Abstract
We have investigated methods of starch dissolution with the aim of finding an optimum method to completely dissolve starch granules to form a molecularly dissolved starch solution without degradation of the polymers. Glycogen was used as a model molecule for amylopectin, to identify the dissolution conditions under which the degradation of the polymers was limited or not present. Dissolution was performed in water with temperatures up to 200 °C, facilitated by the use of heating in an autoclave or a microwave oven, or in dimethyl sulfoxide (DMSO) at 100 °C. Waxy maize starch was chosen due to its high content of amylopectin and very low content of amylose. The degree of starch dissolution under different conditions was determined enzymatically. The effect of different dissolution conditions on the molar mass and root-mean-square radius of the polymers was determined with asymmetrical flow field-flow fractionation coupled to multi-angle light scattering and differential refractive index (AF4-MALS-dRI) detectors under aqueous conditions. The results suggest that reliable and accurate size separation and characterization of amylopectin can be obtained by dissolution of starch granules in an aqueous environment at 140 °C by autoclaving or in DMSO at 100 °C. The results also clearly show an upper limit for heat treatment of starch, above which degradation cannot be avoided.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Starch is one of the most abundant biopolymers on Earth. It is synthesized by higher plants in the form of semi-crystalline granules and used as an energy reserve. Starch consists of a mixture of two glucose polymers: amylose and amylopectin. Amylose is a linear molecule of α(1→4) linked glucose units. Some amylose molecules, particularly the larger ones, are slightly branched by α(1→6) linkages [1]. Amylose is typically reported to have a molar mass (M) between ~105 and 106 g/mol [2]. Amylopectin is highly branched (4–5 % α(1→6) branch linkages [3, 4]) and has a very high molar mass (M > 107 g/mol [5]). It consists of a large number of α(1→4) linked glucose chains that are interlinked by α(1→6) linkages.
Starch is the most important source of energy in human diet. It is widely used in various industries such as the food, pharmaceutical, and paper industries [6]. To optimize the use of starch, it is important to understand the relationship between the properties of the initial material, during processing, and the properties of the final starch product. Thus, the physical and chemical properties need to be characterized. One of the most important properties of starch is its molecular size, as this can affect the viscosity of solutions [5], the adsorption kinetics at interfaces [7], and film properties [8], among others.
It is difficult to determine the molecular size distributions of starch for several reasons. Firstly, it is difficult to obtain a complete dissolution of starch granules without degradation of the individual polymers [2]. This is due, in part, to the semi-crystalline nature of the granules. While the crystalline region of starch requires rather aggressive dissolution conditions, such conditions can degrade the polymers contained in the amorphous regions, as well as already dissolved polymers [9]. Secondly, after aqueous dissolution, starch, in particular amylose, can have a high tendency to aggregate, i.e., retrograde, and thereby to lose solubility [10]. Amylopectin, on the other hand, retrogrades at a much lower rate [11, 12]. Thirdly, starch, in particular amylopectin, has extremely large molecules, presenting a major challenge in size separation techniques.
For accurate and reliable molecular size determination, the dissolution method should ensure the highest possible degree of dissolution of starch granules, while at the same time minimizing degradation. Moreover, the individual polymers thus obtained should remain stable during analysis, i.e., they should not aggregate and they should not be further degraded.
The most common dissolution methods for starch involve the use of elevated temperatures, high pH, or organic solvents such as dimethyl sulfoxide (DMSO) [13]. All these methods have been reported to cause degradation of the starch polymers under certain conditions [14, 9, 15–17]. However, it may be very difficult to determine when degradation starts to occur. Another challenge when choosing a dissolution procedure is that very few comparisons have been made between different methods and there is, thus, a demand for such studies [18].
Size exclusion chromatography (SEC) has been widely used for size separation of starch. However, it is limited by its relatively low exclusion limit with regard to the size of amylopectin [19, 20]. Furthermore, SEC has been shown to cause shear degradation of large amylopectin molecules, and calculations have shown that degradation cannot be avoided [21]. It is possible to obtain size distributions for large starch molecules using alternative size separation methods such as flow field-flow fractionation (FFF) [22, 23]. The shear is much lower in FFF techniques than in SEC [24], and therefore, shear scission of large amylopectin molecules is minimal. Asymmetrical flow field-flow fractionation (AF4), one of the most frequently used techniques of all the FFF techniques, is a powerful separation technique, which has proven to be highly suitable for the separation and characterization of large polydisperse polysaccharides [13]. AF4 coupled with multi-angle light scattering (MALS) provides a direct method of separating and determining the molar mass and the root-mean-square radius (r rms) of macromolecules over a very wide size range [25, 26]. The aim of the present study is to find optimum dissolution conditions for amylopectin using waxy maize starch. Some of the most common dissolution methods used for starch, e.g., dissolution in water at elevated temperatures using either autoclave or microwave heating, and dissolution in DMSO, were investigated and compared. Glycogen was used as a model molecule for amylopectin in order to determine the maximum dissolution temperature at which degradation is minimal or not present. Glycogen was chosen due to its similar structure to amylopectin, i.e., it is an α(1→4) glucan with α(1→6) branches. However, glycogen has a higher degree of branching than amylopectin [3], rendering it highly water soluble at room temperature. Glycogen is thus a suitable model molecule for studies of the potential degradation caused by different dissolution methods. The degree of dissolution of starch granules was assessed enzymatically, and the effect of the dissolution conditions on M and r rms was investigated using AF4 connected to MALS and differential refractive index (dRI) detectors.
