Abstract
Cereal-based beverages contain a complex mixture of various polymeric macromolecules including polysaccharides, peptides, and polyphenols. The molar mass of polymers and their degradation products affect different technological and especially sensory parameters of beverages. Asymmetrical flow field-flow fractionation (AF4) coupled with multi-angle light scattering (MALS) and refractive index detection (dRI) or UV detection (UV) is a technique for structure and molar mass distribution analysis of macromolecules commonly used for pure compound solutions. The objective of this study was to develop a systematic approach for identifying the polymer classes in an AF4//MALS/dRI/UV fractogram of the complex matrix in beer, a yeast-fermented cereal-based beverage. Assignment of fractogram fractions to polymer substance classes was achieved by targeted precipitations, enzymatic hydrolysis, and alignments with purified polymer standards. Corresponding effects on dRI and UV signals were evaluated according to the detector’s sensitivities. Using these techniques, the AF4 fractogram of beer was classified into different fractions: (1) the low molar mass fraction was assigned to proteinaceous molecules with different degrees of glycosylation, (2) the middle molar mass fraction was attributed to protein–polyphenol complexes with a coelution of non-starch polysaccharides, and (3) the high molar mass fraction was identified as a mixture of the cell wall polysaccharides (i.e., β-glucan and arabinoxylan) with a low content of polysaccharide–protein association. In addition, dextrins derived from incomplete starch hydrolysis were identified in all fractions and over the complete molar mass range. The ability to assess the components of an AF4 fractogram is beneficial for the targeted design and evaluation of polymers in fermented cereal-based beverages and for controlling and monitoring quality parameters.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cereal-based (e.g. barley, wheat, rice, maize) fermented beverages represent a complex matrix of various components which includes macromolecules. In general, polymers consist of covalently linked monomers with a varying degree of repetition units, which influence their functionality [1]. They are commonly characterized based on their molar mass and molar mass distribution. Beer, a yeast-fermented beverage, is comprised of polymeric compounds that include proteins, polyphenols, and polysaccharides [2]. These macromolecules affect different techno-functional parameters and especially sensory quality parameters such as mouthfeel and palate fullness of beverages.
Polymeric interactions influence the beverage’s turbidity stability. Permanent beer haze is a precipitation product of a complex of reactions between proteins and polyphenols. The molar mass of beer proteins varies from 10 to 46 kDa, and they are often glycosylated by Maillard reactions [3,4,5,6,7]. Further, a stable foam head is an important quality criterion for consumers [8]. Foam is the result of the interactions between specific beer proteins (LTP 1, protein Z) and isomerized hop-derived α-acids [4, 9,10,11,12].
In addition, polymers are vital for the sensory attributes mouthfeel and palate fullness in cereal-based beverages. It has been shown that polysaccharides [13,14,15] and proteins [3, 5, 15, 16] affect the perception of palate fullness and mouthfeel in beer. The sensory perception of fullness and body depends on the molar mass distribution of the polymers [3, 13, 15]. Apart from cereal-based beverages, it has been shown that polyphenols contribute to fullness or an astringent mouthfeel in wine [17, 18]. Because sensory perceptions are often based on synergistic effects, it is difficult to assign individual substance classes of polymers to specific sensory perception. Therefore, the molar mass distribution is an effective analytical index for the evaluation of palate fullness in beverages [13].
Despite being aware of these influences, body and palate fullness of cereal-based beverages are often inharmonic and not well understood. During malting and manufacturing processes of cereal-based beverages, macromolecules are degraded and modified. These processes are classified into cytolysis (i.e., degradation of cell wall polysaccharides), proteolysis (i.e., degradation of proteins into amino acids/peptides), and amylolysis (i.e., degradation of starch into fermentable carbohydrates). As the molar mass distribution of beer can be influenced by variations in the mashing regime and starch sources [14], there is an increasing demand for the analytical characterization of macromolecules for specifically tailoring beverage quality and sensory parameters.
Asymmetric flow field-flow fractionation (AF4) has been shown to be an effective method for the separation of biopolymers according to their diffusion coefficient. Despite the resemblance to liquid column chromatography, the AF4’s advantage is the absence of a stationary phase. The separation is achieved without shear degradation [19,20,21,22,23,24], which is advantageous, especially for a complex biogenic matrix in cereal-based beverages [25]. Coupling online multi-angle light scattering detection (MALS) with simultaneous concentration detection (refractive index detection (dRI)/UV absorption) allows the determination of molar mass and molar mass distribution without standard calibration. The technique’s underlying principle has been investigated in previous research [22, 26, 27]. Due to different detection principles of the concentration detectors (dRI and UV detection), different classes of polymeric substances can be distinguished. While all kinds of polymers classes are detected by refractive index measurement, only molecules with chromophoric groups show an absorption in the UV spectrum. This simultaneous evaluation of refractive index detection and UV absorption has been successfully applied for analyses of conjugated proteins [28].
The use of AF4/MALS coupled with dRI or UV detection for analyzing (bio)polymers has been a field of active research [13, 14, 24, 29,30,31,32,33,34,35,36], as recently reviewed by Nilsson [19] for food macromolecules and by Malik et al. [37]. These studies can generally be classified by the type of substance class which they researched (mainly polysaccharides and proteins). All of these studies analyzed pure substance solutions isolated from cereal extracts or food. However, only few studies dealing with the separation and characterization of a complex multi-component polymer solution via AF4/MALS have been published [38,39,40,41,42,43,44,45]. AF4 coupled with MALS/dRI/UV detectors has been carried out for the analysis of polysaccharides and proteinaceous polymers in beer by Tügel et al. [39]. The authors declared that the early eluting UV active fraction consists of proteinaceous molecules and they determined β-glucans in the high molar mass fraction [39]. Previous research studied the influence of beer components [13], starch sources, and mashing conditions [14, 46, 47] on the molar mass distribution using an AF4/MALS/dRI/UV system.
