve as a model strain for production of BA by NSLAB; therefore, cultivation temperature (10 ± 1 °C) and also selected factors and their levels were monitored to predict BA formation in fermented dairy products.
When the E. durans was cultivated in anaerobic regime, the maximum tyramine production (A BA) and also the yield factor for tyramine formation (Y TYR/CFU) grew higher in all the tested cultivation media with various compositions. Similar findings on tyramine production by the following bacterial strains are reported in literature: Marcobal et al. [23] studied E. faecium and Lactobacillus brevis, Bover-Cid et al. [15] explored Lactobacillus curvatus and Buňková et al. [13] focused on Lactococcus lactis. All the above studies were implemented under model conditions. Ancín-Azpilicueta et al. [24] also found higher production of the toxic compounds in wines produced by the oxygen-free technology. Therefore, the following hypothesis could be formulated: tyrosine-decarboxylase of many LAB shows higher activity in anaerobic environment.
The higher production of tyramine by E. durans was observed in media with the higher concentrations of NaCl. Similarly, Buňková et al. [13] found that five strains of L. lactis produced more tyramine in media with 20 g/L NaCl than in media containing 10 g/L NaCl and/or in the absence of NaCl. Likewise, gram-negative bacteria of Enterobacter or Morganella genera showed higher production of BA in media with the higher concentration of NaCl than in the environment with lower level of NaCl and/or in its absence [16, 19]. According to findings of Wolken et al. [25] and Pereira et al. [26], the elevated production of tyramine in media with higher concentrations of NaCl can be explained by the fact that Na+ ions are involved in regulation of intracellular pH. The ions are important in sodium/proton antiport system, as they are exchanged with H+ ions that are removed out of cells. Thus, Na+ ions play an essential role in the tyrosine decarboxylation pathway. On the contrary, Gardini et al. [18, 27] noticed higher production of BA by the E. faecalis strain (especially of tyramine a phenylethylamine) under in vitro conditions and also in skim milk or in dry fermented sausages at lower concentrations of NaCl (up to 20 g/L).
Production of tyramine by E. durans was also evaluated in cultivation medium doped by lactose. The reduced growth of cells of bacteria and the lower maximum tyramine production (A BA) were observed when no disaccharide was added to cultivation medium (regardless of the concentration of NaCl). The maximum tyramine production (A BA) by E. durans was found in optimal cultivation medium that is in M17 with addition of 5 g/L lactose. The production of tyramine decreased with additional increase in lactose concentration (at the same concentrations of NaCl). Buňková et al. [13] presented very similar conclusions for the L. lactis strains. Gardini et al. [27] noticed the maximum production of tyramine of E. faecalis after 5 days of ripening of dry fermented sausages doped with glucose; the effect of additions of glucose on production of tyramine (and also on growth of enterococci) became negligible at the end of ripening. Bover-Cid et al. [15] did not mention any significant effect of additions of glucose on production of tyramine by L. curvatus. In the case of E. durans, the higher tyramine production (A BA) in media with the higher concentration of lactose can also be caused by more expressed acidification of the cultivation media. Many studies confirmed the higher production of BA at lower pH values because of their protective function in intracellular pH homeostasis [15, 28]. On the contrary, the highest yield factor for tyramine formation (Y TYR/CFU) was found in the medium without any lactose. The finding can be explained by the absence of sufficient source of energy (i.e., lactose in this case) in the medium, which results in bacteria acquiring the energy necessary for biochemical processes in decarboxylation reactions. Thus, they can serve as alternative sources of energy for the bacteria. Pessione et al. [29] confirmed the hypothesis applying the proteomic approach. They found that E. faecalis bacteria are able to derive energy just due to decarboxylation of amino acids. Molenaar et al. [28] presented very similar conclusions for Lactobacillus buchneri.
Conclusions
Considering the possible toxic action of BA on human organisms, research on all technologically important microorganisms that could be declared as their potential producers and also exploration of the conditions under which they can produce BA are considered highly important. E. durans we studied produced the highest amounts of tyramine under anaerobic conditions in the presence of 20 g/L NaCl and 5 g/L lactose. The model conditions applied in our experiments corresponded to the manufacturing conditions most commonly used in dairy industry.