Materials and methods
Materials
Waxy maize starch was obtained from Lyckeby Stärkelsen (Kristianstad, Sweden). Glycogen from oyster, type II, was supplied by Sigma Chemicals (G8751, Lot No. 116K0675, St. Louis, MO, USA). The moisture content of the waxy maize starch, hereafter referred to as amylopectin, was found to be 10.9 (±0.1) % w/w by drying at 105 °C for 24 h. According to the glycogen certificate of analysis provided by the supplier, the sample loss on drying is 0.1 %, and the material contains 86 % glycogen and 1 % reducing sugars.
Dissolution methods
Sample aqueous solutions (1 mg/mL) were obtained by dissolving the sample in pure water (water purified with the Milli-Q system, Millipore Corp., Bedford, MA, USA) or in DMSO (WVR BDH Prolabo, 23500, Batch 11D010502, Stockholm, Sweden). When using water as the solvent, the sample was dissolved at temperatures ranging from 23 °C (room temperature) to 200 °C. The heating was facilitated using a high-pressure laboratory autoclave (Model II, Carl Roth GmbH & Co KG, Karlsruhe, Germany) or using a microwave-accelerated reaction system (MARS 5, CEM Corporation, Matthews, NC, USA). When DMSO was used as the solvent, the sample was dissolved in DMSO at 100 °C, after which water was added to give an aqueous solution (the percentage of DMSO in the final solution was 3 %). Each dissolution method is described in detail in the next sections.
Autoclave heating
Aqueous glycogen (1 mg/mL) was prepared by dissolving the sample in water and then transferring the sample to the autoclave cylinder. Amylopectin solutions (1 mg/mL) were prepared by weighing 200 mg of sample and then transferring qualitatively quantitatively into the autoclave cylinder with 200 mL of water. Before heating, the sample was flushed with nitrogen gas for 5 min. The solution/suspension was heated from room temperature to the target temperature. Target temperatures for the glycogen solutions were 100, 120, 130, 140, 150, 160, 170, 180, and 200 °C, and for the amylopectin dispersions, 120, 140, 150, 160, and 170 °C, taking 14, 18, 20, 22, and 25 min, respectively, from room temperature. The target temperature was maintained for 20 min, and after heating, the autoclave cylinder was quenched to approximately 90 °C by immersion in cold water.
The glycogen solution thus obtained was immediately injected into the AF4 separation channel. For amylopectin, an aliquot (~15 mL) of the autoclaved sample (1 mg/mL) was filtered with a 0.45-μm regenerated cellulose filter (syringe filter, 25 mm diameter, Spartan 30/B, Ref. No. 463050, Lot. No. BI1097-3, Schleicher & Schuell, Dassel, Germany). Both the filtered and unfiltered solutions were then diluted four times with water and immediately injected into the AF4 separation channel.
Amylopectin solutions were also directly prepared at the different temperatures to give a concentration of 0.25 mg/mL. An aliquot (50 mL) of this sample was centrifuged at 1000g for 10 min (Beckman Coulter Allegra X-15R centrifuge, CA, USA).
Dissolution in DMSO
Glycogen (50 mg) or amylopectin (100 mg) was weighed directly into a glass vial (26 × 76 mm, 25 mL capacity). In the case of glycogen, 1 mL DMSO was immediately added. In the case of amylopectin, the sample was first dispersed in ethanol (0.3 mL, 80 % ethanol in water v/v) with magnetic stirring for 3–5 min, after which DMSO (3 mL) was added while being stirred (it is important to ensure that the amylopectin sample is completely dispersed in the DMSO without the formation of lumps). The respective vials were capped and then heated to 100 °C for 1 h in a boiling water bath with magnetic stirring at 160 rpm (magnetic stirrer with heating, IKA® WERKE, GmbH & Co. KG, Staufen, Germany), after which the sample was cooled to room temperature.
The glycogen solution was quantitatively transferred and diluted with water to 50 mL in a volumetric flask (corresponding to 1 mg/mL) and immediately injected into the AF4 separation channel. The amylopectin solution was quantitatively transferred and diluted with water to 100 mL in a volumetric flask (corresponding to 1 mg/mL). An aliquot (~15 mL) of this solution was filtered with a 0.45-μm regenerated cellulose filter, and both the filtered and unfiltered samples were then diluted four times with water and immediately injected into the AF4 separation channel.
An amylopectin solution was also directly prepared to a concentration of 0.25 mg/mL. An aliquot (50 mL) of this sample was centrifuged at 1000g for 10 min (Beckman Coulter Allegra X-15R centrifuge, CA, USA).
Microwave heating
A control vessel containing 49.5 g pure water and two sample vessels (50 mL capacity, HP-500 Plus with Teflon vessel liners), each containing 49.5 g of the glycogen solution (1 mg/mL), were loaded into the microwave system. A method was created using HP-500 as a sample vessel and a power of 900 W; 2 min ramp time for heating at 100, 120, and 140 °C; 3 min ramp for 150 or 160 °C; 3.2 min ramp for 170 °C; and 6 min ramp for 200 °C. The holding time for all temperatures was 50 s. After heating, the samples were cooled to 100 °C inside the microwave system (5–20 min) and then immediately injected into the AF4 separation channel.