However, there is still a lack of information on the identification of polymers in an AF4 fratogramm of complex matrices containing different polymeric substance classes. Some quality and sensory effects of beverages cannot be ascribed to single polymer classes in a complex medium, as previously mentioned. Therefore, there is a demand for the analytical characterization of polymers in cereal-based beverages without prior purification. This study proposes an analytical method to evaluate polymer composition and molar mass of the complex matrix beer. The objective of this research was to develop a systematic approach for identifying the different polymer classes in a native state within AF4 fractograms of a cereal-based beverage by substance-specific degradation techniques. The identifications were done by comparisons of the elution profiles of bright beer with specifically processed beers and spiked beers. This approach enabled us to draw conclusions about the type of substance class within the native chromatograms and to gain deeper insights for the structural characterization regarding substance class. The method is beneficial for the targeted design of polymers in cereal-based beverages which can be applied for monitoring and controlling of beverages’ quality parameters (e.g., haze stability, foam stability, and the sensory parameters mouthfeel and palate fullness).
Experimental
Approach
Commercial German lager beer (composition is depicted in Table 1) was used as a yeast-fermented cereal-based beverage. It was brewed with 100% malted barley in accordance with the German purity law. Beer samples were degassed by 5 min ultrasonic treatment before further analysis. First, the beer was measured with an AF4/MALS/dRI/UV system in a native state. According to the detector responses of the dRI and UV detectors, predictions were proposed for the respective substance classes in the fractogram. Our predictions were tested by comparing a beer sample to processed beer samples and polymer standards. Processed samples included (1) protein adsorption with stabilization agents and SDS, (2) enzymatic hydrolysis, (3) synthetic protein–polyphenol complex, and (4) spiked beers with polymer standards. The effects of these treatments were compared with the dRI and UV detector signals of the beers in a native state.
Protein adsorption/precipitation methods
Proteinaceous materials were precipitated by the incubation with proteolytic stabilization agents bentonite (PanReac AppliChem, Germany), silicagel (Becosorb®, Eaton, Germany), and tannic acid (Brutan F, S.A. Ajinomoto OmniChem N.V, Belgium). Bentonite was swollen for 24 h in purified water before adding it to the sample. Bentonite was added to beer (2.2% v/v) and stirred for 120 min at room temperature. Samples were incubated with tannin (0.15% w/v) analogously with minor modifications. No swelling of the agent was necessary, and the sample was incubated for 10 min while stirring. Silicagel (1% w/v) treatment was conducted similarly with an incubation time of 10 min while stirring. For all stabilization methods, the precipitated material was removed by filtering through a 0.45-μm membrane before analysis via AF4.
Beer proteins and glycocompounds were precipitated from beer using the KDS and KDS–acetone method according to Mainente et al. [49] with minor modifications. Sodium dodecyl sulfate (SDS; Carl Roth®) was added to beer (0.2% w/v) and was shaken for 30 min at room temperatures. After incubation, the sample was subsequently boiled for 5 min. In total, 3 M KCl was added to get a concentration of 400 mM and the sample was shaken for 30 min. The sample was incubated for 18 h at 4 °C, then centrifuged for 15 min at 4696×g at 4 °C. For AF4 analysis, specimens were collected from the supernatants after the addition of SDS and after the addition of KCl. The glycocompounds were removed from the supernatant after KDS precipitation by addition of acetone (acetone/sample 4:1 v/v), and the sample was incubated for 1 h at 0 °C. The organic solvent was evaporated with a centrifugal vacuum concentrator (Eppendorf® concentrator 5301) at 30 °C for 30 min. The evaporation of acetone was monitored gravimetrically. The pellet was discarded and the supernatant was analyzed via AF4 analysis.
Selective enzymatic hydrolysis
Different enzymes were used for the selective hydrolysis of different substance classes. Substance specificity and incubation conditions of the respective enzymes are depicted in Table 2. Enzyme dosage was chosen in an excess for entire hydrolysis (dosage was adapted after pretrials and based on recommendation of the manufacturer). Activity was tested; pH was monitored and constant. The proteinaceous polymers of lager beer were selectively hydrolyzed by the endopeptidase Pepsin (Carl Roth®, Germany). β-Glucan was hydrolyzed by different commercial enzyme-mixtures: Glucazyme™ (Megazyme, Ireland), Cellulase (Megazyme, Ireland), and Westase (Takara, Japan). Dextrins were hydrolyzed by the amylolytic enzyme mixture Attenuzyme® Flex (Novozymes, Denmark). Enzymes were inactivated by boiling the sample for 15 min after the incubation. The evaporated water was compensated by gravimetrical addition of distilled water to initial weight. All samples were passed through a 0.45-μm membrane filter prior to analysis. Control samples without enzymatic degradation were treated analogously. Refractive index signals and UV absorption of native enzymes (analogous concentrations; separation via AF4) were subtracted from the corresponding detector signals. This enabled the qualitative comparison of the sample signals without background signals of the added enzymes.
Qualitative comparison with pure substance solutions
β-Glucan (barley, medium viscosity) and arabinoxylan (wheat, medium viscosity) were purchased from Megazyme (Ireland) and dissolved in purified water for a 60-min boiling period (0.1% w/v). The evaporated water was compensated by gravimetrical addition of distilled water to initial weight. Beer and polysaccharide solution were mixed 1/1 v/v. The original beer was diluted with distilled water to the same ratio.
Bovine serum albumin (BSA, Carl Roth, Germany) was dissolved in purified water (0.05% w/v) and homogenized. Complexes between BSA and tannin were formed by incubating a BSA solution with tannin (0.015% w/v) while stirring constantly for 60 min at ambient temperature. The sample was passed through a 0.45-μm membrane before analysis.
