Abstract
A simultaneous determination method for the enantiomers of chiral carboxylic acids by the combination of ultraperformance liquid chromatography and mass spectrometry (UPLC–MS/MS) has been developed. (S)(+)-1-(2-Pyrrolidinylmethyl)-pyrrolidine (S-PMP) was used as the derivatization reagent for the high-throughput determination of biological chiral carboxylic acids, i.e., lactic acid (LA) and 3-hydroxybutyric acid (HA). The S-PMP efficiently reacted with the carboxylic acids under mild conditions at room temperature in the presence of 2,2′-dipyridyl disulfide and triphenylphosphine. The resulting S-PMP derivatives were highly responsive in the electrospray ionization (ESI)-MS operating in the positive-ion mode and gave characteristic product ions during the MS/MS, which enabled the sensitive detection using selected reaction monitoring. The derivatization was effective for the enantiomeric separation of the chiral carboxylic acids, and the resolution values of dl-LA and dl-HA were 4.91 and 9.37, respectively. Furthermore, a rapid separation of the derivatives of dl-LA and dl-HA within 7 min was performed using the UPLC system. The limits of detection on the column were in the low femtogram range (5–12 fg). The proposed procedure was successfully applied for the determination of the d- and l-isomers of LA and HA in the saliva of diabetes mellitus (DM) patients and healthy volunteers. The d-LA in DM patients was clearly higher than that in normal subjects. The derivatization followed by UPLC–ESI-MS/MS enabled the enantiomeric separation and detection of trace amounts of LA and HA in human saliva with a simple pretreatment and small sample volume.

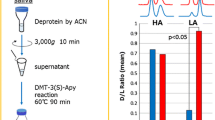
Concentration of HA and LA enantiomers in saliva of DM patients and healthy volunteers (n = 10). DM diabetes mellitus patients, C healthy volunteers
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mass spectrometry has become a popular analytical detection method because of its high sensitivity and specificity compared to other detection methods, such as ultraviolet–visible and fluorescence (FL). Various applications, e.g., impurity analysis and metabolite identification, are utilizing liquid chromatography–mass spectrometry (LC–MS) as one of their main techniques [1]. Besides, the MS/MS determination after enantiomeric separation by LC seems to be a powerful technique for the analysis of chiral carboxylic acids. However, the determination of intact carboxylic acids in biological samples is not always successful in spite of the highly sensitive LC–MS method because the negative ion [M–H]− is usually adopted for the detection. Furthermore, only a nonspecific and/or low-intensity product ion is often formed by collision-induced dissociation (CID) of the negatively charged ion. As a result, selected reaction monitoring (SRM), which is a highly specific technique available using a triple quadrupole mass spectrometer, may not be employed [2]. Furthermore, for the carboxylic acid analysis, the best chromatographic resolution with reversed-phase columns is generally achieved at an acidic pH, in which the ionization of the carboxyl groups is suppressed. Consequently, chemical derivatization suitable for the positive-ion detection is effective for enhancing the detectability of carboxylic acids in electrospray ionization (ESI)-MS/MS [3–9]. For the determination of chiral compounds, the combination of the separation of diastereomers derived from a chiral reagent by reversed-phase chromatography and the detection by MS/MS seems to be effective. However, the number of chiral reagents for the LC–MS/MS analysis is currently very limited [10, 11]. Based on these observations, we searched for chiral amines that are efficient for the separation and detection of chiral carboxylic acids by LC–MS/MS. In a previous study, several chiral amines, i.e., 1-(2-pyrrolidinylmethyl)-pyrrolidine (PMP), 1-aminoindan (AI), and 4-(3-aminopyrrolidin-1-yl)-7-(N,N-dimethylaminosulfonyl)-2,1,3-benzoxadiazole (DBD-APy), were found and used for the determination of nonsteroidal anti-inflammatory drugs (NSAIDs), i.e., ibuprofen, flurbiprofen, and loxoprofen, spiked in rat plasma [12]. A good separation and detection of the resulting diastereomers was achieved by the proposed method using the chiral reagents and LC–MS/MS.
Diabetes mellitus (DM) is one of the diseases of interest due to its increasing worldwide prevalence not only in European–American but also in Asian countries [13, 14]. The DM in humans is a multifactorial disorder based on environmental factors and genetic background. In many cases, DM is asymptomatic for a long period, and the patient is not aware of the disease [15, 16].