Amylopectin dissolution yield
After dissolution, the starch content in the aqueous solutions before and after filtration or centrifugation was determined using an enzymatic total starch analysis kit (Megazyme International Ltd, Co. Wicklow, Ireland).
An aliquot (10 mL) of the amylopectin sample was mixed with acetate buffer (1 mL, 1.2 M, pH 5) and α-amylase (100 μL). The solution was then boiled in a water bath for 6 min. It was then cooled to 50 °C, and amyloglucosidase (100 μL) was added and the solution incubated at 50 °C for 1 h. The solution was then centrifuged at 2000g for 10 min, and duplicate aliquots (100 μL) of the solution supernatant, a standard d-glucose solution (100 μL, 1 mg/mL), and a blank (100 μL water) were each mixed with glucose oxidase/peroxidase reagent (3 mL) and incubated at 50 °C for 20 min. The absorbance of the samples and d-glucose standards was then read against the blank at 510 nm in a Cary 50 Bio UV/Visible Spectrophotometer (Varian, Victoria, Australia).
The degree of dissolution of the starch granules (hereafter referred to as the dissolution yield) was determined using the following equation:
AF4 analysis equipment
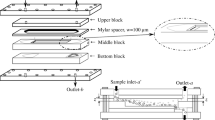
AF4 separation was performed using an Eclipse 3+ separation system (Wyatt Technology Europe, Dernbach, Germany) connected to a Dawn Heleos II MALS detector (Wyatt Technology) operating at a wavelength of 658 nm, and an Optilab T-rEX dRI detector (Wyatt Technology) operating at a wavelength of 658 nm. A short separation channel with trapezoidal geometry (tip-to-tip length 17.4 cm and breadth at the inlet and outlet of 2.17 and 0.37 cm, respectively, Wyatt Technology) and with a nominal thickness of 350 μm was used. The ultrafiltration membrane forming the accumulation wall was made of regenerated cellulose (for amylopectin: Millipore PLGC, Wyatt Technology; for glycogen: Nadir, Microdyn-Nadir GmbH, Wiesbaden, Germany), both with a cutoff of 104 g/mol. An Agilent 1100 series isocratic pump (Agilent Technology, Waldbronn, Germany) with an in-line degasser and an Agilent 1100 series autosampler (Agilent Technology) were used for delivery of the carrier liquid and to inject the sample into the separation channel. A filter holder, with a 0.1-μm pore size polyvinylidene fluoride membrane (Millipore Corp, Bedford, MA, USA), was placed between the pump and the channel to ensure that only particle-free carrier liquid reached the channel. The carrier liquid was 10 mM sodium nitrate (AppliChem, A3125, Darmstadt, Germany) with 0.02 % (w/v) sodium azide (BDH, 10369, Poole, UK) to prevent bacterial growth.
The performance of a newly assembled channel was verified using bromophenol solution, and bovine serum albumin (Sigma, A4378, St. Louis, MO, USA) solution (1 mg/mL) was used to validate the performance of the AF4 system (to obtain a good resolution of the monomer, dimer, and trimer) as described by Nilsson [13].
AF4 separation conditions
Separation was performed using a constant detector flow of 1 mL/min. The sample was injected into the channel at a flow rate of 0.2 mL/min for 4 min. After injection, a 3-min focusing/relaxation step was performed prior to elution using a focus flow rate identical to the initial cross-flow. In order to avoid excessive retention and long elution times, the cross-flow rate was programmed to decay exponentially with time, according to the function:
where Q c(t) is the cross-flow rate as a function of time t after elution starts, Q c(0) is the initial cross-flow at time zero, and t 1/2 is the half-life of the exponential decay. Exponentially decaying cross-flow rates have been shown to give good resolution and higher selectivity across the size distribution of polydisperse macromolecules [27].
For glycogen, elution was started at Q c (0) = 1 mL/min and then decreased exponentially with a t 1/2 of 4 min to 0.07 mL/min, which was maintained for 15 min. For amylopectin, elution was started at Q c (0) = 0.5 mL/min and then decreased exponentially with a t 1/2 of 1 min to 0.07 mL/min, which was then maintained from 3 to 20 min. After elution, the channel was flushed without any cross-flow for 3 min before the next analysis.
AF4 data processing
Data were recorded using Astra software, version 5.3.4.14 (Wyatt technology). M and r rms were obtained using the Berry method [28, 29] by performing a first-order fit to the data obtained at scattering angles of 34.8–100.3° (detectors 5–12). The lowest scattering angles, 14.4° and 25.9° (detectors 3 and 4), were not included, as the data obtained were imprecise. A specific refractive index increment (dn/dc) of 0.146 mL/g was used for amylopectin in water [30]. The same value was used for glycogen. The second virial coefficient was assumed to be negligible.
AF4 mass recovery
The sample recovery was determined from the ratio of the mass eluted from the separation channel (integration of the dRI signal) to the injected mass (based on the starch content).
