Abstract
In this paper, we investigate whether dissolution in water under autoclaving conditions (140 °C, 20 min) or in dimethyl sulfoxide, DMSO (100 °C, 1 h), is preferable for characterization of amylose. Two types of amylose, potato and maize, were dissolved either in water using an autoclave or in DMSO. On the aqueous solutions obtained, the extent of molecular dissolution of the sample (referred to as the dissolution yield) was determined by enzymatic analysis as well as the molecular properties, such as molar mass and root-mean-square radius, obtained with asymmetrical flow field-flow fractionation coupled to multi-angle light scattering and differential refractive index detection (AF4-MALS-dRI). The results showed that both dissolution methods are efficient at dissolving amylose. However, AF4-MALS-dRI analysis revealed substantial differences. Amylose aqueous solutions obtained by dissolution in DMSO were relatively stable over time, but the dissolution method in autoclave caused some degradation of the molecules, and their solutions display a high tendency to retrograde.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Starch granules consist of two types of biopolymers: amylopectin and amylose. Amylopectin is a highly branched molecule comprising a large number of short α(1→4)-linked glucose chains which are joined together by α(1→6) branch linkages (approximately 4–5 % α(1→6) linkages [1, 2]). Amylopectin has a very high molar mass, M (approximately 106 < M < 109 g/mol) [3–7]. Amylose is a linear molecule of α(1→4)-linked glucose units. Some amylose molecules, particularly the larger ones, can be slightly branched [8]. Amylose is typically reported to have a M of approximately 105–106 g/mol [9]. The ratio between amylose and amylopectin depends on the starch species. In starch from non-mutant genotypes, the amylose range is between 10 and 30 %, while in mutant genotypes, unusually high-amylose contents (40 to 70 %) down to essentially 0 % can be found [10].
In aqueous media, amylose has a tendency to aggregate, more specifically to retrograde, and thereby loose solubility. This transformation may change the texture of foods rich in starch by increasing firmness or rigidity. To make the best use of starch in human and animal nutrition, as well as in industrial applications, it is important to gain knowledge and understanding of structure–property relationships. Currently, there is limited understanding of how molecular size affects starch functional properties, partly due to the lack of reproducible methods for characterizing molecular size [9].
Structural characterization of starch polymers is difficult for a number of reasons: Firstly, obtaining molecular dissolutions of the starch granules is difficult—for example, when high-amylose starch varieties are heated in water at 96 °C, they show very poor swelling and low solubility (6–9 % of leached material from the granules) [11], and autoclaving or jet-cooking is necessary for gel formation [10]. Secondly, elevated temperatures [12] or severe shearing [13] used for dissolution can cause molecular degradation of amylose. Thirdly, after aqueous dissolution, starch solutions and particularly amylose can be very unstable; dilute amylose solutions can show turbidity as a result of retrogradation and eventually crystalline precipitation [6, 10]. Fourthly, the broad size distribution can be a challenge for size separation techniques.
Size exclusion chromatography, SEC, and field-flow fractionation, FFF (such as asymmetrical flow field-flow fractionation, AF4, and symmetrical flow field-flow fractionation, SF4), are the most common methods for separation of starch polymers for determination of size and M (Table 1).
SEC has been used to separate and characterize amylose extracted from aqueous leaching [23–25], and reliable size distribution can be obtained essentially free of shear scission [26]. However, SEC is limited by its rather low exclusion limits in terms of the size of amylopectin, which also suffers shear degradation during the passage through the packed column [27, 28]. Cave et al. demonstrated clearly that shear degradation of amylopectin occurs in SEC, and calculations showed that such degradation cannot be avoided under experimental conditions [26].
In contrast, AF4 is a powerful separation technique capable of separating extremely polydisperse molecules/particles over a size range from approximately 2 to >800 nm. The technique minimizes or eliminates degradation and unwanted interaction [29] (i.e., low shear forces and small internal surface area). AF4 coupled with multi-angle light scattering (MALS) has already been applied successfully for M and root-mean-square radius (r rms) determination of starches [5, 7, 15, 16, 30] (Table 1).
To obtain reliable measurements of the size distribution of the starch polymers, the dissolution procedure should fully dissolve starch without degradation. The separation technique should also provide optimal size separation (with good resolution, minimal band broadening, and good signal-to-noise ratio) without loss of the sample during the separation and detection process [9]. It is therefore essential to determine whether the entire starch sample has been dissolved and ensure that the dissolution procedure has not caused significant degradation of the starch polymers. After separation, the recovery should always be determined and reported [9], and the sample should remain stable during the size separation.
Elevated temperature/pressure, high pH, dissolution in DMSO, and/or a combination of these procedures have typically been applied to obtain molecular dissolutions of the starch granules (Table 1). In many cases, the dissolution yield and the recovery have been reported (Table 1). However, the dissolution methods are usually not examined to see whether degradation has occurred during dissolution. Furthermore, the methods have not been compared, so such studies are demanded.
In a recent paper, we reported on dissolving amylopectin from waxy maize starch, using either heating at elevated temperatures (autoclaving or microwave treatment in the temperature range 120 to 170 °C) in aqueous environment or dissolution in DMSO at 100 °C [3]. These methods have been applied previously to dissolve starch (Table 1). The dissolution with autoclave at the different temperatures and dissolution in DMSO were compared and evaluated with AF4-MALS-dRI, together with enzymatic analysis to determine the dissolution yield (Table 1). Glycogen was used to examine whether degradation occurs at the different dissolution conditions. The results showed that dissolution in water by autoclaving at 140 °C and dissolution in DMSO are optimum conditions for amylopectin, since the starch is dissolved to a large extent (see ref [3] in Table 1) with limited degradation of the polymers. Furthermore, heating at 160 °C and above caused considerable degradation of the amylopectin molecule, whereas at 120 °C, dissolution was incomplete.
