Abstract
The macromolecular composition of beer is largely determined by the brewing and the mashing process. It is known that the physico-chemical properties of proteinaceous and polysaccharide molecules are closely related to the mechanism of foam stability. Three types of “American pale ale” style beer were prepared using different mashing protocols. The foam stability of the beers was assessed using the Derek Rudin standard method. Asymmetric flow field-flow fractionation (AF4) in combination with ultraviolet (UV), multiangle light scattering (MALS) and differential refractive index (dRI) detectors was used to separate the macromolecules present in the beers and the molar mass (M) and molar mass distributions (MD) were determined. Macromolecular components were identified by enzymatic treatments with β-glucanase and proteinase K. The MD of β-glucan ranged from 106 to 108 g/mol. In addition, correlation between the beer’s composition and foam stability was investigated (increased concentration of protein and β-glucan was associated with increased foam stability).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Beer is the world’s most popular and consumed drink after water and tea; it is also the oldest alcoholic beverage [1,2,3]. Beer is mainly composed of water, a starch source from malting barley or other cereals, and flavoring from hops. Beer is comprised of complex macromolecules, such as polysaccharides and proteins (polypeptides) [4].
Generally, the process of beer production can be divided into malting and brewing, whereas brewing can also be divided into several principal steps: mashing, boiling, fermenting, and maturation. Among them, mashing is an important step as it determines the total material that can be extracted from the malt and present starch is enzymatically converted into lower molar mass (M) sugars [4], resulting in the sweet wort. Malted barley contains various enzymes, such as α-amylase, β-amylase, β-glucanase, cystase, protease, and others [5, 6], which to various extents will digest malt macromolecules into smaller species. The wort composition is essential for beer quality, and a number of factors influence the mashing process and, thus, wort composition and properties. Important factors are water composition, grist composition, pH, mash thickness, dissolved oxygen, etc. Another key parameter when discussing the properties of the sweet wort (and eventually the finished beer) is the mash profile (i.e., temperatures and time); however, the mash profile can be changed relatively easy compared to other parameters [6].
The quality of beer will begin to decrease depending on the storage time and temperature after packaging. Therefore, it is essential to assess the stability of the beer. The presence of some proteins results in beer that appears hazy, and polysaccharides with high M tend to increase the viscosity and turbidity of beer [6,7,8,9].
Foam stability of beer is often closely related to consumer satisfaction. Several beer constituents are associated with foam formation and stability: hops (iso-α-acids), proteins, and polysaccharides [10,11,12]. The enzymatic activity during mashing will affect proteins and polysaccharides and, thus, change beer properties like viscosity and foam stability. The characterization of beer macromolecules is typically based on methods described by the European Brewery Convention (EBC) [13], e.g., high-performance liquid chromatography (HPLC) and matrix-assisted laser desorption/ionization time of flight mass spectrometry (MALDI-TOF-MS) for the analysis of β-glucan and proteins but they only provide information for relatively low-M materials [14, 15]. β-glucan is commonly analyzed with fluorimetry as described by Munck et al. [13]. However, the type of beer or wort has to be regarded as dark samples can influence the absorbance and, hence, the β-glucan content may be underestimated. Using Calcofluor as labeling agent for β-glucan during fluorimetry might introduce additional errors, as the complex formation between Calcofluor and β-glucan molecules is not quantitative for β-glucan below 104 g/mol, resulting in a lower detector response [16].
Furthermore, most of the EBC-described methods do not provide information on the M, molar mass distribution (MD), and possible aggregation of β-glucan, resulting in high-M species above 106 g/mol. One study assessed the influence of the MD of beer components on the intensity of palate fullness, but this study focused more on low-M (<105) beer components [17]. Another drawback with these techniques mentioned by the EBC is that, due to sample preparation, a non-native state of the constituents might be analyzed. Tomasi et al. developed a simple and suitable method to determine and characterize β-glucan in beer wort, utilizing size exclusion chromatography (SEC), coupled to different detectors such as light scattering, viscometer, and differential refractive index (dRI) detector, to obtain information on, e.g., molecular weight averages, fraction description, hydrodynamic sizes, and intrinsic viscosity [18].