Results
AF4-MALS-dRI results for glycogen
The effects of the various dissolution methods on the retention of glycogen are shown in Fig. 1, where both the dRI fractograms (where the signal is proportional to the analyte mass concentration) and MALS fractograms (where the signal is proportional to the analyte mass concentration and exponentially dependent on analyte size) are plotted as a function of the elution time.
Elution profiles for glycogen dissolved under different conditions and analyzed under aqueous conditions. a dRI (lines), M (dots) and b MALS 90° (lines) vs. elution time for glycogen dissolved in water and autoclaved at different temperatures. c dRI (lines), M (dots) and d MALS 90° (lines) vs. elution time for glycogen dissolved in water and heated to different temperatures in a microwave oven. e dRI (lines), M (dots) and f MALS 90° (lines) vs. elution time for glycogen dissolved in DMSO at 100 °C and in water at room temperature
The elution profiles for glycogen dissolved in water and heated by autoclaving in the temperature range 100–200 °C are shown in Fig. 1a, b. There were no differences in the retention time at temperatures up to 120 °C, as the dRI and MALS signals remain the same under these conditions. A slight decrease in the height of the MALS peak can be observed at 140 °C (Fig. 1b), while heating the sample above 140 °C caused a clear shift towards shorter retention times (Fig. 1a) and a decrease in peak height (Fig. 1b), indicating thermal degradation of the sample. It is interesting to note that as the temperature is increased, the scaling between M and the elution time (i.e., the hydrodynamic size) changes somewhat (Fig. 1a). This can be interpreted as a decrease in the apparent density of the analyte as, for a given elution time, M decreases with increasing temperature (Fig. 1a). This suggests that degradation proceeds in a non-random manner. The experiments using the autoclave to dissolve and heat glycogen were repeated twice, and the results were found to be reproducible (results not shown).
The dissolution of glycogen by microwave heating is shown in Fig. 1c, d. Again, no differences were found in the retention time up to a temperature of 120 °C. At 140 and 150 °C, only a slight change in the dRI signal was observed. However, a clear decrease in retention time was observed at 160 °C and above (Fig. 1c), and the height of the MALS signal decreased considerably (Fig. 1d), indicating degradation. Duplicate AF4-MALS-dRI measurements (for two samples microwaved under the same conditions) did not give reproducible results at temperatures above 160 °C (results not shown). This is believed to be due to non-uniform distribution of the microwave power in the heated material due to electromagnetic wave reflections and interference, resulting in the formation of standing waves, leading to distinct local overheating (hot spots) [31]. Furthermore, a slight variation (substantially smaller than at T > 160 °C) was sometimes observed at lower temperatures (results not shown). The microwave method was thus deemed to have poor repeatability and was not further investigated.
The dissolution of glycogen using water and DMSO as a dissolution solvent is shown in Fig. 1e, f. Minor differences can be observed between the two dissolution conditions. Dissolution in DMSO at 100 °C gives rise to a slightly shorter retention time than dissolution in water at room temperature (Fig. 1e). Experiments using dissolution in DMSO were also carried out in duplicate and showed reproducible results (results not shown).
The difference between different dissolution methods can be clearly seen from the plot of molar mass of glycogen measured at the apex of the dRI peak (M peak) (from Fig. 1) versus temperature, as shown in Fig. 2. No substantial differences were seen between the dissolution of glycogen in DMSO or water at temperatures up to 140 °C. However, a drastic decrease in M peak was seen at temperatures of 160 and 200 °C.
The mass recovery of glycogen from the separation channel was approximately 95 % in the temperature range 23–180 °C. When the temperature was increased to 200 °C, the mass recovery decreased to 66 and 91 % for the autoclaved and microwaved samples, respectively. The high recovery at temperatures between 150 and 180 °C, where degradation of the polymer occurs, suggests that the main mechanism of degradation is by chain scission into shorter chains or oligosaccharides sufficiently large to be retained in the separation channel. The lower recovery at 200 °C is probably the result of extensive degradation of the polymers causing molecules to be either insufficiently retained in the separation channel or lost through the accumulation wall. As no increase in signal could be observed at very short retention times in Fig. 1a, c, the analytes were probably lost through the accumulation wall.
Amylopectin dissolution yield
The dissolution yields, determined enzymatically, for the different dissolution methods after filtration or centrifugation are given in Table 1.
The aqueous starch suspensions heated in the autoclave and those dissolved in DMSO before centrifugation and before filtration all gave very close to 100 % initial starch content (data not shown), indicating that all the granular material remaining after dissolution was accessible to the enzyme. This also shows that there was no oxidation of the glucoside groups during dissolution, which could make glucosides resistant to enzymatic degradation.
The dissolution yield of amylopectin was ≥95 % at 140 °C, while below this temperature, the dissolution yield was low (62 % at 120 °C). Dissolution of amylopectin in DMSO (at 100 °C) showed a dissolution yield ≥90 %. These results show that very high dissolution yields of the starch granules were achieved by autoclaving aqueous solutions at temperatures ≥140 °C and by dissolution in DMSO at 100 °C.
AF4-MALS-dRI results of amylopectin
In order to investigate the effect of the dissolution methods on the size and molar mass of amylopectin, the amount of the amylopectin injected into the AF4 channel needs to be optimized to assure that overloading is eliminated or minimized and that the amylopectin is eluting in normal mode.