Regardless of the initial dissolution procedure, the AF4-MALS-dRI analysis is performed in aqueous solutions, which raises concerns about the stability of the polymer solution (especially with regards to amylose). Dissolved amylopectin typically would not be expected to give rise to instability issues during analysis through aggregation (retrogradation) as such retrogradation occurs slowly [10]. However, amylose retrogradation occurs much more rapidly [10] and could impair the quality of the characterization if it were to occur on experimental time scales. Furthermore, amylose is less studied in literature as the main emphasis is typically on the amylopectin component of starch.
In this paper, the aim is to investigate whether autoclaving in aqueous solvents or dissolution in DMSO is preferable for the characterization of amylose. Another aim was to investigate the time scale over which retrogradation occurs in amylose solutions. Dissolution yields of two types of amylose (potato amylose and high-amylose maize starch) were investigated through enzymatic analysis as well as molecular properties such as M, root-mean-square radius (r rms), and apparent density obtained with AF4-MALS-dRI.
Material and methods
Material
Amylose from corn, practical grade (native high-amylose maize starch, approximately 70 % amylose; A-7043 Sigma), and amylose extracted from potato (10130 Fluka and A05212 Sigma) were obtained from Sigma-Aldrich, St. Louis, MO, USA. The samples were used without further purification. A05212 was used only for the experiment with enzymatic debranching. Moisture content of the potato (10130 Fluka) and maize amylose samples was found to be 8.8 ±2 and 9.8 ± 1 %, respectively, determined by drying at 105 °C for 24 h. All water was purified using a Milli-Q system (Millipore Corp., Bedford, MA, USA)
Dissolution methods
Autoclave method
A high-pressure laboratory autoclave model II (Carl Roth GmbH & Co KG, Karlsruhe, Germany) was used, equipped with a tablet magnetic stirrer and a programmable temperature control unit (WRX 2000). A sample (0.1 g) was accurately weighed and transferred quantitatively into the autoclave cylinder with 100 mL of purified water. Before heating, the suspension was flushed with nitrogen gas for 5 min to avoid oxidative degradation of the sample during heating. The suspension was then heated to 140 °C from room temperature for approximately 14 min, kept at 140 °C for 20 min, quenched to approximately 90 °C by immersing the autoclave cylinder in cold water, and then immediately injected onto the AF4 channel. Injections were also performed every hour for 8 h after dissolution.
DMSO method
DMSO was obtained from WVR, Stockholm, Sweden (BDH Prolabo 23500, Batch 11D010502). A sample (0.05 g) was weighed directly into a glass vial (26 × 76 mm, 25 mL) after which 1 mL DMSO was added. The sample vial was capped and then heated for 1 h in a boiling water-bath during magnetic stirring at 160 rpm (magnetic stirrer with heating, IKA® WERKE, GmbH & CO. KG., Staufen, Germany). After cooling to room temperature, the sample was quantitatively diluted with the AF4 carrier liquid to 50 mL in a volumetric flask and immediately injected onto the AF4 channel. Injections were also performed every hour until 8 h after dissolution.
Dissolution yield
The starch concentration in the potato and maize amylose samples was determined using an enzymatic total starch analysis kit (Megazyme International, Bray, Co. Wicklow, Ireland). After dissolution of the samples, the total starch concentration in the aqueous solution and in the supernatant after centrifugation at 2,000×g for 10 min (Beckman Coulter Allegra X-15R centrifuge, CA, USA) was measured (Megazyme International, Bray, Co. Wicklow, Ireland). An aliquot (10 mL) of the sample, before and after centrifugation, was mixed with acetate buffer (1 mL, 1.2 M, pH 5) and α-amylase (100 μL). The solution was then heated to 100 °C in a water-bath for 6 min, cooled to 50 °C, amyloglucosidase (100 μL) was added, and the solution was incubated at 50 °C for 1 h. The solution was then centrifuged at 2,000×g for 10 min and duplicate aliquots (100 μL) of the supernatant, a standard d-glucose solution (100 μL, 1 mg/mL), and a blank (100 μL of water) were each mixed with glucose oxidase/peroxidase reagent (3 mL) and incubated at 50 °C for 20 min. The absorbance of the samples and d-glucose standards was then read against the blank at 510 nm in a Cary 50 Bio UV/Visible Spectrophotometer (Agilent Technology, Waldbronn, Germany).
The dissolution yield was determined using the following equation:
where C dissolved is the concentration of starch after centrifugation, and C initial is the concentration of starch before centrifugation, i.e., the total enzymatic digestible starch.
Debranching of potato amylose
Potato amylose (0.075 g) was dissolved in DMSO (1.5 mL) at 100 °C for 1 h. An aliquot (500 μL) of this solution was mixed with sodium acetate buffer (4.5 mL, 50 mM, pH 3.5) and then debranched with 20 μL isoamylase from Pseudomonas sp. (1,000 U, Sigma, I5284, Lot BCBC1741) at 38 °C for 24 h. The debranched sample solution was then quantitatively diluted with the AF4 carrier liquid to 25 mL in a volumetric flask and immediately injected onto the AF4 channel. For comparison, a non-debranched sample solution was also prepared. An aliquot (500 μL) from the amylose-DMSO solution was taken and then quantitatively diluted with the AF4 carrier liquid to 25 mL in a volumetric flask before being immediately injected onto the AF4 channel.