References
Foulquié Moreno MR, Sarantinopoulo P, Tsakalidou E, De Vuyst L (2006) The role and application of enterococci in food and health. Int J Food Microbiol 106:1–24
Franz CMAP, Stiles ME, Schleifer KH, Holzapfel WH (2003) Enterococci in foods—a conundrum for food safety. Int J Food Microbiol 88:105–122
Giraffa G (2002) Enterococci from food. FEMS Microbiol Rev 26:163–171
Khan H, Flint S, Yu P-L (2010) Enterocins in food preservation. Int J Food Microbiol 141:1–10
Lauková A, Czikková S (2001) Antagonistic effect of enterocin CCM 4231 from Enterococcus faecium on “bryndza”, a traditional Slovak dairy product from sheep milk. Microbiol Res 156:31–34
Ogier J-C, Serror P (2008) Safety assessment of dairy microorganisms: The Enterococcus genus. Int J Food Microbiol 126:291–301
Kučerová K, Svobodová H, Tůma Š, Ondráčková I (2009) Production of biogenic amines by enterococci. Czech J Food Sci 27:S2-50–S2-55
Ladero V, Sánchez-Llana E, Fernández M, Alvarez MA (2011) Survival of biogenic amine-producing dairy LAB strains at pasteurisation conditions. Int J Food Sci Technol 46:516–521
Pircher A, Bauer F, Paulsen P (2007) Formation of cadaverine, histamine, putrescine and tyramine by bacteria isolated from meat, fermented sausages and cheeses. Eur Food Res Technol 226:225–231
Aymerich T, Martín B, Garriga M, Vidal-Carou MC, Bover-Cid S, Hugas M (2006) Safety properties and molecular strain typing of lactic acid bacteria from slightly fermented sausages. J Appl Microbiol 100:40–49
Buňková L, Buňka F, Hlobilová M, Vaňátková Z, Nováková D, Dráb V (2009) Tyramine production of technological important strains of Lactobacillus, Lactococcus and Streptococcus. Eur Food Res Technol 229:533–538
Buňková L, Buňka F, Mantlová G, Čablová A, Sedláček I, Švec P, Pachlová V, Kráčmar S (2010) The effect of ripening and storage conditions on the distribution of tyramine, putrescine and cadaverine in Edam-cheese. Food Microbiol 27:880–888
Buňková L, Buňka F, Pollaková E, Podešvová T, Dráb V (2011) The effect of lactose, NaCl and an aero/anaerobic environment on the tyrosine decarboxylase activity of Lactococcus lactis subsp. cremoris and Lactococcus lactis subsp. lactis. Int J Food Microbiol 147:112–119
de Palencia PF, Fernández M, Hohedano ML, Ladero V, Quevedo C, Alvarez MA, López P (2011) Role of tyramine synthesis by food-borne Enterococcus durans in adaptation to the gastrointestinal tract environment. Appl Environ Microbiol 77:699–702
Bover-Cid S, Miguélez-Arrizado MJ, Becker B, Holzapfel WH, Vidal-Carou MC (2008) Amino acid decarboxylation by Lactobacillus curvatus CTC273 affected by the pH and glucose availability. Food Microbiol 25:269–277
Emborg J, Dalgaard P (2008) Modeling the effect of temperature, carbon dioxide, water activity and pH on growth and histamine formation on Morganella psychrotolerans. Int J Food Microbiol 128:226–233
Fernández M, Linares DM, Rodríguez A, Alvarez MA (2007) Factors affecting tyramine production in Enterococcus durans IPLA 655. Appl Microbiol Biotechnol 73:1400–1406
Gardini F, Martuscelli M, Caruso MC, Galgano F, Crudele MA, Favati F, Guerzoni ME, Suzzi G (2001) Effects of pH, temperature and NaCl concentration on the growth kinetics, proteolytic activity and biogenic amine production of Enterococcus faecalis. Int J Food Microbiol 64:105–117
Greif G, Greifová M, Karovičová J (2006) Effects of NaCl concentration and initial pH value on biogenic amine formation dynamics by Enterobacter spp. bacteria in model conditions. J Food Nutr Res 45:21–29
Zwietering M, Jongenburge I, Rombouts FM, van′t Riet K (1990) Modeling of the bacterial growth curve. Appl Environ Microbiol 56:1875–1881
Bhardwaj A, Kaur G, Gupta H, Vij S, Malik RK (2011) Interspecies diversity, safety and probiotic potential of bacteriocinogenic Enterococcus faecium isolated form dairy food and human faeces. World J Microbiol Biotechnol 27:591–602
Rea MC, Franz CMAP, Holzapfel WH, Cogan TM (2004) Development of enterococci and production of tyramine during the manufacture and ripening of Cheddar cheese. Irish J Agr Food Res 43:247–258
Marcobal Á, Martín-Álvarez PJ, Moreno-Arribas MV, Muñoz R (2006) A multifactorial design for studying factors influencing growth and tyramine production of the lactic acid bacteria Lactobacillus brevis CECT 4669 and Enterococcus faecium BIFI-58. Res Microbiol 157:417–424
Ancín-Azpilicueta C, González-Marco A, Jiménez-Moreno N (2010) Comparative study of the amine concentration in wines obtained from the traditional fermentation and from a more anaerobic fermentation method. LWT Food Sci Technol 43:771–776
Wolken WAM, Lucas PM, Lonvaud-Funel A, Lolkema JS (2006) The mechanism of the tyrosine transporter TyrP supports a proton motive tyrosine decarboxylation pathway in Lactobacillus brevis. J Bacteriol 188:2198–2206
Pereira CI, Matos D, San Romão MV, Crespo MTB (2009) Dual role for the tyrosine decarboxylation pathway in Enterococcus faecium E17: response to an acid challenge and generation of a proton motive force. Appl Environ Microbiol 75:345–352
Gardini F, Bover-Cid S, Tofalo R, Belletti N, Gatto V, Suzzi G, Torriani S (2008) Modeling the aminogenic potential of Enterococcus faecalis EF37 in dry fermented sausages through chemical and molecular approaches. Appl Environ Microbiol 74:2740–2750
Molenaar D, Bosscher JS, ten Brink B, Driessen AJM, Konings WN (1993) Generation of a proton motive force by histidine decarboxylation and electrogenic histidine/histamine antiport in Lactobacillus buchneri. J Bacteriol 175:2864–2870
Pessione E, Pessione A, Lamberti C, Coïsson DJ, Riedel K, Mazzoli R, Bonetta S, Eberl L, Giunta C (2009) First evidence of a membrane-bound, tyramine and β-phenylethylamine producing, tyrosine decarboxylase in Enterobacter faecalis. A two-dimensional electrophoresis proteomic study. Proteomics 9:2695–2710
Acknowledgments
The financial supports from the Grant Agency of the Czech Republic (Grant No. GA ČR 503/11/1417), from the internal grant of Tomas Bata University in Zlin (No. IGA/12/FT/11/D) and the Ministry of Education, Youth and Sports (MSM 2672286101) are greatly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Buňková, L., Buňka, F., Dráb, V. et al. Effects of NaCl, lactose and availability of oxygen on tyramine production by the Enterococcus durans CCDM 53. Eur Food Res Technol 234, 973–979 (2012). https://doi.org/10.1007/s00217-012-1714-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-012-1714-y