Effect of sample mass load
To find a sample mass load suitable for analysis, a series of separations were performed with different sample loads using the samples obtained by dissolution in water at 140 °C by autoclaving and by dissolution in DMSO at 100 °C. The results are shown in Fig. 3 (traced in red, gray, and black).
Effect of sample mass load on elution time and r rms distributions (lines and dots in red, gray, and black) of amylopectin dissolved a in water at 140 °C and b in DMSO at 100 °C. The effect of sample filtration on elution time and r rms distributions (lines and dots in blue) of amylopectin dissolved a in water at 140 °C and b in DMSO at 100 °C. Analyses were performed under aqueous conditions
The mass load of the samples heated to 140 °C was varied from 1.25 to 5 μg (Fig. 3a). It is evident from this figure that there is a considerable difference in the retention time between 1.25 and 5 μg, suggesting that overloading occurs at the higher mass load. The effect of the higher sample mass load on r rms can also be seen as a function of elution time in Fig. 3a, suggesting that size fractionation could be impaired at a mass load of 5 μg (although it should be noted that AF4 does not fractionate based on r rms). For amylopectin dissolved in DMSO, the sample mass load was varied from 1.25 to 2.5 μg (Fig. 3b). The results show that even a small difference in sample mass load (from 2 to 2.5 μg) leads to a difference in the retention time. Figure 3b also suggests that better size separation is obtained at a mass load of 1.25 μg than at 2 μg as the selectivity on r rms is somewhat higher.
These results indicate that the sample mass load for amylopectin must be very low (i.e., around 1 μg) to ensure good size separation. At higher mass loads, separation appears to be compromised as a result of overloading.
Effect of sample filtration
The r rms values presented in Fig. 3 were obtained according to the Berry method, using the same set of angles and first-order polynomial curve fitting to all eluted fractions. However, for early-eluted fractions in both the autoclaved (140 °C) sample (<7 min, Fig. 3a, shown in black) and the DMSO dissolved sample (<5 min, Fig. 3b, shown in black), the fits were rather poor and showed a strong curvature. Such curvature in light-scattering measurements has previously been interpreted as indicating the presence of aggregates, entangled chains, poorly dissolved polymers, or large impurities [32, 33]. The presence of very low amounts of large species that elute in steric/hyperlayer mode can cause noisy signals, and thus errors in light-scattering measurements. They will contribute substantially to the scattered light intensity, especially at low angles, where the scattering intensity is the highest, and therefore impair the interpretation of data [34]. In the case of AF4, the presence of large sample species may arise from parallel elution in normal mode and in steric/hyperlayer mode [24, 35]. These anomalous curvatures in the Berry plot for the early-eluted fractions could therefore be caused by co-elution of species with substantial differences in size. To investigate this, aqueous samples obtained by autoclaving at 140 °C and by dissolution in DMSO were filtered (0.45 μm) prior to injection into the AF4 separation channel. The resulting fractograms after filtration at a mass load of 1.25 μg are also shown in Fig. 3 (shown in blue), and the mass recovery as a result of filtration is shown in Table 2.
Filtration, as shown in Table 1, removed only ~5 % of the mass of the samples obtained either by autoclaving at 140 °C or dissolution in DMSO at 100 °C, which indicates that most of the sample had dissolved. The mass recovery from the separation channel (Table 2) was obtained using a mass load of 2.5 μg, since at the 1.25 μg sample load the signal-to-noise (S/N) ratio in the dRI detector was quite low, making accurate determination of the mass recovery difficult. The mass recovery for samples autoclaved at 140 °C was 100 % for both filtered and unfiltered samples. The filtered sample originally dissolved in DMSO exhibited 100 % recovery, while the unfiltered sample showed somewhat lower values (92 %). Both high dissolution yield (95 %) and high mass recovery (≥92 %) from the separation channel indicate that the AF4 analysis was quantitative.
Filtration had a considerable effect on the shape of the MALS peak for the sample autoclaved at 140 °C. Before filtration, the 140 °C sample showed an asymmetric peak (Fig. 3a, shown in black), while after filtration, the peak had a more symmetric form (Fig. 3a, shown in blue). Moreover, after filtration, the anomalous curvature in the Berry plot disappeared, and the calculated values of r rms between 3 and 7 min were much smaller (Fig. 3a, shown in blue). The late-eluted fractions (t r > 7 min) were practically unaffected by filtration, since no difference in r rms was observed between filtered and unfiltered samples (the differences in r rms values between filtered and unfiltered samples at 4 and 10 min are given in Table 2). This latter observation is an indication that filtration does not remove large components of the sample eluting in normal mode.
In the case of the samples obtained by dissolution in DMSO, no substantial differences are observed between the MALS fractograms of the filtered and unfiltered samples (Fig. 3b, blue vs. black lines). The difference in peak height may reflect differences in mass recovery (Table 2). Both the filtered and unfiltered samples showed similar r rms distribution (Fig. 3b, blue vs. black dots). At retention times <5 min, the unfiltered sample shows somewhat higher r rms values than the filtered (see also Table 2), as caused by the curvature of the Berry plot (as discussed previously).
