Abstract
Rationale
The effectiveness of cannabidiolic acid (CBDA) was compared with other potential treatments for anticipatory nausea (AN), using a rat model of contextually elicited conditioned gaping reactions.
Objective
The potential of ondansetron (OND), Δ9-tetrahydrocannabinol (THC), chlordiazepoxide (CDP), CBDA, and co-administration of CBDA and tetrahydrocannabinolic acid (THCA) to reduce AN and modify locomotor activity was evaluated.
Materials and methods
Following four pairings of a novel context with lithium chloride (LiCl), the rats were given a test for AN. On the test trial, they received pretreatment injections of the following: vehicle, OND (0.1 or 1.0 mg/kg), THC (0.5 mg/kg), CBDA (0.0001, 0.001, 0.01, 0.1 mg/kg or 1.0 mg/kg), CDP (1, 5, or 10 mg/kg) or co-administration of subthreshold doses of CBDA (0.1 μg/kg), and THCA (5 μg/kg). Immediately following the AN test trial in all experiments, rats were given a 15 min locomotor activity test. Finally, the potential of CBDA (0.001, 0.01, 0.1, and 1 mg/kg) to attenuate conditioned freezing to a shock-paired tone was assessed.
Results
THC, CBDA, and CDP, but not OND, reduced contextually elicited gaping reactions. Co-administration of subthreshold doses of CBDA and THCA also suppressed AN, and this effect was blocked by pretreatment with either a cannabinoid receptor 1 (CB1) receptor antagonist or a 5-hydroxytryptamine 1A (5-HT1A) receptor antagonist. CDP (but not CBDA, THC or CBDA and THCA) also suppressed locomotor activity at effective doses. CBDA did not modify the expression of conditioned fear.
Conclusions
CBDA has therapeutic potential as a highly potent and selective treatment for AN without psychoactive or locomotor effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
When untreated, chemotherapy-induced nausea and vomiting can be characterized by three distinct episodes: (1) acute nausea and vomiting, occurring immediately following the session, (2) delayed nausea and vomiting, beginning approximately 24 h following the session, and (3) anticipatory nausea (AN) and vomiting, occurring upon reexposure to contextual stimuli previously associated with the acute nausea and vomiting (a classically conditioned response). A major advance in the control of acute nausea and vomiting was the discovery that blockade of one subtype of the 5-hydroxytryptamine (5-HT) receptor, the 5-HT3 receptor, could suppress the acute emetic response (retching and vomiting) induced by cisplatin in the ferret and the shrew (Rudd and Naylor 1994, 1996; Sam et al. 2003; Kwiatkowska et al. 2004; Lau et al. 2005). Indeed, ondansetron (OND, a 5-HT3 receptor antagonist) has proven to be highly effective in reducing acute nausea and vomiting in chemotherapy patients (Morrow et al. 1995). OND, however, is less effective in reducing delayed nausea and vomiting and is completely ineffective in reducing instances of AN (Morrow et al. 1995, 1998; Hsu 2010) once they develop. Therefore, an effective and selective treatment for AN is necessary.
AN continues to be a challenge for available pharmacotherapies. The search for an effective treatment for AN has been limited by the lack of selective rodent preclinical models of nausea, because rats and mice do not vomit in response to toxins. Rats, however, display the orofacial reaction of conditioned gaping (rapid, large amplitude openings of the mouth with simultaneous retraction of the corners of the mouth exposing excisors) when reexposed to a flavor using the taste reactivity procedure (Grill and Norgren 1978) or a context (Limebeer et al. 2008) that has previously been paired with an emetic treatment, such as lithium chloride (LiCl). Conditioned gaping responses elicited by a LiCl-paired context is remarkably similar to the phenomenon of AN in humans. Interestingly, shrews, which vomit in response to an emetic treatment, also display a conditioned retching reaction when reintroduced to the context in which they previously vomited (Parker and Kemp 2001; Parker et al. 2006). With the development of animal models of AN, it is possible to evaluate the potential of drug treatments to reduce this side effect of chemotherapy treatment.
In the development of models of AN, Limebeer et al. (2006) initially reported that when rats were repeatedly intraorally infused (6 1-min infusions separated by 5 min) with a novel flavor in a context previously paired with LiCl, they not only gaped during the flavor infusions but also during the inter-infusion intervals. The gaping reactions (during the flavor and inter-infusion interval) were reduced by pretreatment with a low dose (0.5 mg/kg) of Δ9-tetrahydrocannabinol (THC), but not with OND (0.1 mg/kg). Similarly, THC, but not OND, also reduces contextually elicited conditioned retching in shrews (Parker and Kemp 2001; Parker et al. 2006). OND is also ineffective in treating AN in human chemotherapy patients (e.g., Morrow et al. 1995).