A recent study has shown the suitability of asymmetric flow field-flow fractionation (AF4) to analyze the macromolecules for beer foam and liquid [19]. Tügel et al. obtained wide MD and were able to analyze high M species, suggesting that there could also be highly aggregated macromolecules. AF4 is a gentle, flow-based separation technique with the ability of determining M and MD of macromolecules in a broad range (103–1017 g/mol) due to the thin ribbon-like open channel without packing material [20, 21]. The absence of a stationary phase, compared to chromatographic techniques like HPLC and SEC, enables a separation of the macromolecules under gentle and “close to native” conditions. In brief, the separation in AF4 is based on the hydrodynamic size and the individual diffusion coefficients of the analytes that are relaxed after injection onto the channel in a field perpendicular to the accumulation wall during a focusing step. Smaller molecules will be relaxed higher up in the channel, bigger molecules closer to the accumulation wall. Due to the parabolic flow profile during elution, the small analytes will elute earlier as large species. The retention time (t r) is directly related to the hydrodynamic radius of the analyte, and with the help of an in-line multiangle light scattering detector (MALS) and concentration detector, e.g., dRI, M and MD can be obtained. AF4 connected to various detectors has already been proven a powerful technique for the characterization of various food macromolecules [22].
The aim of this study was to further evaluate the applicability of AF4 for the efficient separation and characterization of macromolecules in beer. Furthermore, the aim was to investigate the influence of mashing conditions on the macromolecular properties and the relationship between the macromolecular composition and foam stability.
In this study, we produced beer using three different mashing processes. Each beer underwent enzymatic treatments for semi-quantitative and qualitative analysis of the present macromolecules. AF4 coupled with online ultraviolet (UV) detector, MALS, and dRI detectors was employed for the separation and characterization of M and MD of macromolecules in the beers. In addition, the foam stability for the different beer types was assessed using the Derek Rudin standard method [12, 23] to confirm the correlation between macromolecules and foam stability.
Materials and methods
Materials
Acetic acid, sodium acetate trihydrate, sodium azide (NaN3), β-glucanase, and proteinase K were purchased from Sigma-Aldrich (St. Louis, MO, USA). The β-glucanase and proteinase K activities are ≥1.0 units/mg and ≥30.0 units/mg, respectively. All chemicals were of analytical grade and were used without further purification. The carrier liquid for AF4 was prepared with water purified through a Milli-Q plus purification water system (Millipore Co. Ltd., Billeria, USA, resistance = 18.2 MΩ/cm). The carrier liquid was vacuum-filtered through a 0.1 μm cellulose nitrate membrane filter (Whatman GmbH, Dassel, Germany) before it was used.
The materials used to produce the beer were the following: the grains used were Pale Malt (2 Row barley) UK (5.9 EBC) from Weyermann Malting (Bamberg, Germany), Cara-Pils/Dextrine (3.9 EBC), and Crystal 100 (100.0 EBC) from Viking Malt (Halmstad, Sweden), using a ratio of 18:1:1; the hops varieties used were Cascade (6.90% α-acid) and Amarillo Gold (8.80% α-acid) both grown in the USA and supplied by Humlegården (Sollentuna, Sweden); and the yeast used was Safale US-05 (Fermentis/Lesaffre, Marcq-en-Baroeul, France).
Production of the beer
Three types of beer (reference beer, protein rest beer, and β-glucan rest beer) were produced on a 20 L scale using 20 L Braumeister (Speidel, Ofterdingen, Germany). Reference beer was produced using a typical saccharification rest for American ale production. Protein rest beer and β-glucan rest beer were incubated at temperatures of 50 and 37 °C for 30 min before the saccharification rest, to maintain proteinases and β-amylases at their optimum activity, respectively. An overview of the mashing process conditions can be found in Table 1. After mashing, all beers were boiled at 100 °C for 75 min, and subsequently given time to cool down to room temperature. Then, 11.5 g yeast were added to each batch for fermentation during 1 week at 17 °C. Prior to bottling and a second fermentation step for another week in the cold room, sediments like non-soluble proteins, yeast residues, and insoluble polysaccharides were removed.