AF4 analysis equipment
The AF4 separation was carried out using an Eclipse 3+ separation System (Wyatt Technology Europe, Dernbach, Germany) connected to a Dawn Heleos II MALS detector (Wyatt Technology) operating at a wavelength of 658 nm and an Optilab T-rEX differential refractive index detector (Wyatt Technology) operating at a wavelength of 658 nm. A short separation channel with trapezoidal geometry (tip-to-tip length of 17.4 cm, widths at inlet and outlet of 2.17 and 0.37 cm, respectively, Wyatt Technology) and with a nominal thickness of 350 μm was used. The ultra-filtration membrane forming the accumulation wall was made of regenerated cellulose (Millipore PLGC, Wyatt Technology). For the experiment with enzymatic debranching, a regenerated cellulose membrane (cutoff 104 g/mol) from Microdyn-Nadir GmbH, Wiesbaden, Germany was used. An Agilent 1100 series isocratic pump (Agilent Technologies, Waldbronn, Germany) with an in-line degasser and an Agilent 110 series autosampler (Agilent Technology) were used for delivery of the carrier liquid and for handling the injection of sample onto the channel. A filter-holder, with a 0.1-μm pore size polyvinylidene fluoride membrane (Millipore Corp., Bedford, MA, USA), was placed between the pump and the channel to ensure the carrier liquid reaching the channel was free of particles. The carrier liquid was 10 mM NaNO3 (AppliChem, A3125, Darmstadt, Germany) with 0.02 % (w/v) NaN3 (BDH 10369, Poole, UK) to prevent bacterial growth.
Bovine serum albumin, BSA (Sigma) solution (1 mg/mL), was used to validate the performance of the AF4 system and to determine the channel height [31]. According to a procedure described elsewhere [32], the thickness was determined to be 238 and 321 μm for channels with Millipore and Microdyn-Nadir regenerated cellulose membranes, respectively.
AF4 separation parameters
The separation was performed using a constant detector flow at 1 mL/min throughout the separation. The injection onto the channel was performed at a flow rate of 0.2 mL/min during 4 min. After injection, a 3-min focusing/relaxation step was performed prior to elution with the focus flow rate being identical to the initial cross-flow rate during elution (1 mL/min). In order to avoid excessive retention and long elution times, the cross-flow rate was programmed to decay exponentially with time according to the function:
where Q c (t) is the cross-flow as a function of time t after elution starts, Q c (0) is the initial cross-flow at time zero, and t 1/2 is the half-life of the exponential decay. Exponentially decaying cross-flow rates have been shown to give good resolution and high selectivity across the size distribution of polydisperse macromolecules [33].
The elution was started at Q c (0) of 1 mL/min and then decreased exponentially with time (t 1/2 of 4 min) down to 0.09 mL/min and was then kept constant from 14 to 57 min. After elution, the channel was flushed without any cross-flow for 3 min before the next analysis. To avoid overloading, the amount injected onto the channel was optimized for the potato amylose sample obtained by the DMSO method. The injected volume varied from 30 to 80 μL (corresponding to a mass load of 21 to 56 μg on a dry weight basis) in steps of 10 μL. Confirmation that retention times were independent of the injected amount indicated no overloading, and an injected volume of 80 μL was chosen (giving adequate signal-to-noise ratio).
Data processing
Molar mass (M), root-mean-square radius (r rms), and separation channel mass recovery
Data was obtained using Astra software, version 5.3.4.14 (Wyatt technology). The molar mass, M, and the root-mean-square radius, r rms, were obtained by the Berry method [34, 35] performing a first-order fit to data obtained at scattering angles of 60.0–121.2° (detectors 8–14). The lowest scattering angles, 25.9° and 51.5° (detectors 4–7), were not included, as the data obtained was imprecise. A specific refractive index increment, dn/dc, value of amylose in water of 0.146 mL/g [36] was used, and the second virial coefficient was assumed to be negligible. The sample recovery was determined from the ratio of the mass eluted from the separation channel (integration of the dRI signal) to the injected mass (based on starch concentration).
Apparent density
The apparent densities over the size distribution were obtained from the M and r rms data, assuming homogeneous distribution of mass and a spherical shape. As r rms gives only an approximate description of the volume of possible shapes, the density obtained should be regarded as an apparent property. The apparent density, ρ i , for component i of the sample is calculated from:
where M i is the molar mass of fraction i, V i is the volume of fraction i, N A is the Avogadro number, and α is given by Eq. (4).
The apparent density describes the distribution of mass in volume for the fractions, providing additional information about scaling in a macromolecular population [37].
Results
Potato and maize amylose aqueous solutions were obtained by dissolution in water using an autoclave or in DMSO, and the dissolution yield was determined. The yield is obtained by comparing the concentration in the aqueous solutions after removal of any insoluble particles (starch concentration after centrifugation) with the total concentration of the sample (starch concentration before centrifugation). Samples were injected onto the AF4 channel immediately after dissolution (defined as time zero, t = 0 h). Samples were allowed to age at room temperature, and from, it further injections were performed every hour up to 8 h after dissolution, to investigate the stability of the solutions.
Dissolution yield
The starch concentration of maize amylose sample was 96 % (value corrected for moisture content of the sample), but in potato amylose, the starch concentration was low, around 70 %. The low starch concentration can be caused by the presence of resistant starch which is not accessible for enzymatic degradation. The low value may also have minor contributions from non-starch impurities.