Since Limebeer et al. (2006) demonstrated that contextually elicited gaping reactions were evident even during intervals between flavor exposure, they investigated the potential of LiCl-paired contexts in the absence of a flavor exposure to elicit gaping reactions on their own. Indeed, Limebeer et al. (2008) reported such an effect. Subsequent studies using the contextually elicited conditioned gaping model have found that the non-psychoactive cannabinoid cannabidiol (CBD) found in the marihuana plant also suppresses contextually elicited conditioned gaping in rats (Rock et al. 2008) and conditioned retching in shrews (Parker and Kemp 2001). As well, the fatty acid amide hydrolase (FAAH) inhibitor (URB597) which elevates endogenous anandamide (Fegley et al. 2005) dose-dependently suppresses contextually elicited (as well as flavor elicited, Cross-Mellor et al. 2007) conditioned gaping in rats (Rock et al. 2008) and conditioned retching in shrews (Parker et al. 2006). Most recently, the dual FAAH/monoacylglycerol lipase inhibitor, JZL195, which elevates both anandamide and 2-arachidonoylglycerol was found to effectively reduce contextually elicited conditioned gaping (Limebeer et al. 2014). The suppression of AN by both URB597 and by JZL195 were reversed by the cannabinoid 1 (CB1) receptor antagonist/inverse agonist, SR141716 (SR), which on its own did not modify the strength of the contextually elicited gaping reactions (Limebeer et al. 2014; Rock et al. 2008).
The acidic precursors to CBD and THC, cannabidiolic acid (CBDA, Mechoulam and Gaoni 1965) and tetrahydrocannabinolic acid (THCA, Gaoni and Mechoulam 1964) respectively, are converted to CBD or THC upon application of heat (Potter et al. 2008). CBDA and THCA are more potent than CBD and THC, respectively, in suppressing both acute nausea (conditioned gaping to a LiCl-paired flavor) and AN (contextually elicited conditioned gaping) in rats and vomiting in shrews, suggesting that these compounds may be an effective treatment for both phases of chemotherapy-induced nausea and vomiting (Bolognini et al. 2013; Rock et al. 2013). Both CBD and CBDA act as indirect 5-HT1A receptor agonists in vitro and in vivo to reduce acute nausea (conditioned gaping to a LiCl-paired flavor) and AN by 5-HT1A receptor agonism (Rock et al. 2012; Bolognini et al. 2013). Furthermore, administration of WAY100635 (WAY, a selective 5-HT1A receptor antagonist) blocks CBD’s suppressive effect on conditioned gaping to a LiCl-paired flavor (Rock et al. 2012) and CBDA’s suppressive effect on conditioned gaping to a LiCl-paired flavor and contextually elicited conditioned gaping (Bolognini et al. 2013). Like THC, THCA also acts on the (CB1) receptor to reduce contextually elicited conditioned gaping and vomiting in shrews, as administration of SR blocks these effects (Parker et al. 2004; Rock et al. 2013).
Currently, AN in humans is most often treated with non-specific anti-anxiety drugs (Razavi et al. 1993; Malik et al. 1995); however, both benzodiazepines and THC have sedating side effects, which may diminish their therapeutic effectiveness. Therefore, the present experiments evaluated the potential of CBDA (across a wide range of doses), the benzodiazepine chlordiazepoxide (CDP), OND, and THC to interfere with the expression of contextually elicited conditioned gaping reactions in rats and to modify general locomotor activity. It is expected, based on previous data from our laboratory (Bolognini et al. 2013), that CBDA will potently and dose-dependently reduce contextually elicited conditioned gaping without interfering with locomotor activity. We also expect that THC will reduce contextually elicited conditioned gaping based on previous findings indicating that THC reduced contextually elicited conditioned gaping in which a novel flavor was infused at test within the LiCl-paired chamber (Limebeer et al. 2006). We expected that CDP would effectively reduce contextually elicited conditioned gaping, as benzodiazepines have been shown to reduce AN in human patients (as reviewed by Kamen et al. 2014); however, we expected that CDP may also impair locomotor activity (Haller et al. 2010). On the other hand, it is expected that OND will not be effective in reducing contextually elicited conditioned gaping based on previous work from our laboratory in which a novel flavor was infused at test within the LiCl-paired chamber (Limebeer et al. 2006).