Preparation of the beer samples
All types of beer were transferred into 2-mL plastic tubes and sonicated using an FS60 sonicator (Fisher Scientific, Ottawa, Canada) for 30 min for degassing at room temperature and 130 W. The effect of sonication on beer macromolecules was investigated in a previous paper and no influence on the sample was noticed [19].
Enzymatic treatments of the beer samples
Enzymatic treatments of the beer samples were carried out with β-glucanase and proteinase K to confirm the composition of macromolecules in beer. Beer samples were prepared in 1 mL amounts filled into a centrifuge tube, and 10 μL of enzyme/mL was added to the beer. Reaction times were optimized to reach the optimal enzyme activities for the samples, resulting in 24 h at 60 °C for β-glucanase and 12 h at 37 °C for proteinase K, respectively. Effects of the temperature treatment on the macromolecules of the beer were investigated to make sure that the temperature changes during enzymatic treatment did not affect the macromolecules. No difference was observed in AF4 fractograms and MD without enzyme but with the same temperature treatments.
AF4 analysis of the beer
The AF4 system used in this work was an Eclipse 2 system (Wyatt Technology Europe, Dernbach, Germany), coupled online with a S 4245 UV detector (Schambeck SFD GmbH, Bad Honnef, Germany) operated at a wavelength of 280 nm, a DAWN EOS MALS (Wyatt Technology, Santa Barbara, CA, USA) operated at a wavelength of 690 nm, and a RI-101 dRI detector (Shodex, Tokyo, Japan). The carrier liquid was pumped into the AF4 channel using an Agilent 1100 HPLC pump (Agilent Technologies, Waldbronn, Germany) with an in-line vacuum degasser, and the flow rate was measured by an OptiFlow 1000 Liquid Flowmeter (Agilent Technologies, Palo Alto, CA, USA). The analysis channel was an Eclipse AF4 long channel (Wyatt Technology, Europe, Dernbach, Germany) assembled with a regenerated cellulose membrane having a molecular weight cut-off (MWCO) of 10 kDa (Millipore, Bedford, USA) and a 350-μm-thick Mylar spacer. The actual channel thickness (= 234.6 μm) was determined from t r of bovine serum albumin (BSA, MW = 66 kDa, Sigma-Aldrich, St. Louis, MO, USA) using the FFFHydRad 2.0 software [24]. The channel geometry was trapezoidal with a tip-to-tip length of 26.5 cm and a width at the inlet and the outlet of 2.2 and 0.6 cm, respectively. A 50-μL beer sample was injected into the AF4 channel at an injection flow rate of 0.2 mL/min for 3 min, and the focusing time was 3 min at 4.0 mL/min. The channel flow was kept constant at 1.0 mL/min, while the cross flow was exponentially decreasing from 4.0 to 0.2 mL/min with a half-life of 5 min and then kept at a constant cross flow of 0.2 mL/min for 30 min. The carrier liquid used was 50 mM of acetate buffer at a pH of 4.4, approximating the pH of beer. All AF4 experiments were performed at room temperature. The M and MD were determined by online AF4-MALS using the Berry method [25, 26], and all data processing was performed using ASTRA software (Version 6.1.1, Wyatt Technology). The dn/dc values of 0.185 and 0.150 mL/g were used to characterize the proteins (population 1) and polysaccharides (populations 2 and 3), respectively [27,28,29,30].
Assessment of beer foam stability
The foam stability was determined using Derek Rudin standard method [12, 23]. The device for the measurement of foam stability is shown in Fig. S1 in the Electronic Supplementary Material (ESM). The foam collapse rate was determined as follows: degassed beer was added up to the 6 cm mark and foamed by injecting nitrogen (N2) or carbon dioxide (CO2) gas at a rate of about 100 mL/min and a constant temperature of 20 °C until the foam reached the 40 cm mark. The time needed for the foam liquid interface to migrate from the 5 cm mark to the 2 cm mark was measured and, hence, the collapse rate was determined. Each measurement was repeated five times. As the foam stability is directly associated to the cleanliness of the glass, a washing step with ethanol was done prior to the next analysis, and the glass was given time to dry at room temperature.