The results of these experiments therefore suggest that small amounts of large sample components elute in steric/hyperlayer mode.
Effect of dissolution method on amylopectin retention
Amylopectin solutions were obtained by dissolution in water at 120, 140, 150, 160, and 170 °C under autoclaving and by dissolution in DMSO at 100 °C. The samples were filtered prior to injection into the AF4 system. The optimum sample mass load was determined (the results are included as additional data in the Electronic supplementary material, ESM). Injected masses of 5, 2.5, and 2 μg could be used for samples autoclaved at 170, 160, and 150 °C, respectively, without causing overloading. At the lower temperatures of 140, 120, and 100 °C, an injected mass of 1.25 μg was used as higher amounts caused overloading and lower amounts would cause insufficient dRI detection. The elution profiles, together with M and r rms at the different dissolution temperatures, are shown in Fig. 4a–f. For all temperatures, the M and r rms increased with elution time, indicating the separation took place in normal mode. The comparison of elution profiles at the different dissolution temperatures can be seen in Fig. 4g. Different retention times show characteristic differences in hydrodynamic size. Between dissolution in DMSO and dissolution by autoclaving at 140 °C, minor differences in amylopectin retention are observed; the latter shows a slightly lower retention time than the former. However, substantial differences are observed at temperatures above 140 °C; the MALS fractograms shift towards shorter retention times, reflecting a decrease in hydrodynamic radius and, thus, indicating degradation. The effect of temperature on M peak and r rms-peak (i.e., the maximum values of M and r rms at the apex of the MALS peak) is shown in Fig. 4h. The results resemble those obtained for glycogen (Fig. 2) and show that M peak and r rms-peak remain roughly constant between 100 and 140 °C, but decrease considerably above 140 °C. Table 3 shows values of peak retention time, r rms-peak, z-average r rms, weight-average M, and mass recovery of amylopectin at the different dissolution temperatures.
The effect of dissolution temperatures on the M and r rms of amylopectin. a–f show the MALS signal fractograms as well as the M and r rms vs. elution time for aqueous solutions of amylopectin obtained from dissolution at 170, 160, 150, 140, and 120 °C in an autoclave, and in DMSO at 100 °C, respectively. g The effect of the different dissolution conditions on the retention time. h M peak (filled symbols) and r rms-peak (open symbols) vs. temperature for the aqueous solutions after autoclaving (blue symbols) and after DMSO dissolution (green symbols). Analyses were performed under aqueous conditions
Sample recovery from the separation channel
The sample recovery was determined from the ratio of the mass eluted from the separation channel (integration of the dRI signal) to the injected mass (based on the starch content). The mass recovery of amylopectin was above 92 % in all cases (Tables 2 and 3), which indicates that AF4 analysis was quantitative. However, at a sample load of 1.25 μg, the S/N ratio in the dRI detector was rather low, making accurate determinations of the mass recovery and molar mass very difficult. Averaging of multiple injections has been suggested as a method of obtaining reliable characterization despite the low sample mass load [33].
Discussion
The dissolution of glycogen using different methods at various temperatures showed that aqueous solutions using autoclaving and microwave heating at temperatures between 150 and 200 °C led to degradation of glycogen. It has been reported that starch can be hydrolyzed when heated in water at temperatures between 180 and 240 °C (hydrolytic properties of water affect starch under hydrothermal conditions) [36, 37]; however, the results of the present study suggest that thermal hydrolysis of the glycosidic linkages also occurs at temperatures <180 °C. The degradation products of glycogen at temperatures between 150 and 180 °C were found to be sufficiently large to be retained in the separation channel (95 % mass recovery), while at 200 °C, 44 % was lost through the accumulation wall membrane of the separation channel due to their small size. This is consistent with previous findings on starch [36, 37], which reported that at 180 °C, the main degradation products of starch are short chains (without monosaccharides, disaccharides, or decomposition products such as 5-hydroxymethylfurfural and furfural), whereas at 200 °C, starch is mainly converted into relatively low molecular mass species (the major product was glucose with ~500 g glucose/kg starch).
The dissolution of glycogen in water at 140 °C under autoclaving and the dissolution in DMSO at 100 °C caused only a slight shift in retention compared with the dissolution in water at room temperature. The dissolution of amylopectin using these methods gave close to complete molecular dissolutions (≥95 %). When amylopectin sample solutions are AF4 analyzed, the very large, possibly undissolved species present in low amounts (~5 %) eluted first together with the very small species in a combined mechanism, i.e., normal and steric/hyperlayer mode occurring in parallel, impairing the interpretation of the data for the early-eluting fraction.
Filtration of amylopectin sample solutions prior to AF4 analysis effectively removed undissolved species, while not affecting those eluting later in normal mode. However, the filter must be chosen with great care as there is a risk that not only large undissolved particles but also part of the sample polymer will be removed if the pore size is too small. Also, filtration could have shear effects on analytes, and if the filter material is inappropriate, part of the sample could be adsorbed on the filter [34, 38]. It has been reported, using thermal FFF, that filtration of poly(vinyl acetate) samples changed the molar mass distribution dramatically (reducing it by an order of magnitude) and reduced the mass recovery [39]. Filtration of a β-glucan sample prior to injection into the AF4 separation channel has been reported to affect the conformation of large aggregates, but not the mass recovery [40].