In addition, we examined the effectiveness of co-administration of subthreshold doses of CBDA and THCA, as well as their mechanisms of action in the expression of contextually elicited conditioned gaping reactions. Since both non-psychoactive compounds are in the cannabis plant before heating and both are effective in reducing AN in this model, it is conceivable that administration of doses of CBDA and THCA that are ineffective on their own, when combined, will reduce contextually elicited conditioned gaping. We also anticipated that administration of either SR or WAY would block the combined suppressive effect of CBDA and THCA on contextually elicited conditioned gaping, as CBDA has been shown to produce this effect by acting as a 5-HT1A receptor agonist (Bolognini et al. 2013) and THCA’s effects have been blocked by SR, a CB1 receptor antagonist (Rock et al. 2013).
Finally, since anti-anxiety drugs are currently used in human patients to treat AN, the potential of CBDA to reduce anxiety (as has been shown with CBD, Zuardi et al. 1993) was also assessed using the expression of conditioned freezing to a shock-paired tone. As CBD exhibits anti-anxiety-like effects, we expected that CBDA may also produce such effects.
Materials and methods
Animals
Animal procedures complied with the Canadian Council on Animal Care and the protocols were approved by the Institutional Animal Care Committee at University of Guelph. A total of 199 naive male Sprague–Dawley rats, obtained from Charles River Laboratories (St Constant, Quebec), were used for assessment of AN. They were pair-housed in shoebox cages, subjected to an ambient temperature of 21 °C and a 12/12 h light–dark schedule (lights off at 7 am), and maintained on food (Highland Rat Chow [8640]) and water ad libitum. Their body weights ranged from 300 to 440 g on the day of testing for AN.
Drugs
LiCl (Sigma-Aldrich) was prepared in a 0.15 M solution with sterile water and was administered intraperitoneally (ip) at a volume of 20 ml/kg (127.2 mg/kg dose). OND (Sigma-Aldrich) was prepared in saline (SAL) and was administered subcutaneously (sc) at a volume of 1 ml/kg at concentrations of 0.1 mg/ml (0.1 mg/kg) or 1.0 mg/ml (1.0 mg/kg). CDP (provided by NIDA) was prepared in SAL and administered ip at a volume of 1 ml/kg at concentrations of 1 mg/ml (1 mg/kg), 5 mg/ml (5 mg/kg), and 10 mg/ml (10 mg/kg); at doses of 5–10 mg/kg, CDP produces anxiolytic-like responses in animal measures of anxiety, such as the light–dark immersion test (Merlo Pich and Samanin 1989; Chaouloff et al. 1997). THC, THCA, and CBDA (GW Pharmaceuticals, UK) were prepared in 1:1:18 vehicle (VEH) solution of ethanol/cremophor (Sigma)/physiological SAL. At test, THC was administered ip at a volume of 1 ml/kg at a concentration of 0.5 mg/ml (0.5 mg/kg); this dose has been shown to be effective in suppressing acute nausea (conditioned gaping to a LiCl-paired flavor) in rats (Limebeer and Parker 1999) and to interfere with gaping to a novel sucrose solution presented in a context previously paired with nausea (Limebeer et al. 2006). Each dose of CBDA was administered ip at a volume of 1 ml/kg at concentrations of 0.0001 mg/ml (0.0001 mg/kg), 0.001 mg/ml (0.001 mg/kg), 0.01 mg/ml (0.01 mg/kg), 0.1 mg/ml (0.1 mg/kg), or 1.0 mg/ml (0.1 mg/kg). In experiment 4, THCA was administered ip at a volume of 2 ml/kg at a concentration of 0.25 μg/ml (5 μg/kg) and CBDA was administered ip at a volume of 1 ml/kg at a concentration of 0.1 μg/ml (0.1 μg/kg). The doses of 0.1 μg/kg of CBDA (based on experiment 2 results) and 5 μg/kg of THCA (Rock et al. 2013) were used, as they are below those that suppress contextually elicited gaping on their own. SR at 1 mg/kg—a dose that on its own does not potentiate conditioned gaping over that of VEH (Bolognini et al. 2013)—was prepared in a VEH consisting of a 1:1:18 mixture of ethanol, cremophor, and SAL and administered ip in a volume of 1 ml/kg at a concentration of 1 mg/ml. WAY (0.1 mg.kg) was mixed in SAL and administered ip in a volume of 1 ml/kg at a concentration of 0.1 mg/ml (Rock et al. 2012; Bolognini et al. 2013). These doses of SR and WAY were selected based on previous work indicating that neither of these compounds alone interferes with contextually elicited conditioned gaping (Rock et al. 2012; Bolognini et al. 2013).