The dissolution yield results are shown in Table 2. For both samples, the dissolution yield was high (98–100 %) regardless of the dissolution method used for dissolution.
Sample mass recovery from the AF4 channel
The sample mass recovery from the AF4 channel for potato and maize amylose is shown in Table 2. For potato amylose solutions obtained by the autoclave method, mass recovery increased with time after dissolution; from 81 % at t = 0 h to 96 % 8 h after dissolution. Conversely, when potato amylose was dissolved with the DMSO method, the mass recovery at time 0 after dissolution was ∼93 %, and this value remained constant over time (from 0 to 8 h after dissolution).
For maize amylose solutions obtained by the autoclave or DMSO method, recovery values were low and remained roughly constant over time (from 0 to 8 h after dissolution). For the autoclaved sample, the recoveries at 0 and 8 h after dissolution were 60 and 62 %, respectively, while for maize amylose dissolved with DMSO method, the corresponding values were 55 and 60 % (Table 2). The reason for such low mass recoveries was presumably due to the loss of low molar mass fractions of the polymer through the accumulation wall membrane.
Amyloses from cereals are generally smaller molecules than those of other origin, especially high-amylose maize starch [8]. Thus, the observed higher mass loss of maize amylose through the accumulation wall in comparison with potato amylose may be related to this size difference.
A lesser contribution to the low recovery was that the void peaks (t 0) were excluded in the integration of the dRI signal. This was because samples obtained with the DMSO method gave void peaks that were much higher than those of autoclaved samples. The void peak was only included for the calculation of the recovery of the autoclaved potato amylose sample.
AF4-MALS-dRI results for amylose using DMSO during dissolution
Potato amylose
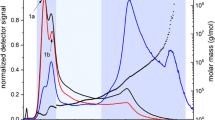
Figure 1 shows the MALS 90° and dRI fractograms for potato amylose (0–8 h after dissolution) dissolved with the DMSO method. The fractograms and the molar mass remained unchanged in the investigated time range. The dRI signal shows a peak followed by a short, low signal tail, while the MALS signal clearly reveals the presence of two populations. The main population, eluting early and representing the dRI peak, has a M ranging from 105 to 106 g/mol, and a second population in the dRI tail has M in the range 2 · 106 to 2 · 107 g/mol. Rice amylose samples [38], amyloses from different botanical origin prepared by aqueous leaching [24], and synthetic amylose [39] have also shown the presence of two populations of molecules (bimodal chromatograms) when examined by size exclusion chromatography. The second population could possibly be an intermediate component between amylose and amylopectin [40], a low M amylopectin contaminating the amylose sample [38], or aggregated and retrograded amylose [24].
Iso-amylolysis of potato amylose
One way of obtaining information about the identity of the second population is to use isoamylase enzyme, which is highly selective and only depolymerizes α(1→6)-glucosidic linkages. Consequently, the enzyme was incubated with the potato amylose solution before being injected onto the AF4 separation channel. As control sample, a sample prepared under the same conditions but without the enzyme was used. The resulting fractograms are presented in Fig. 2.
Comparison of non-debranched (shown in black) and debranched (shown in red) potato amylose (A05212 SIGMA) solution obtained by the DMSO dissolution method. a MALS 90° fractogram and r rms vs. elution time. b dRI fractogram vs. elution time. Note that retention times differ compared to Fig. 1 as a different membrane supplier (Nadir) was use for the channel in Fig. 2, as described in the “Materials and methods” section. This resulted in somewhat longer retention times and broader peaks
After enzymatic debranching, the second MALS peak disappeared (Fig. 2a), and the dRI peak displayed a narrower shape and considerably less tailing compared to the non-debranched sample (Fig. 2b). These results show that the later-eluting population is comprised of branched material. The sample solution after incubation with isoamylase contained small amounts of precipitated material, and its solution appearance was somewhat opaque. This is probably the result of aggregation of the short chains produced during amylolysis [41–43]. These aggregates were most probably eluted in the so-called steric/hyperlayer mode of AF4 [44] as the first MALS peak with the highest intensity (Fig. 2a). It can be seen in Fig. 2a that the early eluting peak is associated with a larger size (r rms) which supports the suggestion that this peak contains aggregated short chains which co-elute in reverse order. The mass recovery was lower for the debranched sample (80 %) than for the non-debranched (90 %), which may be caused by loss of relatively low molar mass material through the accumulation wall membrane (cutoff 104 g/mol).
Maize amylose
The fractograms of maize amylose dissolved by the DMSO method are shown in Fig. 3a, b. Like the potato sample, the dRI signal (Fig. 3a) shows a major peak with M in the range 105 to 106 g/mol and a significantly longer tail (up to approximately 20 min) containing higher concentrations than that of the potato amylose sample (Figs. 1 and 2). The MALS signal shows one main MALS peak corresponding to the population contained in the dRI peak tail (Fig. 3b). This second population displayed an M from 2 · 106 to approximately 109 g/mol.
Maize amylose dissolved by the DMSO method. Fractograms obtained 0, 1, 2, and 8 h after dissolution. a dRI signal (lines) and M (symbols) vs. elution time. b MALS 90° signal (lines) and r rms (symbols) vs. elution time. M and r rms obtained 0, 1, 2, and 8 h after dissolution are denoted by open squares, down triangles, circles, and closed squares, respectively
For maize amylose, the fractograms (dRI and MALS) remained unchanged for the first 2 h after dissolution (Fig. 3a, b). After that, the second population peak tail (Fig. 3a) started to increase slightly (Fig. 3a, b). The result is in contrast with the results for potato amylose for which the aqueous solutions obtained with the DMSO dissolution method were stable in the investigated time range (Fig. 1).