Results and discussion
Characterization of the beer using AF4-UV-MALS-dRI
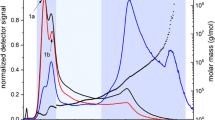
With the help of AF4-UV-MALS-dRI, the M and MD of each fraction were determined. Figure 1 shows AF4-UV-MALS-dRI fractograms for the three types of beer with their MD. It can be seen from Fig. 1a that there are three major populations of macromolecules in each beer. Protein rest and β-glucan rest beers have similar elution behaviors and M ranges for each population. In all beers, population 1 elutes during t r of 2–6 min and has a M range of 2.0–4.0 × 104 g/mol. However, the dRI and UV fractograms (Figs. 2b, c) show different responses and, hence, indicate different amounts of low-M substances for each beer, whereas the reference beer shows the lowest dRI response but highest for UV and protein rest beer the highest dRI response but lowest for UV. In the case of the protein rest beer, the high dRI response compared to the reference beer could be expected as higher β-glucanase activity is present due to the protein rest at 50 °C. This may result in an increased concentration of the low-M substances and, hence, the increase in dRI response. Population 2 was eluted with a t r of 6–21 min, and all beers (reference, protein rest, and β-glucan rest) had a similar elution pattern but different M ranges of 5 × 104 to 7 × 106, 5 × 104 to 3 × 106, and 4 × 104 to 2 × 106 g/mol, respectively. Population 3 was eluted between 21 and 50 min and each beer had a different signal pattern from MALS signal as well as different M ranges of 1 × 107 to 3 × 108, 2.5 × 106 to 5.5 × 106, and 2 × 106 to 1 × 107 g/mol, respectively. For all beers, the dRI is strongest for population 1, weaker for population 2, and very weak for population 3 indicating that these are present at lower concentrations (Figs. 2b, c). However, populations 2 and 3 are readily detectable with the MALS detector as the light scattering intensity is high for large analytes. The results show that the mashing conditions in the brewing process have a significant influence on the concentration and M range of beer components. The M range and the MALS response in the reference beer are higher than those of the protein rest and β-glucan rest beers which is likely to be a result of the higher enzymatic activity in the two latter. Accordingly, enzymatic treatments of the beers were used to investigate the chemical composition of the macromolecules.
Characterization of enzymatically treated beer
Reference beer was produced using the optimal (or general) mashing conditions for “American pale ale” style beer (see Table 1). Figure 2 shows the AF4 fractograms and MD of reference beer before and after enzymatic treatment with either β-glucanase or proteinase K. A significant change is observed in the MALS response (see Fig. 2a); moreover, the maximum M of populations 2 and 3 decreased after β-glucanase treatment, from 3 × 108 to 2.3 × 106 g/mol, but no significant change was observed after treatment with proteinase K (only the MALS intensity decreased), suggesting that the ultra-high M macromolecules (>107 g/mol) in populations 2 and 3 are considerably degraded by β-glucanase treatment (see symbol in Fig. 2a); thus, these analytes are to a large extent composed of β-glucan, and the M range of β-glucan was confirmed to be approximately 106 to 108 g/mol. The precise M range of proteins before and after enzymatic treatment was difficult to determine from AF4 results because there were only minor changes in the UV-absorbance, but not in t r. Due to the differences, the signal could be identified as coming from proteinaceous matter, but the M range did not change.
Furthermore, proteins will affect the macromolecules’ aggregation. Results demonstrated that proteinase K-treatment does not change the M range of populations 2 and 3 (Fig. 2a), but t r of the peak maximum of population 3 increased with about 5 min, indicating a higher hydrodynamic size (r hyd) of the species, which could indicate some aggregation of β-glucan in beer as no change in UV response is observed.