AF4 analysis equipment and separation conditions
Degassed samples were injected directly into the AF4 separation channel (long channel, Wyatt Technology Europe, Germany). The height of the inserted spacer was 350 μm, and the width was 21.5 mm at the widest position.. For separation, a regenerated cellulose ultrafiltration membrane with a nominal cutoff of 10 kDa (Millipore, PLGC membrane) was used. The carrier liquid was supplied by an isocratic pump (Agilent 1200 series). Eluent was 50 mM NaNO3 with 0.025% (w/v) NaN3 added to prevent bacterial growth. A membrane filter (0.1 μm, Supor, Pall Corporation) was placed between the pump and the auto-sampler to remove particles from the eluent. The samples (100 μL) were automatically injected by an auto-sampler (Agilent 1200 series). The AF4 instrument (Eclipse, Wyatt Technology Europe) provides the control of the flow management for elution and focusing within the separation channel. The injection flow was 0.2 mL/min for 2 min. After injection, the sample was focused for 8 min with a focus flow of 4.0 mL/min. The channel flow of the following elution was set to 1.0 mL/min. Fig. S1 in the Electronic Supplementary Material (ESM) illustrates the applied cross-flow gradient according to good fractogram practice [53]. The applied cross-flow was set to 4.0 ml/min for 5 min and decreased linearly in two steps. First, cross-flow was reduced to 0.2 mL/min in 10 min and reduced to zero in the following 10 min. The channel was then rinsed with eluent for 21 min without cross-flow.
AF4 was coupled online with a DAD detector (Agilent series 1200, set at 280 nm), a MALS detector (DAWN HELEOS, Wyatt Technology Europe), and a dRI detector (Agilent series 1260 RID VIS-Lamp). Data were recorded by the software ASTRA (Wyatt Technology Europe, version 6.1.2). Blank runs were subtracted from the corresponding detector signals. Baseline subtraction eliminated signal drifts caused by fluctuations in the channel pressure or in the eluent’s salt concentration. The volume delay between the applied detectors was eliminated by alignment to BSA [22]. Molar masses were calculated using Berry method for data obtained at 57.0°–126.0° scattering angles and dn/dc values were set to 0.185 mL/g (fraction 1) and 0.146 mL/g (fraction 2 and 3), according to previous studies [39, 46].
Results
Predictions of substance classes
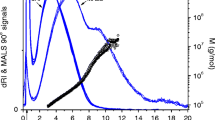
Based on the separation principle of AF4, the low molar mass components elute prior to fractions with higher molar mass as shown in the AF4 fractogram of unprocessed bright beer (Fig. 1). For all samples, blank runs were subtracted to achieve stable baselines. The fractogram was classified into distinct fractions according to the elution profile of the refractive index and UV absorption at 280 nm based on previous studies [39, 46] and known beer composition. The maximum signal intensity of both signals was detected in the interval between 2 and 5 min immediately following the void peak. Two partial peak maxima were observed within this fraction and were classified into peak 1a and peak 1b (Fig. 1), respectively. Due to the uniformity of refractive index and UV absorption, the elution of proteins was assumed for fraction 1. The comparable reduced UV signal (in relation to dRI signal) in the interval between 5 and 12 min was attributed to the elution of protein–polyphenol complexes (fraction 2). The gap between dRI signal and UV absorption indicated the presence of another substance class and a partial dRI maximum was observed at 15 min elution time (faction 3) which was assigned to the elution of non-chromophoric cell wall polysaccharides. Further, the presence of non-fermentable dextrins from an incomplete starch hydrolysis was assumed. Each predicted fraction was then verified by correlating the elution profile of a bright beer with specifically processed beer or with specific spiked beers.
Overlay of the normalized detector signals (black line refractive index, red line UV absorption obtained at 280 nm, and blue line MALS signal at 90° scattering angle) and black triangle molar mass (right axis) of bright beer after separation via AF4. The fractogram was classified into three fractions according to their detector-specific signal behavior: fraction 1: low molar mass fraction (10–40 kDa); fraction 2: middle molar mass fraction (60–400 kDa); fraction 3: high molar mass fraction (400–1200 kDa)
Proteins
The low molar mass fraction of beer’s elution profile with high UV absorption and intense dRI signal was classified as proteins in previous studies [39]. This allocation was confirmed in this study using a modified AF4 separation method with baseline subtraction (Fig. 1). Since the dRI signal is not substance-specific and proteins are UV active, the uniformity of both detectors, dRI and UV, indicates the elution of proteinaceous material in fraction 1. This is further supported by comparison with a protein standard. Bovine serum albumin was used due to its similar molar mass compared to beer proteins. Thereby the detector responses were compared using identical separation conditions. The uniformity of dRI and UV signals of a pure BSA solution (fractogram not shown) showed an elution behavior resembling to the detector signals in fraction 1 (Fig. 1). As the molar mass of beer proteins is more or less comparable to the monomer of BSA (molar mass 66 kDa), this allocation was in agreement to previous results.
The selective removal of proteins by specific adsorption and precipitation techniques (Fig. 2) confirmed this classification. All proteolytic stabilization techniques caused a decrease in the UV and dRI signals of fraction 1. However, the extent of the signal decrease differed among the applied agents and their specificity. For example, the dRI and the UV signals in fraction 1 were merely attenuated by protein adsorption with silicagel. The stabilization agents tannin and bentonite removed low molar mass proteins almost completely (fraction 1), which correlated to an almost complete elimination of UV absorption and the marked decrease in the dRI signal in fraction 1. In addition, the decrease in the dRI signal and UV signal in fraction 2 and 3 was observed due to dilution by the aqueous bentonite solution.
The denaturation of proteins via SDS caused a decrease in the UV and dRI signals of peak 1b (Fig. 3). After decreasing solubility with KCl, a subsequent decrease of peak 1a was obtained. As a result, non-glycosylated proteins were precipitated. The remaining UV and dRI signals were attributed to the glycosylated proteins in beer. These glycocompounds were precipitated from the KDS supernatants by acetone according to a published method [49]. In this study, the organic solvent was evaporated after precipitation. Hence, we were able to draw qualitative conclusions from the AF4 analysis of the resulting supernatants. The UV absorption and dRI signals were almost eliminated after the acetone precipitation (Fig. 3). Thus, the elution of proteins in fraction 1 was evident; however, two partial signals were detected at 5 min in the UV/dRI signal and at 10 min in the dRI signal. The resuspension of the pellet gave a qualitatively similar detector response (data not shown). However, a partial elution of polyphenols and polysaccharides in the supernatant was likely the cause.