AF4-MALS-dRI results for amylose dissolved with the autoclave method
Potato amylose
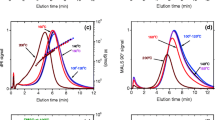
The dRI and MALS fractograms of potato amylose dissolved with the autoclave method and immediately injected (t = 0 h) are shown in Fig. 4a, b. The fractograms are compared with those obtained by dissolution in DMSO.
Comparison of autoclave (black) and DMSO (blue) dissolution methods for the potato amylose (the shaded areas correspond to apparent density plots in Fig. 5). a dRI signal (line) and molar mass (symbols) vs. elution time; b MALS signal (lines) and r rms (symbols) vs. elution time for samples injected immediately after dissolution; c dRI signal (lines) and molar mass (symbols) vs. elution time; d MALS signal (lines) and r rms (symbols) vs. elution time for samples injected 0–8 h after dissolution
The autoclaved sample displays a somewhat narrower dRI peak and shows more material eluting in the peak tail compared to the sample dissolved by the DMSO method (Fig. 4a). The differences in peaks are reflected in their mass recoveries, being 81 and 93 % for the autoclaved sample and DMSO-dissolved sample, respectively, at t = 0 h (Table 2).
The somewhat narrower dRI peak shown in the autoclaved sample could be an indication that slight degradation occurred during dissolution. The degradation products could be of sufficiently small size to be lost through the accumulation wall (cutoff 104 g/mol), giving rise to a mass recovery of 81 % at t = 0 h (Table 2). In our previous study [3], amylopectin showed a slightly lower retention time when it was dissolved at 140 °C compared with the one dissolved in DMSO, which is in agreement with what we observed here for amylose. However, the mass recovery for amylopectin was higher (95 %) than that reported here (81 %) for potato amylose dissolved by autoclaving.
The differences observed in the tails of the dRI peak between autoclaved and DMSO-dissolved samples become clearly visible by looking at the corresponding MALS signals (Fig. 4b). When DMSO is used, the late-eluting population shows a MALS signal that appears as a shoulder of the main peak, while in the autoclaved sample, the MALS signal for the late eluting population appears as a broader and higher signal, incompletely resolved from the early eluting population. Differences in M and r rms are also evident in Fig. 4a, b. The autoclaved sample shows broader M and r rms distributions than those observed from dissolution with the DMSO method.
In Fig. 4c, d, the dRI and MALS fractograms obtained every hour (0–8 h) after dissolution are shown. The autoclaved sample shows significant changes occurring in the tail of the dRI peak, which grows with time and eventually becomes a distinct second peak (Fig. 4c). In contrast, the dRI and MALS fractograms of samples dissolved with the DMSO method remained unchanged with time.
Sections of the fractograms from Fig. 4 were chosen to compare the autoclave and DMSO methods at t = 0 h and t = 8 h. Population 1 (P1) consisted of fractions eluting between 3.4 and 6 min, and population 2 (P2) consisted of fractions eluting between 8 and 14 min for the autoclave method and between 8 and 11 min for the DMSO method. Weight average M and z average r rms of P1 and P2 as well as over the entire size distribution are given in Table 3. No notable differences in M and r rms were found for P1 between dissolution methods or between 0 and 8 h after dissolution. For P2, however, a striking difference in M between dissolution methods was found, i.e., the M of the autoclaved sample is approximately four times higher than that obtained with the DMSO method.
Apparent density of potato amylose
For sections shown as shaded areas in Fig. 4 (P1 and P2), Fig. 5 shows the apparent density values. For P1, there are only minor differences in apparent density between dissolution methods. The apparent densities of P1 are rather low (approximately 4 kg/m3).
Apparent density vs. molar mass for P1 and P2 (corresponding to shaded areas in Fig. 4) for potato amylose dissolved by the DMSO (blue) and autoclave method (black). Data obtained 0 h (open circles), 1 h (down triangles), and 8 h (closed circles) after dissolution
The P2 of potato amylose dissolved with the DMSO method displays an apparent density between 10 and 20 kg/m3, while the P2 of the one dissolved with the autoclave method displays apparent densities between 23 and 40 kg/m3. As the autoclaved sample ages, it can also be observed that the apparent densities increase and show a clear dependence on M (Fig. 5). Such relatively compact structures with density increasing over time are not present when the sample is dissolved with DMSO method. One explanation could be that P2 of the autoclaved potato amylose sample comprises retrograded amylose. These results were in agreement with visual observation of the solutions, i.e., for potato amylose dissolved with DMSO, the solution remained clear for several days while for potato amylose dissolved by autoclaving the solution appearance changed from clear (at t = 0 h after dissolution) to opaque 8 h after dissolution.
Maize amylose
The dRI and MALS fractograms of the autoclaved maize amylose 0 h after dissolution (Fig. 6a, b) are compared with those obtained with the DMSO method.