Elution profiles at different dissolution temperatures as well as the effect of the temperature on the peak M and peak r rms were obtained as a means of comparison between dissolution methods/conditions for amylopectin. The temperature of dissolution affected the values of r rms and M, but the differences observed in r rms and M, between 100, 120, and 140 °C, were not as great as those at the higher temperatures of 160 and 170 °C. At 160 °C, the molar mass is almost one order of magnitude smaller than those obtained at temperatures below 140 °C. The value of r rms at 160 °C is also substantially reduced to less than half of the values obtained at temperatures below 140 °C. These results also suggest that the main degradation mechanism of amylopectin and glycogen at temperatures between 150 and 180 °C is chain scission, while depolymerization is likely observed at a dissolution temperature of 200 °C.
Conclusions
Glycogen was found to be a suitable model molecule for amylopectin. Glycogen showed no measurable degradation on dissolution in water at 140 °C using an autoclave or by dissolution in DMSO at 100 °C. Under these conditions, amylopectin molecules are almost completely dissolved. Dissolution using microwave heating (i.e., shorter heat treatment) showed poor reproducibility and comparable results to that of autoclaving. Thus, no benefit was found from using the shorter heat treatment times of the microwave and the dissolution method is, hence, deemed less suitable.
The study of different dissolution temperatures for amylopectin using AF4-MALS-dRI in combination with other analyses revealed that the dissolution in an aqueous environment at 140 °C and in DMSO at 100 °C yields similar results, and therefore, both methods are suitable for the dissolution of amylopectin. At temperatures above 140 °C, degradation of the molecules starts to occur. The results in this paper also clearly illustrate the upper limit for dissolution by heat treatment of starch in an aqueous environment.
References
Hizukuri S, Abe J, Hanashiro I (2006) In: Eliasson A-C (ed) Carbohydrate in food, 2nd edn. Taylor & Francis Group, Boca Raton, Florida
Gidley MJ, Hanashiro I, Hani NM, Hill SE, Huber A, Jane J-L, Liu Q, Morris GA, Rolland-Sabaté A, Striegel AM, Gilbert RG (2010) Reliable measurements of the size distributions of starch molecules in solution: current dilemmas and recommendations. Carbohydr Polym 79(2):255–261
Fernandez C, Rojas C, Nilsson L (2011) Size, structure and scaling relationships in glycogen from various sources investigated with asymmetrical flow field-flow fractionation and 1H NMR. Int J Biol Macromol 49(4):458–465
Nilsson GS, Gorton L, Bergquist K-E, Nilsson U (1996) Determination of the degree of branching in normal and amylopectin type potato starch with 1H-NMR spectroscopy improved resolution and two-dimensional spectroscopy. Starch-Stärke 48(10):352–357
Rojas C, Wahlund K-G, Bergenståhl B, Nilsson L (2008) Macromolecular geometries determined with field-flow fractionation and their impact on the overlap concentration. Biomacromolecules 9(6):1684–1690
Eliasson A-C, Gudmundsson M (2006) In: Eliasson A-C (ed) Carbohydrate in food, 2nd edn. Taylor & Francis Group, Boca Ratón-Florida
Nilsson L, Leeman M, Wahlund K-G, Bergenståhl B (2007) Competitive adsorption of a polydisperse polymer during emulsification: experiments and modeling. Langmuir 23(5):2346–2351
Schwartz D, Whistler R (2009) In: BeMiller J, Whistler R (eds) Starch, chemistry and technology, 3rd edn. Elsevier Inc., New York
Radosta S, Haberer M, Vorwerg W (2001) Molecular characteristics of amylose and starch in dimethyl sulfoxide. Biomacromolecules 2(3):970–978
Miles MJ, Morris VJ, Ring SG (1985) Gelation of amylose. Carbohydr Res 135(2):257–269
Aberle T, Burchard W, Vorwerg W, Radosta S (1994) Conformational contributions of amylose and amylopectin to the structural properties of starches from various sources. Starch-Stärke 46(9):329–335
Ring SG, Colonna P, I’Anson KJ, Kalichevsky MT, Miles MJ, Morris VJ, Orford PD (1987) The gelation and crystallisation of amylopectin. Carbohydr Res 162(2):277–293
Nilsson L (2013) Separation and characterization of food macromolecules using field-flow fractionation: a review. Food Hydrocoll 30(1):1–11
Han J-A, Lim S-T (2004) Structural changes of corn starches by heating and stirring in DMSO measured by SEC-MALLS-RI system. Carbohydr Polym 55(3):265–272
Han J-A, Lim S-T (2004) Structural changes in corn starches during alkaline dissolution by vortexing. Carbohydr Polym 55(2):193–199
Vorwerg W, Radosta S, Leibnitz E (2002) Study of a preparative-scale process for the production of amylose. Carbohydr Polym 47(2):181–189
Takeda Y, Hizukuri S, Juliano BO (1986) Purification and structure of amylose from rice starch. Carbohydr Res 148(2):299–308
Nilsson L (2012) In: Williams K, Caldwell K (eds) Field-flow fractionation in biopolymer analysis. Springer-Verlag/Wien, New York