Figures 3 and 4 show the AF4 fractograms and M of protein rest and β-glucan rest beers before and after enzymatic treatments with β-glucanase and proteinase K. In these cases, the enzymatic treatment results differ from those of the reference beer. M of populations 1 and 3 increased after β-glucanase treatment and M of populations 2 and 3 increased after proteinase K treatment. Protein rest beer had a lower level of proteins than the reference beer and β-glucan rest beer (Table 1). Proteinase K treatments for protein rest and β-glucan rest beers showed different results regarding MD, compared to the untreated beers, i.e., the maximum M increased. The results show the presence of denser aggregates (i.e., higher M at the same t r) which suggests that the protein plays an important role in determining the structure of the macromolecular aggregates. In addition, the void peak of the MALS detector increased after β-glucanase treatment for all beers, suggesting two possible explanations for this phenomenon: Firstly, aggregation of degraded β-glucan from β-glucanase treatment and secondly, aggregation of proteins might cause co-elution in steric/hyperlayer mode without retention in the separation channel [31]. The relative amounts of macromolecules, such as protein and β-glucan, were determined from the area of MALS responses from 8 to 50 min (populations 2 and 3) for each beer. Table 1 shows the results for the relative amounts of protein and β-glucan in the macromolecules of each beer. As the results indicate, the reference beer has a higher amount (about 68%) of β-glucan than the other beers, and β-glucan rest and reference beers have more similar amounts (about 32%) of protein than protein rest beer. However, these values should be considered apparent as a direct comparison assumes that the apparent density and/or conformation are the same at same t r in all three types of beer. AF4 separation occurs based on r hyd and therefore, it might be possible that after an enzymatic treatment, species with similar r hyd but with different M elute at the same t r.
The concentrations of proteins and β-glucan are, in regard to literature, closely related to the foam stability of beer [7, 10,11,12]. Therefore, the foam stability of the beers was measured to investigate the correlation between stability and the amount of the respective macromolecules.
Assessment of foam stability
It is known from literature that the foam stability is dependent on the concentrations of proteins and polysaccharides, e.g., β-glucan in the sample, and high-gravity beer has poorer foam stability [6, 12]. The specific gravities (SG), alcohol content, and viscosity values of the produced beers (reference, protein rest, and β-glucan rest beers) can be found in Table 1. The values for the original gravity show some variation which is probably originating from the different mashing profiles and the temperature effect on enzymatic activity. The variation should be considered small and the difference in alcohol content is not expected to play a large role for the aim of this paper. Figure 5 shows the assessment of foam stability for the three types of beer using different gases (N2 and CO2). The results show that the reference beer has the highest foam stability, followed by β-glucan rest beer and protein rest beer. Furthermore, it can be seen that the stability differed depending on the type of gas; for the reference and β-glucan rest beer, more stable foams were obtained when N2 gas was used, whereas for protein rest beer, the result is not conclusive due to an insignificant difference. The lower foam stability for protein rest beer seems reasonable as the protein content is lower than in the other beers. However, higher foam stability is expected for N2 gas, as it has a lower solubility in water than CO2 gas and, thus, foam collapse originating from Ostwald ripening (disproportionation) is considerably slower [11, 12].
The foam stability results for the three types of beer based on the Derek Rudin standard method. The analysis of each beer was repeated five times and the average values are shown including error bars (standard error of the mean with a 95% confidence interval). The injection flow rate of gas = 100 mL/min, temperature = 20 °C
Furthermore, results imply that higher concentrations of protein and β-glucan were associated with higher foam stability (see Table 1 and Fig. 5) as the reference beer shows the highest foam stability. This is most probably due to good foam stabilizing properties of proteins. From the results (Fig. 5), it can be concluded that the effect of protein on the foam stability is higher compared to the effect of β-glucan. Nevertheless, higher β-glucan content increases the viscosity of beer. In turn, the increased viscosity slows down drainage of liquid from the lamellae in the foam and, thus, gives rise to higher stability. Comparing the results from protein rest to β-glucan rest beer, a higher content of protein (= β-glucan rest beer) seems to have a greater impact on foam stability than a higher content of β-glucan (= protein rest beer).