Comparison of autoclave (black) and DMSO (blue) dissolution methods for the maize amylose. a dRI signal (lines) and molar mass (symbols) vs. elution time; b MALS signal (lines) and r rms (symbols) vs. elution time for samples injected immediately after dissolution; c dRI signal (lines) and molar mass (symbols) vs. elution time; d MALS signal (lines) and r rms (symbols) vs. elution time for samples injected 0 h (open symbols), 1 h, and 8 h (closed symbols) after dissolution
Similar elution behavior and M and r rms distributions are observed for both autoclave and DMSO dissolution methods. The major differences are that the autoclaved sample shows higher and broader dRI peak with a less pronounced tail than that of the DMSO-dissolved sample (Fig. 6a). The less pronounced dRI peak tail also corresponds to a lower MALS signal intensity when compared with the sample dissolved with DMSO (Fig. 6b).
Figure 6c, d shows fractograms obtained 0, 1, and 8 h after dissolution. It is interesting to note that, for the autoclaved sample, the changes in the shape of the fractograms occur relatively quickly over time. The trend is as follows: the tail of the dRI peak increases with time, while a decrease and a narrowing of the main peak occurs compared to what is observed 0 h after dissolution (Fig. 6c). The trend suggests that the relatively small analytes, contained in the main peak, aggregate with time, causing the signal to decrease. In turn, the aggregates cause the increase in signal of the peak tail (at approximately 12 min, Fig. 6c). The shift to longer elution times is more readily observed in the MALS signal, which also shows that changes occur more rapidly in the autoclaved sample within 0–1 h after dissolution than with the DMSO method (Fig. 6d). The MALS signal obtained 8 h after dissolution is higher than those at 0 h for both autoclave and DMSO dissolution methods, indicating the instability of maize amylose sample with time. The differences in M and r rms between 0 and 8 h after dissolution are given in Table 4. The weight average M of the maize sample obtained after autoclaving show higher values with time (0 vs. 8 h) than the one obtained with the DMSO method.
Apparent density of maize amylose
Figure 7 shows the apparent density vs. M for maize amylose dissolved with the DMSO method (Fig. 7a) or the autoclave method (Fig. 7b). It can be observed that the apparent density varies somewhat with M but is rather similar between the two dissolution methods. For the largest M species in the autoclaved sample, the apparent density increases with increasing M which could indicate the presence of incompletely dissolved species.
Discussion
The purpose of the study was to investigate methods to dissolve amylose that result in solutions stable against retrogradation on experimental time scales. The dissolution method should maximize the dissolution yield to be representative and should not cause molecular degradation. The two dissolution methods investigated in this paper yield, from one perspective, similar results when applied to potato and maize amylose. It should be noted that the use of commercial samples of amylose has some limitations, in that the amylose is likely to be retrograded in commercial samples causing it to be difficult to dissolve. The dissolution yield obtained is similar for both potato and maize amylose (>99 %) when the two dissolution methods are compared (Table 2), indicating that both methods are similarly efficient at dissolving amylose. For potato amylose, the concentration of starch in the sample was 70–71 %, and it may be the presence of amylose resistant to enzymatic degradation that gives rise to such a low value. Depending on the molecular order within retrograded amylose, dissolution in water will require temperatures in the range 160–180 °C [6]. Furthermore, to a lesser extent, the oxidation of glucose at elevated temperatures during the original amylose extraction could also contribute to a low concentration of starch in the potato amylose sample [8].
When the autoclaved potato amylose solutions were allowed to age pre-injection, an increase in separation channel mass recovery was observed as well as an increase in detector signals for the late-eluting population. This is also reflected in an increase in average M with increasing time. Together, these results all suggest a relatively fast retrogradation of the autoclave dissolved potato amylose, i.e., a growth in size and mass. Furthermore, aggregation of low M amylose chains that on their own would be lost through the accumulation wall could explain the increased mass recovery observed with time. For the potato amylose dissolved in DMSO, no indication of retrogradation could be observed in the investigated time range (up to 8 h).
Both the autoclave method and the DMSO method resulted in maize amylose solutions susceptible to aggregation (Fig. 6c, d). However, the results suggested that DMSO-dissolved maize amylose remained stable somewhat longer than maize amylose dissolved by autoclave, as very minor changes in the elution profile were observed after 1 h, while larger changes were observed for the autoclaved sample. This again suggested that there were differences induced by the dissolution method
Thus, when the amylose was initially dissolved in DMSO, the solutions appeared more stable over time, so the DMSO dissolution is preferable. The results also showed that samples should be analyzed fresh in order to obtain reliable characterization. It is interesting, and somewhat puzzling, that the stability of the aqueous amylose solutions depended on the initial dissolution method used. The P2 of autoclaved potato amylose showed higher apparent density values than P2 of potato amylose dissolved with the DMSO method already at t = 0 h after dissolution (Fig. 5, P2). A possible interpretation of this in relation to the instabilities of the solutions, discussed above, is that the higher apparent densities reflect incompletely dissolved species. In turn, the presence of such species could serve as nucleation seeds in the aggregation (retrogradation) of the amylose chains. The retrogradation would be considerably faster when these poorly dissolved species are present compared to when they are absent.
Both potato and maize amyloses retrograde faster when dissolved by autoclave than when they are dissolved in DMSO. By comparing autoclaved maize and potato amyloses, it can be seen that potato amylose retrogrades faster than maize amylose as a second peak is formed in the former within 8 h after dissolution, while the formation of a second peak in the latter takes approximately 72 h (data not shown). This difference may be the result of the presence of amylopectin. In a study of amylose chain association and the effect of amylopectin on that process (in mixtures of different ratios of amylose and amylopectin, analyzed by differential scanning calorimetry [45]), it was found that increasing the amount of amylopectin restricted the chain association of amylose. Such effects have been attributed to either to dilution or steric hindrance effects. These effects may contribute to the relatively fast retrogradation of the autoclaved potato amylose sample since it only contains small amounts of branched material, whereas the maize amylose sample contains substantial amounts of amylopectin.