Another method for identifying the different substance classes in the fractograms was by using targeted enzymatic hydrolysis. Samples were treated with acidic peptidase pepsin, which hydrolyzes peptide bonds as an endo-enzyme, prior to AF4 analysis. Based on the decrease of molar mass, hydrolysis products were discarded during focusing. Thus, the products with molar mass below the cutoff of the channel’s ultrafiltration membrane did not affect the detector responses. The enzymes used for hydrolysis are proteins with a molar mass comparable to beer proteins; therefore, blanks of hydrolysis enzymes were subtracted from the corresponding detector signals. Figure 4 shows the effect of enzymatic digestion on proteinaceous macromolecules. A marked decline in refractive index and UV absorption was obtained for fraction 1.
Protein–polyphenol complexes
Proteins and polyphenols from malt or hops often form complexes which can cause turbidity in beer [54]. We predicted that the plateau of the UV signal in fraction 2 was assigned to those complexes. A model solution of protein–polyphenol complexes was produced by incubation of a protein (BSA) with tannic acid. These experiments merely allowed qualitative assertions.
BSA was separated into the monomer, dimer, and trimer fractions via AF4, and these structures were detected by uniform detector signals (Fig. 5). After incubation with tannic acid, the formation of synthetic protein–polyphenol complexes was detected in the fractogram by the dRI and the UV detector (Fig. 5). The aggregation increased over time as evidence by a signal increase in peak 2 and 3. Additionally, a shift of the peak towards a higher retention time was observed which indicated an increase in the molar mass occurred. The signals of the separated BSA monomer, dimer, and trimer structures decreased concomitantly. Since tannic acid’s molar mass is below the cutoff of the ultrafiltration membrane, no signal was detected for the pure substance in solution (fractograms not shown). Thus, we were able to establish that the presence of complexes was due to interactions with BSA and tannic acid. The differences in both signal intensities were attributed to an increasing amount of chromophoric tannic acid in the complex. Therefore, the intensity of UV absorption was higher than the dRI signal. According to the elution profile of the synthesized compounds (Fig. 5), fraction 2 was identified as a partial elution of protein–polyphenol complexes.
AF4 fractograms of normalized refractive index (a) and UV absorption signals (280 nm, b). Overlay of black line BSA (0.015% w/v) and a protein-polyphenol complex, which was synthetically created by the addition of tannic acid: red line BSA + tannic acid (0 h), blue line BSA + tannic acid (24 h), and green line BSA + tannic acid (48 h)
Cell wall polysaccharides
Previous research assigned the high molar mass fraction of beer to β-glucan by enzymatic treatment [39]. Arabinoxylan is another cell wall polysaccharide; however, it could not be identified by treatment with a xylanolytic enzyme previously [39]. In this study, we qualitatively assigned fraction 2 and 3 to the cell wall polysaccharides arabinoxylan and β-glucan. A correlation procedure was carried out by comparison of the detector response of native beers and spiked beers. Therefore, the cell wall polysaccharides β-glucan (Fig. 6) and arabinoxylan (Fig. 7) were spiked into base beer. Pure polysaccharides were eluted at retention times between 5 and 23 min. Due to spiking, differences in the elution profiles were observed at retention times between 10 and 23 min. As predicted, spiking beer with polysaccharides solely resulted in an increase in the dRI signal. The slight UV absorption of arabinoxylan at higher retention times (22 min) indicated the presence of chromophoric ferulic acid (Fig. 7) [55] and by scattering of the UV signal. Ferulic acid can be covalently bound to some arabinofuranosyl residues within the arabinoxylan structure [56]. Additionally, the slight UV signal in the low molar mass fraction of β-glucan (Fig. 6) was ascribed to impurities of the commercial standard with proteinaceous molecules (0.12%, as specified by the supplier [57]). However, the UV absorption signals caused by protein impurities and ferulic acid were neglected. Since variations were only detected in the refractive index signals, the elution of cell wall polysaccharides was evident.
Similar to verifying the assignment of proteins by enzymatic hydrolysis, different enzyme cocktails for cytolytic degradation were used. Hydrolysis products with molar masses below the cutoff of the ultrafiltration membrane were again discarded during focusing. In addition, enzymes were inactivated by boiling. Boiling had no effect on the UV and dRI signals (results of pretests not shown). This step was crucial as it prevented hydrolysis of the ultrafiltration membrane within the AF4 separation channel. The elution profiles of lager beer and lager beer hydrolyzed with different β-glucanases and cytolytic enzymes are shown in Fig. 8. For all hydrolytic enzymes, a marked decrease in the dRI signal for fraction 2 and 3 was observed, while the UV signal remained almost constant. Thus, the partial elution of β-glucans in the high molar mass range (5–20 min) was verified due to the detector responses after enzymatic hydrolysis. However, slight differences were observed according to the respective specificities of the applied enzymes. The slight decrease of UV absorption in the high molar mass range is based on the hydrolysis of fibers, which are partly associated with proteins or polyphenols [58]. Even though these protein−polyphenol complexes are present in minor concentrations compared to the native fibers [58], they contribute to the decrease in UV absorption.
AF4 fractograms of normalized refractive index (a) and UV absorption signals (280 nm, b). Overlay of black line bright beer and beer hydrolyzed with cytolytic enzymes (red line cellulase, blue line glucazyme, green line westase). The signals caused by the native enzyme were subtracted from the corresponding baseline to inhibit background signals
Dextrins
Dextrins are degradation products of an incomplete starch hydrolysis, which cannot be assimilated by yeast. We evaluated the dextrin fraction in the fractogram by enzymatic hydrolysis similar to previous classifications. Therefore, an amylolytic enzyme cocktail including glycoamylases and pullulanases was used for degradation. Figure 9 shows the effect of this degradation. A decrease of the dRI and UV signal was observed for all fractions; however, the intensity of the UV reduction was less pronounced. The decrease in UV absorption might be assigned to the cleavage of glycosylated proteins by amylolytic enzymes. These results support the inference that dextrins contribute to beer’s polymeric substances and that their molar mass is broadly distributed.