Kim W, Eum CH, Lim S, Han J, You S, Lee S (2007) Separation of amylose and amylopectin in corn starch using dual-programmed flow field-flow fractionation. Bull Kor Chem Soc 28(12):2489
Messaud FA, Sanderson RD, Runyon JR, Otte T, Pasch H, Williams SKR (2009) An overview on field-flow fractionation techniques and their applications in the separation and characterization of polymers. Prog Polym Sci 34(4):351–368
Cave RA, Seabrook SA, Gidley MJ, Gilbert RG (2009) Characterization of starch by size-exclusion chromatography: the limitations imposed by shear scission. Biomacromolecules 10(8):2245–2253
You S, Stevenson SG, Izydorczyk MS, Preston KR (2002) Separation and characterization of barley starch polymers by a flow field-flow fractionation technique in combination with multiangle light scattering and differential refractive index detection. Cereal Chem 79(5):624–630
van Bruijnsvoort M, Wahlund K-G, Nilsson G, Kok WT (2001) Retention behaviour of amylopectins in asymmetrical flow field-flow fractionation studied by multi-angle light scattering detection. J Chromatogr A 925(1–2):171–182
Giddings JC (2000) In: Schimpf M, Caldwell K, Giddings JC (eds) Field-flow fractionation handbook. Wiley-Interscience, New York
Wittgren B, Wahlund K-G (1997) Fast molecular mass and size characterization of polysaccharides using asymmetrical flow field-flow fractionation-multiangle light scattering. J Chromatogr A 760(2):205–218
Wyatt PJ (1993) Light scattering and the absolute characterization of macromolecules. Anal Chim Acta 272(1):1–40
Leeman M, Wahlund K-G, Wittgren B (2006) Programmed cross flow asymmetrical flow field-flow fractionation for the size separation of pullulans and hydroxypropyl cellulose. J Chromatogr A 1134(1–2):236–245
Berry GC (1966) Thermodynamic and conformational properties of polystyrene. I. Light-scattering studies on dilute solutions of linear polystyrenes. J Chem Phys 44(12):4550–4564
Andersson M, Wittgren B, Wahlund K-G (2003) Accuracy in multiangle light scattering measurements for molar mass and radius estimations. Model calculations and experiments. Anal Chem 75(16):4279–4291
Brandrup J, Immergut EH, Grulke EA (eds) (1999) Polymer handbook, 4th edn. Wiley, New York
Cherbański R, Rudniak L (2013) Modelling of microwave heating of water in a monomode applicator—influence of operating conditions. Int J Therm Sci 74:214–229
Ring SG, L’Anson K, Morris VJ (1985) Static and dynamic light scattering studies of amylose solutions. Macromolecules 18(2):182–188
Leeman M, Islam MT, Haseltine WG (2007) Asymmetrical flow field-flow fractionation coupled with multi-angle light scattering and refractive index detections for characterization of ultra-high molar mass poly(acrylamide) flocculants. J Chromatogr A 1172(2):194–203
Andersson M, Wittgren B, Wahlund K-G (2001) Ultrahigh molar mass component detected in ethylhydroxyethyl cellulose by asymmetrical flow field-flow fractionation coupled to multiangle light scattering. Anal Chem 73(20):4852–4861
Caldwell K (2000) In: Schimpf M, Caldwell K, Giddings JC (eds) Field-flow fractionation handbook. Wiley-Interscience, New York
Nagamori M, Funazukuri T (2004) Glucose production by hydrolysis of starch under hydrothermal conditions. J Chem Technol Biotechnol 79(3):229–233
Orozco R, Redwood M, Leeke G, Bahari A, Santos R, Macaskie L (2012) Hydrothermal hydrolysis of starch with CO2 and detoxification of the hydrolysates with activated carbon for bio-hydrogen fermentation. Int J Hydrog Energy 37(8):6545–6553
Andersson M, Wittgren B, Schagerlöf H, Momcilovic D, Wahlund K-G (2004) Size and structure characterization of ethylhydroxyethyl cellulose by the combination of field-flow fractionation with other techniques. Investigation of ultralarge components. Biomacromolecules 5(1):97–105
Ratanathanawongs Williams SK, Lee D (2006) Field-flow fractionation of proteins, polysaccharides, synthetic polymers, and supramolecular assemblies. J Sep Sci 29(12):1720–1732
Ulmius M, Önning G, Nilsson L (2012) Solution behavior of barley β-glucan as studied with asymmetrical flow field-flow fractionation. Food Hydrocoll 26(1):175–180
Acknowledgments
Financial support from the Swedish International Development Agency (SIDA) is gratefully acknowledged. We are also grateful to Professor Karl-Gustav Wahlund for his valuable comments on the results.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in the topical collection Field- and Flow-based Separations with guest editors Gaetane Lespes, Catia Contado, and Bruce Gale.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 202 kb)
Rights and permissions
About this article
Cite this article
Perez-Rea, D., Bergenståhl, B. & Nilsson, L. Development and evaluation of methods for starch dissolution using asymmetrical flow field-flow fractionation. Part I: Dissolution of amylopectin. Anal Bioanal Chem 407, 4315–4326 (2015). https://doi.org/10.1007/s00216-015-8611-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-015-8611-8