In the literature, autoclaving at between 150 °C [12] and 155 °C [6] was used for achieving molecular dissolution of high-amylose starch. Such conditions can however cause degradation of the starch polymers [3]. On the other hand, DMSO was reported to dissolve high-amylose starch varieties more completely than high amylopectin starches [23, 46]. But in excess of heating and stirring, the dissolution in DMSO can also cause degradation of the starch [13]. The dissolution condition in DMSO that was used here has shown not to cause degradation of amylopectin [3], and the studies on amylose reported here confirmed those findings.
Conclusions
The results in this paper show that, of the two investigated dissolution methods, initial dissolution in DMSO is the preferred method when preparing samples of amylose for molecular characterization, such as SEC or AF4 analysis. The DMSO dissolution method generates solutions with high dissolution yield and minimum remaining aggregates, with no signs of degradation, and the solutions generated appear stable over typical experimental times. These results also indicate that the dissolution in DMSO can be used for reliable measurements of the size distribution of starches containing amylose in general.
References
Nilsson GS, Gorton L, Bergquist K-E, Nilsson U (1996) Determination of the degree of branching in normal and amylopectin type potato starch with 1H-NMR spectroscopy improved resolution and two-dimensional spectroscopy. Starch-Stärke 48(10):352–357
Fernandez C, Rojas C, Nilsson L (2011) Size, structure and scaling relationships in glycogen from various sources investigated with asymmetrical flow field-flow fractionation and 1H NMR. Int J Biol Macromol 49(4):458–465
Perez-Rea D, Bergenståhl B, Nilsson L (2015) Development and evaluation of methods for starch dissolution using asymmetrical flow field-flow fractionation. Part I. Dissolution of amylopectin. Accepted for publication in Anal Bioanal Chem. doi:10.1007/s00216-015-8611-8
Rojas C, Wahlund K-G, Br B, Nilsson L (2008) Macromolecular geometries determined with field-flow fractionation and their impact on the overlap concentration. Biomacromolecules 9(6):1684–1690
Rolland-Sabaté A, Guilois S, Jaillais B, Colonna P (2011) Molecular size and mass distributions of native starches using complementary separation methods: asymmetrical flow field flow fractionation (A4F) and hydrodynamic and size exclusion chromatography (HDC-SEC). Anal Bioanal Chem 399(4):1493–1505
Aberle T, Burchard W, Vorwerg W, Radosta S (1994) Conformational contributions of amylose and amylopectin to the structural properties of starches from various sources. Starch-Stärke 46(9):329–335
Wahlund K-G, Leeman M, Santacruz S (2011) Size separations of starch of different botanical origin studied by asymmetrical-flow field-flow fractionation and multiangle light scattering. Anal Bioanal Chem 399(4):1455–1465
Hizukuri S, J-i A, Hanashiro I (2006) In: Eliasson A-C (ed) Carbohydrate in food, 2nd edn. Taylor & Francis Group, Boca Ratón
Gidley MJ, Hanashiro I, Hani NM, Hill SE, Huber A, Jane J-L, Liu Q, Morris GA, Rolland-Sabaté A, Striegel AM, Gilbert RG (2010) Reliable measurements of the size distributions of starch molecules in solution: current dilemmas and recommendations. Carbohydr Polym 79(2):255–261
Eliasson A-C, Gudmundsson M (2006) In: Eliasson A-C (ed) Carbohydrate in food, 2nd edn. Taylor & Francis Group, Boca Ratón
Colonna P, Mercier C (1985) Gelatinization and melting of maize and pea starches with normal and high-amylose genotypes. Phytochemistry 24(8):1667–1674
Vorwerg W, Radosta S, Leibnitz E (2002) Study of a preparative-scale process for the production of amylose. Carbohydr Polym 47(2):181–189
Han J-A, Lim S-T (2004) Structural changes of corn starches by heating and stirring in DMSO measured by SEC-MALLS-RI system. Carbohydr Polym 55(3):265–272
Roger P, Baud B, Colonna P (2001) Characterization of starch polysaccharides by flow field-flow fractionation–multi-angle laser light scattering–differential refractometer index. J Chromatogr A 917(1–2):179–185
van Bruijnsvoort M, Wahlund KG, Nilsson G, Kok WT (2001) Retention behaviour of amylopectins in asymmetrical flow field-flow fractionation studied by multi-angle light scattering detection. J Chromatogr A 925(1–2):171–182
You S, Stevenson SG, Izydorczyk MS, Preston KR (2002) Separation and characterization of barley starch polymers by a flow field-flow fractionation technique in combination with multiangle light scattering and differential refractive index detection. Cereal Chem 79(5):624–630
Rolland-Sabaté A, Colonna P, Mendez-Montealvo MG, Planchot V (2007) Branching features of amylopectins and glycogen determined by asymmetrical flow field flow fractionation coupled with multiangle laser light scattering. Biomacromolecules 8(8):2520–2532
Juna S, Williams PA, Davies S (2011) Determination of molecular mass distribution of amylopectin using asymmetrical flow field-flow fractionation. Carbohydr Polym 83(3):1384–1396
Fishman ML, Rodriguez L, Chau HK (1996) Molar masses and sizes of starches by high-performance size-exclusion chromatography with on-line multi-angle laser light scattering detection. J Agric Food Chem 44(10):3182–3188