Lactic acid (LA) and 3-hydroxybutyric acid (HA), which are biologically active carboxylic acids, exist in living organisms. LA in mammals is mainly composed of the l-enantiomer which is produced from pyruvic acid under anaerobic conditions. However, its optical isomer, d-LA, also exists in mammals even though the amount is relatively low compared to l-LA. The endogenous d-LA is produced from methyglyoxal mediated by glyoxalases I and II in the metabolic pathway of glucose [17, 18]. The d-LA concentration is significantly increased in the serum of diabetic animals [19, 20] and patients [21]. This has been considered to be due to the accelerated production of its precursor, methyglyoxal, which causes the patient to develop diabetic complications during the diabetic stage [22–26].
On the other hand, HA, one of the ketone bodies, has been a useful marker for diabetic acidosis in clinical diagnosis because the increase in HA is known to be caused by the enhanced β-oxidation of fatty acid in diabetics. However, the ratio of d- and l-HA enantiomers in DM patients has not been previously reported. Based on these results, we plan the determination of both enantiomers of LA and HA by LC–MS/MS. The strategy for the determination is based upon the diastereomer formations with chiral amines (PMP, DBD-APy, and AI) which are selected as the derivatization reagents for the chiral carboxylic acid, as previously described [12]. The proposed method is also applied to the determination of both enantiomers of LA and HA and their d/l ratios in the saliva of DM patients and healthy volunteers.
Experimental
Materials and chemicals
(S)(+)-1-(2-Pyrrolidinylmethyl)-pyrrolidine (S-PMP), (S)(−)- and (R)(+)-4-(3-aminopyrrolidin-1-yl)-7-(N,N-dimethylaminosulfonyl)-2,1,3-benzoxadiazoles, (S)(+)- and (R)(−)-1-aminoindans, triphenylphosphine (TPP), and 2,2′-dipyridyl disulfide (DPDS) were obtained from Tokyo Kasei (Tokyo, Japan) (Fig. 1). The d- and l-lactic acids (dl-LA; Sigma-Aldrich; St. Louis, MO) and d- and l-3-hydroxybutyric acids (dl-HA; Kanto Chemicals; Tokyo, Japan) were used. The sodium salts of dl-lactic acid-3,3,3-d 3 (dl-LA-d 3) and dl-3-hydroxybutyric acid-3,4,4,4-d 4 (dl-HA-d 4; C/D/N Isotopes; Quebec, Canada) were used as the internal standard (I.S.) (Fig. 1). Acetonitrile, methanol (MeOH), and formic acid (HCOOH) of LC–MS grade were purchased from Kanto Chemicals. Deionized and distilled water (H2O) was used throughout the study (Aquarius PWU-200 automatic water distillation apparatus, Advantec, Tokyo, Japan). All other reagents and solvents were of analytical grade.
A 10-μmol/mL solution of the carboxylic acids (d-, l-, dl-enantiomers of LA or HA) in acetonitrile was prepared as the stock solution. The deuterium-labeled I.S. solutions (dl-LA-d 3 and dl-HA-d 4) were also prepared at the same concentration. The working solutions were prepared by sequential dilutions with acetonitrile.
UPLC–ESI-MS/MS analysis
The UPLC–ESI-MS/MS analysis was performed using a Xevo™ TQ-S triple quadrupole-mass spectrometer (Waters, Milford, MA) connected to an ACQUITY ultraperformance liquid chromatograph (UPLC I-class, Waters). An ACQUITY UPLC BEH C18 column (1.7 μm, 100 × 2.1 mm i.d.; Waters) was used at the flow rate of 0.35 mL/min and 40 °C. The diastereomers of dl-LA and dl-HA, derivatized from a chiral amine, were analyzed by UPLC–ESI-MS/MS in the positive-ion mode unless otherwise stated. The separation and detection conditions were as follows: Mobile phase, methanol–acetonitrile–water mixture containing 0.1 % (v/v) HCOOH; capillary voltage, 3.00 kV; cone voltage, 50 V; desolvation gas flow, 1,000 L/h; cone gas flow, 150 L/h; nebuliser gas flow, 7.0 L/h; collision gas flow, 0.15 mL/min; collision energy, 20–25 eV; collision cell exit potential, 5 V; desolvation temp, 500 °C. Analytical software (MassLynx, version 4.1) was used for the system control and data processing.