Discussion
Asymmetrical flow field flow fractionation is a robust and non-destructive method for separation of bio-macromolecules. Combining these techniques with MALS and simultaneous concentration detection provides the possibility to obtain detailed information on molar mass and conformation of polymers in a native state without the need of calibration. However, this technique has been rarely applied to the separation of complex multi-component solutions. The purpose of this study was to develop a systematic approach for identifying different substance classes in fermented cereal-based beverages in particular beer. We used an AF4 separation technique coupled to MALS and two different concentration detectors (refractive index detection and UV absorption at 280 nm).
Lager beer was separated via AF4 without prior purification and classified into one broadly distributed fraction and three distinct fractions based on elution behavior and detector specificities. Thereby, separation conditions with a flow rate of 4 mL/min for focusing flow and initial cross-flow were used. This delivered a maximum resolution especially for the low molar mass fraction, which elutes first. Baselines obtained from blank samples were subtracted from the corresponding detector signals. Hence, signal drifts caused by fluctuations of pressure and eluent concentration were eliminated. Qualitative and quantitative information (quantitative determination of peak areas) was collected from the resulting fractograms.
Collecting the results of fraction 1, the polymers eluting first were assigned to beer proteins. This assignment was verified using substance-specific degradation techniques adapted from established stabilizing techniques for beer. A decrease in signal intensity of dRI and UV signal was observed for all applied stabilization agents. The specific precipitation with KDS and KDS acetone and the enzymatic degradation of proteinaceous material confirmed this classification. Here, a similar decline in both detector signals was obtained. Considering the detector’s specificities, the decreases of both detector signals were plausible. These results are in accordance with earlier studies [39]. However, Tügel et al. classified the proteinaceous materials solely according to detector specificity without further purification treatments [39]. In this study, the elimination of fraction 1 was incomplete due to selective precipitation properties of each stabilizing agent. Silicagel specifically adsorbs haze-forming proteins, while the foam-positive ingredients are not affected [3, 59]. Further, commercial lager beer used was pre-stabilized with a lower amount of silica gel by the manufacturer. Hereby, the haze-active proteins were already precipitated by the brewer but with a less exhaustive technique. Thus, the low attenuation of both detector signals for fraction 1 was explained by silica gel’s high specificity. Since complexation with tannic acid and adsorption to bentonite are less specific, the decline in both detector responses was more pronounced in fraction 1. A decline in the signal intensities was also observed for fraction 2, since this area was qualitatively assigned to protein–polyphenol complexes, which were partly precipitated by the less selective agents bentonite and tannic acid.
The selective precipitation using the KDS/KDS acetone method [49] enables a distinction between non-glycosylated and glycosylated proteins. Applying the KDS method, the reduction of the detector signals was almost identical compared to the selective stabilization techniques. An almost complete elimination of the UV signal was achieved after the precipitation of glycosylated proteins by acetone. Thus, fraction 1 was classified as proteinaceous material using the KDS/KDS acetone method.
Enzymatic hydrolysis of proteinaceous material by the endoprotease pepsin resulted in a distinctive decrease in both detector signals in fraction 1. However, the reduction of both signals was incomplete due to a partial resistance of glycosylated proteins to pepsin hydrolysis [60, 61].
In general, the classification of proteinaceous material in fraction 1 was verified. The applied method gave a maximum resolution especially in the low molar mass fraction (fraction 1). This enabled us to gain more insights compared to earlier research [39]. Thus, we were even able to differentiate two partial peaks 1a and 1b, which were assigned to beer proteins differing in size and translational diffusion coefficient (Fig. 1). The discrepancy in signal height for peak 1a and 1b (RI/UV ratio) was detectable due to different degrees of glycosylation. Since glycosyl substituents are not UV active, different degrees of glycosylation were observed at peak 1a and 1b (Fig. 1).
In fraction 2 and 3, the dRI and UV signal exhibited a non-uniform and subsequently diverging detector signals (partial dRI maximum at 16 min). Thus, a coelution of chromophoric (protein–polyphenol complexes) and non-chromophoric substance classes (cell wall polysaccharides) was assumed for the middle (fraction 2) and high molar mass fraction (fraction 3) of the fractogram. The presence of protein–polyphenol complexes was verified for fraction 2 and 3 by a qualitative alignment with a synthetic solution of BSA and tannic acid. The formed complexes were detected by the dRI and UV detectors. Hence, the plateau of the UV absorption in fraction 2 and 3 was attributed to protein–polyphenol complexes by an alignment of elution time and detector responses. In contrast to the plateau and the following decrease in UV absorption in fraction 2 and 3, the dRI signal exhibited a divergent detector response. As polysaccharides are non-chromophoric and were verified in the high molar mass fraction, this gap between dRI and UV signal was apparent. β-Glucans, as the major cell wall polysaccharides, were detected in the high molar mass fraction (fraction 2 and 3) by enzymatic treatment in accordance with previous research [39]. Due to different specificities of the applied enzymes, β-glucans in the high molar mass fraction could be assigned to malt and yeast origin. The alignment of spiked beers confirmed the results from the enzymatic hydrolysis. Another cell wall polysaccharide arabinoxylan (purified from wheat) exhibited similar results. Thus, the high molar mass fraction was attributed to the elution of cell wall polysaccharides.
Protein–polyphenol complexes exist in beer, depending on the type and intensity of the brewer’s stabilization technology, vary in molar mass, and increase during beer storage. They can be precipitated by the addition of the stabilization agents, bentonite and tannic acid. Thus, the decrease in both detector signals in fraction 2 after treatment with bentonite and silica gel (as mentioned before) was in accordance with the assignment of fraction 2 to protein–polyphenol complexes.