Bello-Pérez LA, Roger P, Baud B, Colonna P (1998) Macromolecular features of starches determined by aqueous high-performance size exclusion chromatography. J Cereal Sci 27(3):267–278
Praznik W, Mundigler N, Kogler A, Pelzl B, Huber A, Wollendorfer M (1999) Molecular background of technological properties of selected starches. Starch-Stärke 51(6):197–211
Radosta S, Haberer M, Vorwerg W (2001) Molecular characteristics of amylose and starch in dimethyl sulfoxide. Biomacromolecules 2(3):970–978
Zhong F, Yokoyama W, Wang Q, Shoemaker CF (2006) Rice starch, amylopectin, and amylose: molecular weight and solubility in dimethyl sulfoxide-based solvents. J Agric Food Chem 54(6):2320–2326
Roger P, Colonna P (1993) Evidence of the presence of large aggregates contaminating amylose solutions. Carbohydr Polym 21(2–3):83–89
Roger P, Colonna P (1996) Molecular weight distribution of amylose fractions obtained by aqueous leaching of corn starch. Int J Biol Macromol 19(1):51–61
Cave RA, Seabrook SA, Gidley MJ, Gilbert RG (2009) Characterization of starch by size-exclusion chromatography: the limitations imposed by shear scission. Biomacromolecules 10(8):2245–2253
Kim W, Eum CH, Lim S, Han J, You S, Lee S (2007) Separation of amylose and amylopectin in corn starch using dual-programmed flow field-flow fractionation. Bull Korean Chem Soc 28(12):2489
Messaud FA, Sanderson RD, Runyon JR, Otte T, Pasch H, Williams SKR (2009) An overview on field-flow fractionation techniques and their applications in the separation and characterization of polymers. Prog Polym Sci 34(4):351–368
Nilsson L (2012) In: Williams K, Caldwell K (eds) Field-flow fractionation in biopolymer analysis. Springer-Verlag/Wien, NewYork
Lee S, Nilsson P-O, Nilsson GS, Wahlund K-G (2003) Development of asymmetrical flow field-flow fractionation–multi angle laser light scattering analysis for molecular mass characterization of cationic potato amylopectin. J Chromatogr A 1011(1–2):111–123
Nilsson L (2013) Separation and characterization of food macromolecules using field-flow fractionation: A review. Food Hydrocoll 30(1):1–11
Håkansson A, Magnusson E, Bergenståhl B, Nilsson L (2012) Hydrodynamic radius determination with asymmetrical flow field-flow fractionation using decaying cross-flows. Part I. A theoretical approach. J Chromatogr A 1253:120–126
Leeman M, Wahlund K-G, Wittgren B (2006) Programmed cross flow asymmetrical flow field-flow fractionation for the size separation of pullulans and hydroxypropyl cellulose. J Chromatogr A 1134(1–2):236–245
Berry GC (1966) Thermodynamic and conformational properties of polystyrene. I. Light-scattering studies on dilute solutions of linear polystyrenes. J Chem Phys 44(12):4550–4564
Andersson M, Wittgren B, Wahlund K-G (2003) Accuracy in multiangle light scattering measurements for molar mass and radius estimations. Model calculations and experiments. Anal Chem 75(16):4279–4291
Brandrup J, Immergut EH, Grulke EA (eds) (1999) Polymer handbook, 4th edn. Wiley, New York
Nilsson L, Leeman M, Wahlund K-G, Bergenståhl B (2006) Mechanical degradation and changes in conformation of hydrophobically modified starch. Biomacromolecules 7(9):2671–2679
Takeda Y, Hizukuri S, Juliano BO (1986) Purification and structure of amylose from rice starch. Carbohydr Res 148(2):299–308
Roger P, Colonna P (1992) The influence of chain length on the hydrodynamic behaviour of amylose. Carbohydr Res 227:73–83
Vilaplana F, Gilbert RG (2010) Two-dimensional size/branch length distributions of a branched polymer. Macromolecules 43(17):7321–7329
Gidley MJ, Bulpin PV (1989) Aggregation of amylose in aqueous systems: the effect of chain length on phase behavior and aggregation kinetics. Macromolecules 22(1):341–346
Gidley MJ, Bulpin PV (1987) Crystallisation of malto-oligosaccharides as models of the crystalline forms of starch: minimum chain-length requirement for the formation of double helices. Carbohydr Res 161(2):291–300
Hizukuri S, Takeda Y, Yasuda M, Suzuki A (1981) Multi-branched nature of amylose and the action of debranching enzymes. Carbohydr Res 94(2):205–213
Caldwell K (2000) In: Schimpf M, Caldwell K, Giddings JC (eds) Field-flow fractionation handbook. Wiley, New York
Sievert D, Wüsch P (1993) Amylose chain association based on differential scanning calorimetry. J Food Sci 58(6):1332–1335
Jackson DS (1991) Solubility behavior of granular corn starches in methyl sulfoxide (DMSO) as measured by high performance size exclusion chromatography. Starch-Stärke 43(11):422–427
Acknowledgments
Financial support from the Swedish International Development Agency (Sida/SAREC) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in the topical collection Field- and Flow-based Separations with guest editors Gaetane Lespes, Catia Contado, and Bruce Gale.
Rights and permissions
About this article
Cite this article
Perez-Rea, D., Bergenståhl, B. & Nilsson, L. Development and evaluation of methods for starch dissolution using asymmetrical flow field-flow fractionation. Part II: Dissolution of amylose. Anal Bioanal Chem 408, 1399–1412 (2016). https://doi.org/10.1007/s00216-015-8894-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-015-8894-9