Recommended reaction conditions of LA and HA with chiral amines
The freshly prepared solutions of TPP (10 mM) in acetonitrile (100 μL), DPDS (10 mM) in acetonitrile (100 μL), and a chiral amine (1.0 mM) in acetonitrile (100 μL) were vigorously mixed with the carboxylic acid samples containing I.S. (LA, 10 pmol and HA, 1 pmol) in acetonitrile (100 μL). The reaction mixture was stored for 90 min at room temperature. After removal of the solvent, the residues were dissolved in the mobile phase, and then an aliquot (2 μL) was subjected to UPLC–ESI-MS/MS.
Separation and detection of the resulting diastereomers
The separation and detection efficiency of chiral amines as the derivatization reagents for LA and HA was evaluated by the capacity ratio (k’), separation factor (α), resolution value (Rs) and limit of detection (LOD). The LOD (S/N = 3) was calculated from the comparison of the peak area ratios of the injected amounts of the carboxylic acids and the baseline noise.
Analytical validation
Calibration curve
The fixed concentrations of both enantiomers of LA (2.5–250 ng/mL) and HA (0.5–50 ng/mL) were prepared by sequential dilutions of the stock solutions. The working solutions (100 μL) were pretreated and derivatized as described in the “Recommended reaction conditions of LA and HA with chiral amines” section. Each 2 μL of the derivatization solutions was then subjected to the UPLC–ESI-MS/MS system. The calibration curves were constructed by plotting the peak area ratio of S-PMP-LA or -HA to I.S. (y) versus the concentration of LA or HA (x, nanograms per milliliter using linear regression 1/x weighting (n = 5).
Intra-day and inter-day assays
The precisions of intra- and inter-day assays were determined by the repeated measurement (n = 5) of the saliva obtained from two volunteers on 1 day and over 5 days, respectively. The precision was evaluated as the relative standard deviation (RSD, percentage).
Recovery test
The amounts of the l- and d-enantiomers of LA (450 and 900 pg) and HA (104 and 208 pg) in acetonitrile (10 μL) were spiked in 90 μL of a saliva sample from the volunteers (n = 5). The spiked- and unspiked samples were then pretreated and subjected to UPLC–ESI-MS/MS. The concentrations of the d- and l-enantiomers of LA and HA were determined from each calibration curve. The recovery percentage was defined as F/(F 0 + A) × 100 (%), where F is the concentration of LA or HA in the spiked sample, F 0 is the concentration of the LA or HA in the unspiked sample, and A is the spiked concentration.
Pretreatment for LA and HA analyses in saliva
Saliva (ca. 1 mL) was directly collected into a collecting tube (without a collection device) and stored at −80 °C until used. The DM patients (sex, five males and five females; age, 44–69; n = 10) and healthy volunteers (sex, six males and four females; age, 42–61; n = 10) ingested no food and beverage within at least 1 h prior to the sample collection. They also did not brush their teeth within 1 h prior to sample collection to avoid blood contamination. They understood the purpose and significance of this experiment and donated saliva after agreement.
After thawing, the saliva sample was centrifuged at 3,000×g for 10 min to precipitate the denatured mucins. To the supernatant (0.1 mL), 0.3 mL of acetonitrile containing I.S. [dl-LA-d 3, 930 pg (10 pmol) and dl-HA-d 4, 108 pg (1 pmol)] was immediately added, vigorously mixed for 30 s and centrifuged again at 3,000×g for 5 min. The supernatant was deproteinized using the Sirocco Protein Precipitation Plate (Waters, Milford, MA). After connecting the filter plate to a vacuum manifold and maintaining at 10 mmHg for 15–20 min, the collected filtrate (0.35 mL) was dried using a Personal Evaporator (EZ-2, Genevac Co., New York, USA). The residues were dissolved in 0.1 mL of acetonitrile and reacted with 0.1 mL of a chiral amine (1 mM) in the presence of TPP and DPDS, according to the procedures described in the “Recommended reaction conditions of LA and HA with chiral amines” section. After removal of the solvent, the residues were redissolved in 0.4 mL of the initial mobile phase and then a 2-μL aliquot was subjected to the UPLC–ESI-MS/MS. The separation and detection conditions are the same as those listed in Table 1.