Dextrins were identified by enzymatic hydrolysis within the whole fractogram area. Previous research did not examine the macromolecular dextrin fraction in beer [39]. These polysaccharides derive from an incomplete starch hydrolysis during the mashing process [62]. Glycooligosaccharides and dextrins are not fermented by yeast during fermentation. Thus, they remain in the medium through the whole process and influence especially sensory attributes in beer [13]. Their concentration and molar mass can be influenced by mashing regime [14].
This polydisperse dextrin fraction overlay the previously classified fractions. Generally, there is no sharp cutoff between different substance classes in the beer fractograms. Coelution between classified fractions has to be taken into consideration. The classification of the beer fractogram was assigned by the major distinctions in the detector responses of the applied detectors. Thus, a partial (low molecular weight) amount of protein–polyphenol complexes is also evident in fraction 1. Since these protein–polyphenol complexes are resistant to pepsin hydrolysis [63], the incomplete decline of both detector signals after the hydrolysis with pepsin was apparent.
Based on these results, it was possible to characterize polymers in complex multi-component solutions according to their class of substance, structure, and size. This analysis provides a tool for the specific characterization of macromolecules in cereal-based beverages. The gained insights are beneficial for the monitoring and technological adjustment of hydrolysis processes. Thus, beverages’ quality parameters foam, haze stability, and especially sensory profiles (palate fullness and mouthfeel) can be modified during the manufacturing process. Moreover, the insights can be applied for the analytical characterization of lactic acid fermented cereal-based beverages and non-alcoholic beers.
Conclusions
The objective of this study was to develop a systematic approach for identifying different polymer substance classes in beer fractograms by substance-specific degradation techniques. The AF4 fractogram of beer was classified into three distinct fractions and one fraction, which is distributed over the whole fractogram. The low molar mass fraction (fraction 1) was aligned to the elution of proteinaceous material in beer deriving from malt and hops with different degrees of glycosylation. The second fraction was classified into protein–polyphenol complexes with a partial coelution of cell wall polysaccharides. Finally, the high molar mass fraction (fraction 3) was identified as cell wall polysaccharide, β-glucan, and arabinoxylan with a low amount protein association. Dextrins derived from incomplete starch hydrolysis were verified for all fractions in the fractogram. Thus, the molar mass distribution of the dextrin fraction is polydisperse, and they overlay the previously classified distinct fractions.
This approach allows specific analysis of beverages’ polymers: proteinaceous material in beer can be analyzed with degradation methods (stabilizing agents, KDS precipitation, enzymatic hydrolysis). Glycosylated proteins were precipitated by KDS acetone precipitation. Cell wall polysaccharides were hydrolyzed by cytolytic enzymes. Thus, different polymer classes can be analyzed targeted in cereal-based beverages.
These findings are beneficial for the design of polymers in cereal-based beverages. It provides a tool for monitoring and controlling the polymer-dependent quality aspects (e.g., haze stability, foam) and especially sensory profiles of beverages (palate fullness and mouthfeel). The results enable a high application potential for the use of an AF4 multi-detection system for brewing studies.
References
Chassenieux C, Durand D, Jyotishkumar P, Thomas S. Biopolymers: state of the art, new challenges, and opportunities. Handbook of Biopolymer-Based Materials. Wiley. KGaA; 2013. p. 1–6.
Gresser A. Properties and Quality. Handbook of Brewing. Wiley. KGaA; 2009. p. 359–97.
Steiner E, Gastl M, Becker T. Protein changes during malting and brewing with focus on haze and foam formation: a review. Eur Food Res Technol. 2011;232(2):191–204. doi:10.1007/s00217-010-1412-6.
Steiner E, Arendt E, Gastl M, Becker T. Influence of the malting parameters on the haze formation of beer after filtration. Eur Food Res Technol. 2011;233(4):587–97. doi:10.1007/s00217-011-1547-0.
Steiner E, Becker T, Gastl M. Turbidity and haze formation in beer—insights and overview. J Inst Brew. 2010;116(4):360–8. doi:10.1002/j.2050-0416.2010.tb00787.x.
Leiper KA, Stewart GG, McKeown IP. Beer polypeptides and silica gel part I. Polypeptides involved in haze formation. J Inst Brew. 2003;109(1):57–72. doi:10.1002/j.2050-0416.2003.tb00594.x.
Leiper KA, Stewart GG, McKeown IP. Beer polypeptides and silica gel part II. Polypeptides involved in foam formation. J Inst Brew. 2003;109(1):73–9. doi:10.1002/j.2050-0416.2003.tb00595.x.
Neugrodda C, Gastl M, Becker T. Comparison of foam analysis methods and the impact of beer components on foam stability. J Am Soc Brew Chem. 2015;73(2):170–8.
Evans DE, Bamforth CW. 1 - Beer foam: achieving a suitable head. Beer. San Diego: Academic Press; 2009. p. 1–60.
Almaguer C, Schönberger C, Gastl M, Arendt EK, Becker T. Humulus lupulus—a story that begs to be told. A review J Inst Brew. 2014;120(4):289–314. doi:10.1002/jib.160.
Schönberger C, Kostelecky T. 125th anniversary review: the role of hops in brewing. J Inst Brew. 2011;117(3):259–67. doi:10.1002/j.2050-0416.2011.tb00471.x.
Evans DE, Sheehan MC. Don't be fobbed off: the substance of beer foam—a review (1). J Am Soc Brew Chem. 2002;60(2):47–57.
Rübsam H, Gastl M, Becker T. Influence of the range of molecular weight distribution of beer components on the intensity of palate fullness. Eur Food Res Technol. 2012;236(1):65–75. doi:10.1007/s00217-012-1861-1.
Rübsam H, Gastl M, Becker T. Determination of the influence of starch sources and mashing procedures on the range of the molecular weight distribution of beer using field-flow fractionation. J Inst Brew. 2013:139–48. doi:10.1002/jib.69.
Langstaff SA, Lewis MJ. The mouthfeel of beer—a review. J Inst Brew. 1993;99:31–7.