Conclusions
In this study, three types of beer were produced using different mashing processes, and the M range of macromolecules for reference, protein rest, and β-glucan rest beers were determined as 2.0 × 104–3 × 108, 2.0 × 104–5.5 × 106, and 2.0 × 104–1 × 107 g/mol, respectively. In addition, the M of β-glucan was confirmed to be in the range of about 106–108 g/mol. The relative amount and M range of macromolecules in each beer were determined using an AF4-UV-MALS-dRI set-up before and after enzymatic treatment of the beers. For each beer (reference, protein rest, and β-glucan rest beers), the relative amount of protein (32, 24, and 33%) and β-glucan (68, 48, and 45%) was determined. Our results show that beer has a complex aggregated macromolecular composition consisting of proteins and polysaccharides. We were also able to make a direct connection between the AF4 results and the foam stability of the samples; with higher concentration of protein and β-glucan, the foam stability increased, whereas protein had a greater impact on foam stability than β-glucan. The results presented in this paper should be helpful for analyzing beers on the macromolecular scale.
References
Arnold JP. Origin and history of beer and brewing: From prehistoric times to the beginning of brewing science and technology; A critical essay. Alumni Ass'n of the Wahl-Henius Institute of Fermentology; 1911.
Michel RH, McGovern PE, Badler VR. Chemical evidence for ancient beer. Nature. 1992;360(6399):24. doi:10.1038/360024b0.
Max N. The Barbarian's Beverage: A History of Beer in Ancient Europe. 1st ed. Taylor & Francis; 2005.
Bamforth C. Beer: tap into the art and science of brewing. 3rd ed. Oxford University press; 2009.
Esslinger HM. Handbook of brewing: processes, technology, markets. Wiley; 2009.
Priest FG, Stewart GG. Handbook of brewing. CRC Press; 2006.
Asano K, Shinagawa K, Hashimoto N. Characterization of haze-forming proteins of beer and their roles in chill haze formation. J Am Soc Brew Chem. 1982;40(4):147–54. doi:10.1094/ASBCJ-40-0147.
Siebert KJ, Carrasco A, Lynn PY. Formation of protein–polyphenol haze in beverages. J Agric Food Chem. 1996;44(8):1997–2005. doi:10.1021/jf950716r.
Steiner E, Becker T, Gastl M. Turbidity and haze formation in beer—insights and overview. J Inst Brew. 2010;116(4):360–8.
Bamforth CW, Kanauchi M. Interactions between polypeptides derived from barley and other beer components in model foam systems. J Sci Food Agric. 2003;83(10):1045–50. doi:10.1002/jsfa.1503.
Bamforth CW. The relative significance of physics and chemistry for beer foam excellence: theory and practice. J Inst Brew. 2004;110(4):259–66. doi:10.1002/j.2050-0416.2004.tb00620.x.
Bamforth CW. Foam: Practical Guides for Beer Quality. 1st ed. American Society of Brewing Chemists; 2012.
Munck L, Jorgensen K, Ruud-Hansen J, Hansen K. The EBC methods for determination of high molecular weight β-glucan in barley, malt, wort and beer. J Inst Brew. 1989;95(2):79–82. doi:10.1002/j.2050-0416.1989.tb04612.x.
Anderson I. The effect of β-glucan molecular weight on the sensitivity of dye binding assay procedures for β-glucan estimation. J Inst Brew. 1990;96(5):323–6. doi:10.1002/j.2050-0416.1990.tb01038.x.
Laštovičková M, Mazanec K, Benkovská D, Bobáľová J. Utilization of the linear mode of MALDI-TOF mass spectrometry in the study of glycation during the malting process. J Inst Brew. 2010;116(3):245–50. doi:10.1002/j.2050-0416.2010.tb00427.x.
Kim S, Inglett GE. Molecular weight and ionic strength dependence of fluorescence intensity of the Calcofluor/β-glucan complex in flow-injection analysis. J Food Compos Anal. 2006;19(5):466–72. doi:10.1016/j.jfca.2005.11.006.