Result and discussion
The determination of d-LA in blood has typically been performed by utilizing d-lactic acid dehydrogenase (d-LDH), which transforms d-LA into pyruvic acid. In the enzymatic assay using d-LDH, the concentration of d-LA is estimated as the increase in absorption [27–30], fluorescence [31], or amperometric potential [32] of NADH formed from NAD+, which is a co-factor in the enzymatic reaction using d-LDH. Although the enzymatic assay provides a rapid determination, l-LDH is also necessary for determining l-LA. Therefore, the simultaneous determination of d-LA and l-LA is difficult using the enzymatic assay. As the separation methods without using the enzymes, chiral ligand-exchange chromatography, a chiral mobile phase additive, and a chiral stationary phase (CSP) column were reported by Oi et al. [33], Olieman et al. [34], and Norton et al. [35], respectively. Although the enantiomeric separation is possible by these methods, the detection sensitivity is insufficient for the determination of small quantities due to the use of the refractive index and UV absorption in the short wavelength range. To overcome this disadvantage, Imai’s group [36–38] developed a column-switching HPLC method using a CSP separation following pre-column fluorescence derivatization using 4-(N,N-dimethylaminosulfonyl)-7-piperazino-2,1,3-benzoxadiazole [36] and 4-nitro-7-piperazino-2,1,3-benzoxadiazole [37, 38]. The highly sensitive determination of d- and l-LA in the serum was carried out by the HPLC–FL methods using derivatization and the CSP column. However, the operating procedure is complicated, and some interference possibly occurs during the determination of a trace amount in complex matrices. Besides, the determination in saliva may be difficult due to lack of a detection sensitivity. On the other hand, the enantiomeric separation of dl-HA in biological specimens has not been reported. Based on these observations, we developed a separation and detection method for the dl-LA and dl-HA enantiomers by LC–MS/MS. The proposed method was also used for the determination in the saliva from DM patients and healthy volunteers.
Derivatization of LA and HA with chiral amines
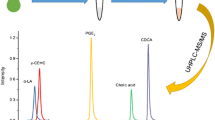
Carboxylic acids are usually labeled with a chiral amine in the presence of activation reagents, such as 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide. The condensation reaction proceeds under mild conditions, such as room temperature. In our previous study, the combination of DPDS and TPP was used for the condensation of NSAIDs with chiral amines, such as PMP and AI (Fig. 1) [12]. The chiral amines reacted with the NSAIDs to produce the corresponding amide-type diastereomers in the presence of the activation reagents [39]. In the present study, the same activation reagents (DPDS and TPP) and solvent (acetonitrile) were employed for the derivatization reactions of dl-LA and dl-HA with chiral amines. The reaction time of the derivatization with S-PMP was first optimized with LA and HA. As shown in Fig. 2, the formation of the derivatives gradually increased with the increase in the reaction times. The peak areas of the derivatives reached a plateau after a 90-min reaction and were constant over a 180-min period. Furthermore, the time courses of the derivatization reactions are very similar for both enantiomers. The results showed that no difference in the reactivity was observed in both enantiomers. The time courses obtained in this study agreed with those in a previous report [12]. Therefore, the 90-min reaction at room temperature in acetonitrile was selected for the derivatization of the LA and HA enantiomers with the chiral amines in the presence of DPDS and TPP. When the resulting derivatives were dissolved in the respective mobile phases, they were stable at room temperature for at least 1 day and at 4 °C for at least 5 days.
UPLC–ESI-MS/MS analysis of dl-LA and dl-HA
The separation of both enantiomers of LA and HA after derivatization with the chiral amines, i.e., PMP, DBD-APy, and AI, was attempted by reversed-phase chromatography using the isocratic elution of a water–methanol–acetonitrile mixture containing 0.1 % HCOOH. In this study, an ODS column packed with small particle silica gel (1.7μm) was used for the high-throughput separation. As shown in Table 1, the diastereomers of LA and HA derived from S-PMP were well separated under an isocratic elution condition. A good separation of HA derivatives was also observed using S-AI (Table 1). However, the separations of the diasteromers, produced from DBD-APy, were insufficient (Table 1). Therefore, the chiral amines, S-PMP and S-AI, were adopted for the following experiments.