Steiner E, Auer A, Becker T, Gastl M. Comparison of beer quality attributes between beers brewed with 100% barley malt and 100% barley raw material. J Sci Food Agr. 2012;92(4):803–13. doi:10.1002/jsfa.4651.
Vidal S, Francis L, Williams P, Kwiatkowski M, Gawel R, Cheynier V, et al. The mouth-feel properties of polysaccharides and anthocyanins in a wine like medium. Food Chem. 2004;85(4):519–25. doi:10.1016/S0308-8146(03)00084-0.
Hufnagel JC, Hofmann T. Orosensory-directed identification of astringent mouthfeel and bitter-tasting compounds in red wine. J Agr Food Chem. 2008;56(4):1376–86. doi:10.1021/jf073031n.
Nilsson L. Separation and characterization of food macromolecules using field-flow fractionation: a review. Food Hydrocoll. 2013;30(1):1–11. doi:10.1016/j.foodhyd.2012.04.007.
Wagner M, Holzschuh S, Traeger A, Fahr A, Schubert US. Asymmetric flow field-flow fractionation in the field of nanomedicine. Anal Chem. 2014;86(11):5201–10. doi:10.1021/ac501664t.
Yohannes G, Jussila M, Hartonen K, Riekkola ML. Asymmetrical flow field-flow fractionation technique for separation and characterization of biopolymers and bioparticles. J Chromatogr A. 2011;1218(27):4104–16. doi:10.1016/j.chroma.2010.12.110.
Podzimek S. Light scattering, size exclusion chromatography and asymmetric flow field flow fractionation. Wiley; 2011.
Kavurt UB, Marioli M, Kok WT, Stamatialis D. Membranes for separation of biomacromolecules and bioparticles via flow field-flow fractionation. J Chem Technol Biotechnol. 2015;90(1):11–8. doi:10.1002/jctb.4473.
Roda B, Zattoni A, Reschiglian P, Moon MH, Mirasoli M, Michelini E, et al. Field-flow fractionation in bioanalysis: a review of recent trends. Anal Chim Acta. 2009;635(2):132–43. doi:10.1016/j.aca.2009.01.015.
Rübsam H, Krottenthaler M, Gastl M, Becker T. An overview of separation methods in starch analysis: the importance of size exclusion chromatography and field flow fractionation. Starch - Stärke. 2012:683–95. doi:10.1002/star.201100188.
Runyon JR, Ulmius M, Nilsson L. A perspective on the characterization of colloids and macromolecules using asymmetrical flow field-flow fractionation. Colloid Surface A. 2014;442(0):25–33. doi:10.1016/j.colsurfa.2013.04.010.
Wyatt PJ. Light scattering and the absolute characterization of macromolecules. Anal Chim Acta. 1993;272(1):1–40. doi:10.1016/0003-2670(93)80373-S.
Rebolj K, Pahovnik D, Žagar E. Characterization of a protein conjugate using an asymmetrical-flow field-flow fractionation and a size-exclusion chromatography with multi-detection system. Anal Chem. 2012;84(17):7374–83. doi:10.1021/ac3010378.
Perez-Rea D, Bergenståhl B, Nilsson L. Development and evaluation of methods for starch dissolution using asymmetrical flow field-flow fractionation. Part I: dissolution of amylopectin. Anal Bioanal Chem. 2015;407(15):4315–26. doi:10.1007/s00216-015-8611-8.
Perez-Rea D, Bergenståhl B, Nilsson L. Development and evaluation of methods for starch dissolution using asymmetrical flow field-flow fractionation. Part II: Dissolution of amylose. Anal Bioanal Chem. 2015:1–14. doi:10.1007/s00216-015-8894-9.
Ulmius M, Önning G, Nilsson L. Solution behavior of barley β-glucan as studied with asymmetrical flow field-flow fractionation. Food Hydrocoll. 2012;26(1):175–80. doi:10.1016/j.foodhyd.2011.05.004.
Kupetz M, Sacher B, Becker T. Impact of flavouring substances on the aggregation behaviour of dissolved barley β-glucans in a model beer. Carbohydr Polym. 2016;143:204–11. doi:10.1016/j.carbpol.2016.01.070.
Dou H, Zhou B, Jang H-D, Lee S. Study on antidiabetic activity of wheat and barley starch using asymmetrical flow field-flow fractionation coupled with multiangle light scattering. J Chromatogr A. 2014;1340:115–20. doi:10.1016/j.chroma.2014.03.014.
Håkansson A, Ulmius M, Nilsson L. Asymmetrical flow field-flow fractionation enables the characterization of molecular and supramolecular properties of cereal β-glucan dispersions. Carbohydr Polym. 2012;87(1):518–23. doi:10.1016/j.carbpol.2011.08.014.
Pitkänen L, Sontag-Strohm T, Kanerva P. Enhanced separation and characterization of gluten polymers by asymmetrical flow field-flow fractionation coupled with multiple detectors. J Cereal Sci. 2014;59(2):126–31. doi:10.1016/j.jcs.2013.11.009.
Fvd K, Legros S, Hofmann T, Larsen EH, Loeschner K. Separation and characterization of nanoparticles in complex food and environmental samples by field-flow fractionation. Trends Analyt Chem. 2011;30(3):425–36. doi:10.1016/j.trac.2010.11.012.
Malik MI, Pasch H. Field-flow fractionation: new and exciting perspectives in polymer analysis. Prog Polym Sci. 2016; doi:10.1016/j.progpolymsci.2016.03.004.
Runyon JR, Nilsson L, Alftrén J, Bergenståhl B. Characterization of oat proteins and aggregates using asymmetric flow field-flow fractionation. Anal Bioanal Chem. 2013;405(21):6649–55. doi:10.1007/s00216-013-7115-7.
Tügel I, Runyon JR, Gómez Galindo F, Nilsson L. Analysis of polysaccharide and proteinaceous macromolecules in beer using asymmetrical flow field-flow fractionation. J Inst Brew. 2015:44–8. doi:10.1002/jib.195.