Rübsam H, Gastl M, Becker T. Influence of the range of molecular weight distribution of beer components on the intensity of palate fullness. Eur Food Res Technol. 2013;236(1):65–75.
Tomasi I, Marconi O, Sileoni V, Perretti G. Validation of a high-performance size-exclusion chromatography method to determine and characterize β-glucans in beer wort using a triple-detector array. Food Chem. 2017;214:176–82. doi:10.1016/j.foodchem.2016.06.092.
Tügel I, Runyon JR, Gómez Galindo F, Nilsson L. Analysis of polysaccharide and proteinaceous macromolecules in beer using asymmetrical flow field-flow fractionation. J Inst Brew. 2015;121(1):44–8. doi:10.1002/jib.195.
Giddings JC. Field flow fractionation: a versatile method for the characterization of macromolecular and particulate materials. Anal Chem. 1981;53(11):1170A–8A.
Wahlund KG, Giddings JC. Properties of an asymmetrical flow field-flow fractionation channel having one permeable wall. Anal Chem. 1987;59(9):1332–9.
Nilsson L. Separation and characterization of food macromolecules using field-flow fractionation: a review. Food Hydrocoll. 2013;30(1):1–11. doi:10.1016/j.foodhyd.2012.04.007.
Rudin AD. Measurement of the foam stability of beers. J Inst Brew. 1957;63(6):506–9. doi:10.1002/j.2050-0416.1957.tb06291.x.
Håkansson A, Magnusson E, Bergenståhl B, Nilsson L. Hydrodynamic radius determination with asymmetrical flow field-flow fractionation using decaying cross-flows. Part I. A theoretical approach. J Chromatogr A. 2012;1253:120–6. doi:10.1016/j.chroma.2012.07.029.
Berry GC. Thermodynamic and conformational properties of polystyrene. I. Light-scattering studies on dilute solutions of linear polystyrenes. J Chem Phys. 1966;44(12):4550–64. doi:10.1063/1.1726673.
Andersson M, Wittgren B, Wahlund K-G. Accuracy in multiangle light scattering measurements for molar mass and radius estimations. Model Calculations and Experiments Anal Chem. 2003;75(16):4279–91. doi:10.1021/ac030128+.
Theisen A. Refractive increment data-book for polymer and biomolecular scientists: Nottingham University Press; 2000.
Steiner E, Gastl M, Becker T. Protein changes during malting and brewing with focus on haze and foam formation: a review. Eur Food Res Technol. 2011;232(2):191–204. doi:10.1007/s00217-010-1412-6.
Ulmius M, Önning G, Nilsson L. Solution behavior of barley β-glucan as studied with asymmetrical flow field-flow fractionation. Food Hydrocoll. 2012;26(1):175–80. doi:10.1016/j.foodhyd.2011.05.004.
Grimm A, Krüger E, Burchard W. Solution properties of β-D-(1, 3)(1, 4)-glucan isolated from beer. Carbohydr Polym. 1995;27(3):205–14. doi:10.1016/0144-8617(95)00056-D.
Myers MN, Giddings JC. Properties of the transition from normal to steric field-flow fractionation. Anal Chem. 1982;54(13):2284–9.
Acknowledgements
The authors acknowledge the support provided by the National Research Foundation (NRF) of Korea (NRF-2013K2A3A1000086 and NRF-2016R1A2B4012105), support from Hannam University Research Fund, and support from the Swedish foundation for international cooperation for research and higher education (STINT).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
There are no competing interests present and there are no patents, products in development or marketed products to declare. Furthermore, we agree with the policies on sharing data and materials, as in the guide for authors.
Electronic supplementary material
ESM 1
(PDF 94 kb)
Rights and permissions
About this article
Cite this article
Choi, J., Zielke, C., Nilsson, L. et al. Characterization of the molar mass distribution of macromolecules in beer for different mashing processes using asymmetric flow field-flow fractionation (AF4) coupled with multiple detectors. Anal Bioanal Chem 409, 4551–4558 (2017). https://doi.org/10.1007/s00216-017-0393-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-017-0393-8