The mass spectral features of the S-PMP derivatives, obtained from tandem triple quadrupole MS, are shown in Fig. 3. The mass spectra patterns of the dl-LA-d 3 and dl-HA-d 4 were almost the same as those of dl-LA and dl-HA, except for 3 and 4 m/z differences (Fig. 3A). The specific and characteristic cleavages were also observed from the CID of the protonated molecule, [M + H]+ (Fig. 3B). The MS transitions (precursor and productions) and the limits of detection obtained from the SRM chromatograms are shown in Table 1. The SRM transition for S-PMP-LA and S-PMP-HA was m/z 227.2 → 156.0 and 241.1 → 170.1, respectively. The typical SRM chromatograms are shown in Fig. 4A. Each pair of diastereomers derived from S-PMP was clearly separated within 7 min by the reversed-phase chromatography (dl-LA, Rs = 4.91; dl-HA, Rs = 9.37). The peaks corresponding to dl-LA-d 3 and dl-HA-d 4 also appeared at the same elution positions (Fig. 4A). Thus, a high-throughput separation was obtained by UPLC using a small particle silica gel column. Furthermore, a highly sensitive detection at 5–12 fmoles was obtained from the SRM chromatograms of the S-PMP derivatives (Table 1). The peaks of dl-HA derived from S-AI were also successfully separated by the reversed-phase chromatography (Rs = 2.64). Although the separation efficiency was different in dl-LA and dl-HA, the S-PMP and S-AI reagents seem to be applicable for the enantiomeric separation of LA and HA in saliva.
Mass spectral features of the derivatives obtained from S-PMP. A Mass spectra; B MS/MS spectra. 1 S-PMP-LA, 2 S-PMP-LA-d 3, 3 S-PMP-HA, 4 S-PMP-HA-d 4. The MS/MS spectra were recorded by the collisional activation of [M+H]+ of the respective derivatives. The other conditions are described in the “Experimental” section and Table 1
SRM chromatograms of the diastereomers obtained from authentic carboxylic acids and saliva of a healthy volunteer. A Authentic carboxylic acids (injection amount, 25 fmol each); B Saliva. The UPLC–ESI-MS/MS conditions are described in the “Experimental” section and Table 1
Analysis of dl-LA and dl-HA in saliva
Saliva has recently been attracting attention as a new biological specimen in clinical examinations and therapeutic drug monitoring [40, 41] because saliva offers easy, noninvasive, stress-free, and real-time repeated sampling when blood collection is either undesirable or difficult. However, a major disadvantage of using saliva is the low analyte concentration. Consequently, a highly sensitive and selective detection technique is required for the utilization.
In general, the determinations of LA and HA in DM patients are carried out using blood and urine. However, their use as a diagnosis sample is not suitable as previously mentioned. Hygienic practice during collection and handling is another consideration. In contrast, saliva is relatively clean and the samples can be quickly and noninvasively collected and easily stored. Furthermore, the simultaneous determinations of both enantiomers of LA and HA have not yet been performed in biological specimens. Thus, their separation assay in saliva was performed in DM patients and healthy volunteers.
One hundred microliters of saliva collected from the DM patients and healthy volunteers was pretreated as described in the “Pretreatment for LA and HA analyses in saliva” section. Figure 4A shows the SRM chromatograms of authentic LA and HA derived from S-PMP. The typical SRM chromatograms obtained from the saliva of a normal subject are also shown in Fig. 4B. The peak corresponding to both enantiomers of LA and HA was clearly separated at 1.93 (l-LA), 2.71 (d-LA), 3.81 (l-HA), and 6.20 min (d-HA).
Validation
The calibration curves of the d- and l-isomers of LA and HA after derivatization with S-PMP were first studied. As shown in Table 2, a good linearity was obtained from the concentrations of the carboxylic acids versus the ratios of intensity of the carboxylic acid to deuterium-labeled I.S. The RSD values of the repeated assays of LA and HA in saliva were less than 9.89 % for the intra-day and 9.27 % for the inter-day analyses [Electronic supplementary material (ESM) Table S1]. Besides, the recovery values of LA and HA spiked in the saliva samples were more than 97.7 % (ESM Table S2). The RSD values of intra-day and inter-day analyses of HA obtained from S-AI reagent were less than 8.28 and 8.82, respectively. The recovery values of HA using S-AI were more than 97.4 % (detailed data not shown). These values are an acceptable range in bioanalysis. Based on these results, the proposed method is not only accurate but also precise. Therefore, the present method seems to be applicable for the determination of dl-LA and dl-HA in saliva.