Pellaud J, Leemans C, Carruthers A, Melotte L. Use of FFF-MALLS to analyse colloids in beer. Proc Congr - Eur Brew Conv. 2005;30:119/1- /13.
Dou H, Magnusson E, Choi J, Duan F, Nilsson L, Lee S. Study on aggregation behavior of low density lipoprotein in hen egg yolk plasma by asymmetrical flow field-flow fractionation coupled with multiple detectors. Food Chem. 2016;192:228–34. doi:10.1016/j.foodchem.2015.07.019.
Ulmius M, Adapa S, Önning G, Nilsson L. Gastrointestinal conditions influence the solution behaviour of cereal β-glucans in vitro. Food Chem. 2012;130(3):536–40. doi:10.1016/j.foodchem.2011.07.066.
Pasch H, Makan AC, Chirowodza H, Ngaza N, Hiller W. Analysis of complex polymers by multidetector field-flow fractionation. Anal Bioanal Chem. 2014;406(6):1585–96. doi:10.1007/s00216-013-7308-0.
Coelho C, Parot J, Gonsior M, Nikolantonaki M, Schmitt-Kopplin P, Parlanti E et al. Asymmetrical flow field-flow fractionation of white wine chromophoric colloidal matter. Anal Bioanal Chem. 2017:1–10. doi:10.1007/s00216-017-0221-1.
Zielke C, Teixeira C, Ding H, Cui S, Nyman M, Nilsson L. Analysis of β-glucan molar mass from barley malt and brewer’s spent grain with asymmetric flow field-flow fractionation (AF4) and their association to proteins. Carbohydr Polym. 2017;157:541–9. doi:10.1016/j.carbpol.2016.10.045.
Choi J, Zielke C, Nilsson L, Lee S. Characterization of the molar mass distribution of macromolecules in beer for different mashing processes using asymmetric flow field-flow fractionation (AF4) coupled with multiple detectors. Anal Bioanal Chem. 2017:1–8. doi: 10.1007/s00216-017-0393-8.
Rübsam H, Becker T, Gastl M. Analytical characterization of the hydrolysis of barley malt macromolecules during enzymatic degradation over time using AF4/MALS/RI. J Food Sci. 2017;82(6):1326–32. doi:10.1111/1750-3841.13716.
MEBAK Brautechnische Analysenmethoden: Würze, Bier, Biermischgetränke; Methodensammlung der Mitteleuropäischen Brautechnischen Analysenkommission. Freising: Selbstverl. der MEBAK; 2012.
Mainente F, Simonato B, Zoccatelli G, Rizzi C. A method for the preparative separation of beer proteins and glycocompounds. J Inst Brew. 2011;117(3):435–9. doi:10.1002/j.2050-0416.2011.tb00490.x.
Megazyme. Data Booklet: Enzymatic Yeast beta glucan—assay procedure. Megazyme International Ireland; 2014.
Megazyme. Data Booklet: cellulase (endo-,4-β-D-glucanase) from A. niger Megazyme International Ireland; 2014.
Takara Bio Inc. Data Booklet: Westase (digestive enzyme of yeast cell walls of yeast). Takara Clontech; 2013.
Wahlund K-G. Flow field-flow fractionation: critical overview. J Chromatogr A. 2013;1287:97–112. doi:10.1016/j.chroma.2013.02.028.
Siebert KJ. Haze formation in beverages. LWT-Food Sci Technol. 2006;39(9):987–94. doi:10.1016/j.lwt.2006.02.012.
Köhnke T, Pujolras C, Roubroeks JP, Gatenholm P. The effect of barley husk arabinoxylan adsorption on the properties of cellulose fibres. Cellulose. 2008;15(4):537. doi:10.1007/s10570-008-9209-5.
Steiner J, Procopio S, Becker T. Brewer’s spent grain: source of value-added polysaccharides for the food industry in reference to the health claims. Eur Food Res Technol. 2015;241(3):303–15. doi:10.1007/s00217-015-2461-7.
Megazyme. Data Booklet: barley beta-glucan (medium viscosity) Megazyme International Ireland; 11/12.
Goñi I, Díaz-Rubio ME, Saura-Calixto F. 28—dietary fiber in beer: content, composition, colonic fermentability, and contribution to the diet. In: Preedy V, editor. Beer in health and disease prevention. San Diego: Academic Press; 2009. p. 299–307.
Rehmanji M, Gopal C, Mola A. Beer stabilization technology—clearly a matter of choice. MBAA TQ. 2005;42(4):332–8.
Lindorff-Larsen K, Winther JR. Surprisingly high stability of barley lipid transfer protein, LTP1, towards denaturant, heat and proteases. FEBS Lett. 2001;488(3):145–8. doi:10.1016/s0014-5793(00)02424-8.
Marciniak-Darmochwal K, Kostyra H. Influence of nonenzymatic glycosylation (glycation) of pea proteins (pisum stivum) on their susceptibility to enzymatic hydrolysis. J Food Biochem. 2009;33(4):506–21. doi:10.1111/j.1745-4514.2009.00234.x.
Ferreira IMPLVO. 27—beer carbohydrates. In: Preedy VR, editor. Beer in Health and Disease Prevention. San Diego: Academic Press; 2009. p. 291–8.
Onyango C, Noetzold H, Bley T, Henle T. Proximate composition and digestibility of fermented and extruded uji from maize–finger millet blend. LWT-Food Sci Technol. 2004;37(8):827–32. doi:10.1016/j.lwt.2004.03.008.
Acknowledgements
This research project was supported by the German Ministry of Economics and Technology (via AiF), Project AiF 18563N.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Fig. S1
(PDF 148 kb)
Rights and permissions
About this article
Cite this article
Krebs, G., Becker, T. & Gastl, M. Characterization of polymeric substance classes in cereal-based beverages using asymmetrical flow field-flow fractionation with a multi-detection system. Anal Bioanal Chem 409, 5723–5734 (2017). https://doi.org/10.1007/s00216-017-0512-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-017-0512-6