Concentration of LA and HA enantiomers in saliva of DM patients and healthy volunteers
Ten saliva samples of DM patients and healthy volunteers were each analyzed by the proposed procedure. Figure 5 shows the d- and l-HA concentrations and the d/l ratios obtained from S-PMP and S-AI reagents. The average concentrations of l-HA and d-HA obtained from S-PMP reagent were calculated as 14.3 and 10.2 ng/mL in the DM patients, and 9.72 and 6.71 ng/mL in the normal subjects, respectively (Table 3). The amounts of both enantiomers were slightly higher in the DM patients than in the normal subjects, but the d/l ratio was almost comparable in both samples (74.3 versus 81.5). Almost the same results were also observed from the use of the S-AI reagent (Table 3 and Fig. 5B). Therefore, the determinations of d-LA and l-LA were carried out using only the S-PMP reagent. These results are shown in Fig. 6 and Table 3. No large difference of the l-LA concentration was observed between the DM patients (Av. 64.7 ng/mL) and normal subjects (Av. 52.4 ng/mL). On the contrary, the concentration of d-LA was obviously higher in the DM patients (41.6 versus 6.02 ng/mL). The increase in the total LA (dl-LA) in the serum of diabetic patients has been reported by Avogaro et al. [42, 43]. Furthermore, Imai et al. [38] compared the d/l ratios in serum of diabetic patients and normal subjects. Although the ratio of the d-enantiomer in the diabetic patients was slightly higher than that of the normal subjects, no significant difference was observed in the serum analysis. In our study, a similar higher concentration of LA in the DM patients is obtained from the saliva analysis. Besides, the d/l ratio in the DM patients was significantly higher than that in the normal subjects, which is different from the results in the serum [38]. It is currently not obvious that this large difference is due to the difference in the sample type (serum versus saliva). Thus, a detailed study is planned to verify the results.
Conclusion
We developed a simple and practical derivatization procedure for the enhanced detection and enantiomeric separation of LA and HA using LC–ESI-MS/MS. The detectability was increased with the derivatization by three to four orders of magnitude over the intact carboxylic acids [35]. Furthermore, the retention efficiency of LA and HA, which are not only water soluble but also hydrophilic carboxylic acids, increased with the derivatization. As a result, the diastereomers derived from S-PMP were satisfactorily separated by reversed-phase chromatography (Rs = 4.91 in dl-LA and Rs = 9.37 in dl-HA). The Rs values were obviously larger than those in the use of CSP column [38]. Furthermore, a high-throughput separation within 7 min was carried out by the use of a UPLC and an anti-pressurized column packed with 1.7-μm particle ODS silica gel.
The enantioselective and trace analyses of LA and HA in saliva of DM patients were achieved by derivatization followed by UPLC–ESI-MS/MS. This is the first reported instance for the simultaneous and enantioselective analysis of LA and HA in the saliva of DM patients and healthy volunteers. The present method is highly sensitive and has enough specificity and practicality for biological sample analysis. Consequently, the proposed procedure is useful for the noninvasive salivary diagnosis of DM patients. The proposed method seems to be applicable for the enantioselective determination of LA and HA in various biological specimens. Further study of the chiral analysis of LA and HA is currently underway in our laboratory.
References
Toyo’oka T (2008) J Chromatogr Sci 46:233–247
Gagné S, Crane S, Huang Z, Li CS, Bateman KP, Lévesque JF (2007) J Lipid Res 48:252–259
Tsukamoto Y, Santa T, Saimaru H, Imai K, Funatsu T (2005) Biomed Chromatogr 19:802–808
Pettinella C, Lee SH, Cipollone F, Blair IA (2007) J Chromatogr B 850:168–176
Kretschmer A, Giera M, Wijtmans M, de Vries L, Lingeman H, Irth H, Niessen WMA (2011) J Chromatogr B 879:1393–1401
Guo K, Li L (2010) Anal Chem 82:8789–8793
Bollinger JG, Thompson W, Lai Y, Oslund RC, Hallstrand TS, Sadilek M, Turecek F, Gelb MH (2010) Anal Chem 82:6790–6796
Higashi T, Ichikawa T, Inagaki S, Min JZ, Fukushima T, Toyo’oka T (2010) J Pharm Biomed Anal 52:809–818
Li X, Franke AA (2011) Anal Chem 83:3192–3198
Nozawa Y, Sakai N, Arai K, Kawasaki Y, Harada K (2007) J Microbiol Methods 70:306–311
Fujii K, Ikai Y, Mayumi T, Oka H, Suzuki M, Harada K (1997) Anal Chem 69:3346–3352
Tsutsui H, Fujii S, Sakamoto T, Min JZ, Todoroki K, Toyo’oka T (2012) J Sep Sci 35:1551–1559
Zimmet P, Alberti KGMM, Shaw J (2001) Nature 414:782–787
Yoon KH, Lee JH, Kim JW, Cho JH, Choi YH, Ko SH (2006) Lancet 368:1681–1688
Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA (2002) N Engl J Med 346:393–403
Tuomilehto J, Lindstrom J, Eriksson JG, Valie TT, Hamalainen H, Ilanne-Parikka P (2001) N Engl J Med 344:1343–50
Racker E (1951) J Biol Chem 190:685–696
Thornalley PJ (1990) Biochem J 269:1–11
Kondoh Y, Kawase M, Kawamaki Y, Ohmori S (1992) Res Exp Med 192:407–414
Christopher MM, Broussard JD, Fallin CW, Drost NJ, Peterson ME (1995) Metab Clin Exp 44:287–290
McLellan AC, Plillips SA, Thornalley PJ (1992) Anal Biochem 206:12–16
Thornalley PJ, Hooper NI, Jennings PE, Florkowski CM, Jones AF, Lunec J, Barnett AH (1989) Diabetes Res Clin Prac 7:115–120
McLellan AC, Plillips SA, Thornalley PJ (1992) Anal Biochem 206:17–23
Thornalley PJ (1994) Amino Acids 6:15–23
McLellan AC, Thornalley PJ, Benn J, Sonksen PH (1994) Clin Sci 87:21–29
Thornalley PJ, McLellan AC, Lo TWC, Benn J, Sonksen PH (1996) Clin Sci 91:575–582
Haschke-Becher E, Baumgartner M, Bachmann C (2000) Clin Chim Acta 298:99–109
Wellmer A, Prange J, Gerber J, Zysk G, Lange P, Michel U, Eiffert H, Nau R (2001) Scand J Infect Dis 33:909–913
Marti R, Varela E, Segura RM, Alegre J, Surinach JM, Pascual C (1997) Clin Chem 43:1010–1015
Gutheil WG (1998) Anal Biochem 259:62–67
McLellan AC, Plillips SA, Thornalley PJ (1992) Anal Biochem 206:12–16
Avramescu A, Noguer T, Magearu V, Marty JL (2001) Anal Chim Acta 433:81–88
Oi N, Kitahara H, Aoki F (1994) J Chromatogr A 666:457–462
Olieman C, DeVries ES (1988) Neth Milk Dairy J 42:111–120
Norton D, Crow B, Bishop M, Kovalcik K, George J, Bralley JA (2007) J Chromatogr B 850:190–198
Fukushima T, Adachi S, Ichihara H, Al-Kindy S, Imai K (1999) Biomed Chromatogr 13:418–424
Fukushima T, Lee J-A, Korenaga T, Ichihara H, Kato M, Imai K (2001) Biomed Chromatogr 15:189–195
Hasegawa H, Fukushima T, Lee J-A, Tsukamoto K, Moriya K, Ono Y, Imai K (2003) Anal Bioanal Chem 377:886–891
Toyo’oka T, Ishibashi M, Takeda Y, Nakashima K, Imai K (1991) J Chromatogr 588:61–71
Chiappin S, Antonelli G, Gatti R, De Palo EF (2007) Clin Chim Acta 383:30–40
Gröschl M (2008) Clin Chem 54:1759–1769
Avogaro A, Toffolo G, Miola M, Valerio A, Cobelli C, Del Prato S (1996) J Clin Invest 98:108–115
Avogaro A, Crepalgi C, Miola M, Maran A, Pengo V, Tiengo A, Del Prato S (1996) J Endocrinol Invest 19:99–105
Acknowledgments
This study was supported in part by a Grant-in-Aid for Scientific Research (C) from the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 100 kb)
Rights and permissions
About this article
Cite this article
Tsutsui, H., Mochizuki, T., Maeda, T. et al. Simultaneous determination of dl-lactic acid and dl-3-hydroxybutyric acid enantiomers in saliva of diabetes mellitus patients by high-throughput LC–ESI-MS/MS. Anal Bioanal Chem 404, 1925–1934 (2012). https://doi.org/10.1007/s00216-012-6320-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-012-6320-0