Apparatus
The distinctive context used for AN conditioning was constructed of black Plexiglas and sat on a table with a clear Plexiglas top as previously described (Limebeer et al. 2014). A mirror beneath the chamber on a 45° angle facilitated viewing of the ventral surface of the rat. A Sony video camera (Handycam, model DCR-HC28, Henry’s Camera, Waterloo, ON, Canada) was used to videotape the rats from the mirror beneath the chamber. The videotapes were later scored using “The Observer” Event recording software (Noldus Information Technology, Leesburg, VA, USA).
The activity chamber was constructed of white Plexiglas with the dimensions of 60 cm × 25 cm × 25 cm and located in a different room than the AN chamber, illuminated with a red light. A video camera mounted on an extension pole captured the activity of the rat which was sent to a computer for analysis of distance (cm) traveled using the Ethovision software program (Noldus Information Technology, Leesburg, VA, USA).
For the fear conditioning experiment, the rats were conditioned in one of four chambers (30 × 24 × 40 cm; MED-Associates, Burlington, VT, USA) constructed of aluminum (rear and side walls) and Plexiglas (door and ceiling), which were housed in sound-attenuating cabinets. The chambers were equipped with a speaker to present discrete auditory stimuli and a solid-state grid scrambler to deliver foot shocks. Background noise (65 dB) was provided by a ventilation fan that was housed in each cabinet. The activity of each rat was continuously recorded by a near-infrared-based imaging camera that was connected to a computer. Freezing behavior was assessed using “Video Freeze” (MED-Associates; Burlington, VT, USA) software (30 fps, movement threshold: 150, min freeze duration: 90), and percent freezing behavior was calculated as the amount of time each rat was immobile per minute. The original chamber context (context A) was used for habituation and conditioning, whereas a novel context (context B) was created for the purpose of specifically assessing conditioned behavior to the auditory stimulus alone. Context B consisted of chambers that were equipped with an opaque white plastic insert over the floor bars and an opaque black plastic teepee overtop. The chambers were wiped down with a 1 % acetic acid solution to yield a novel odor. To further distinguish from the original context, the testing room was illuminated by a dim red light (40 W), whereas the sound-attenuating cabinets were illuminated by white ambient light.
Procedures
Effect of pretreatments on contextually elicited conditioned gaping and locomotor activity
All rats received four conditioning trials, with 72 h between trials. On each conditioning trial, each rat was injected with LiCl and immediately placed in the distinctive context for 30 min. The test trial occurred 72 h after the final conditioning trial. In experiment 1, on the test trial, the rats were randomly assigned to one of four pretreatment groups (n = 8/group): VEH, 0.1 mg/kg OND, 1.0 mg/kg OND, or 0.5 mg/kg THC. In experiment 2, on the test trial, the rats were assigned to one of six preteatment groups: VEH (n = 10), 0.0001 mg/kg CBDA (n = 8), 0.001 mg/kg CBDA (n = 8), 0.01 mg/kg CBDA (n = 8), 0.1 mg/kg CBDA (n = 7), and 1.0 mg/kg CBDA (n = 6). In experiment 3, on the test trial, the rats were assigned to one of four pretreatment groups (n = 8/group): VEH, 1 mg/kg CDP, 5 mg/kg CDP, or 10 mg/kg CDP.
Thirty minutes following OND, THC, or CDP and 45 min following CBDA, the rats received an ip injection of SAL and were placed in the conditioning chamber for 5 min while their orofacial reactions were videotaped from the mirror beneath the chamber. VEH-injected rats display the most contextually elicited gaping reactions during the first 5 min of a longer test trial (Limebeer et al. 2008; Rock et al. 2008). The videotapes were later scored for the number of gapes (large amplitude openings of the mouth with simultaneous retractions of the corners of the mouth exposing incisors). Immediately following the test trial, the rats were placed in the novel activity chamber for 15 min and their locomotor activity was automatically video tracked.
Effect of co-administration of subthreshold doses of CBDA and THCA on contextually elicited conditioned gaping and locomotor activity
Procedures were the same as above, except as indicated. On the test trial, rats received a first pretreatment of CBDA or VEH. Fifteen minutes later, rats received a second pretreatment of THCA or VEH. To investigate the mechanism of action, additional groups of rats were injected with either SR or WAY prior to the first pretreatment. This resulted in the following pretreatment groups (n = 8/group): VEH-VEH, VEH-THCA, CBDA-VEH, CBDA-THCA, SR-CBDA-THCA, and WAY-CBDA-THCA.
Effect of CBDA on expression of conditioned freezing to a shock-paired tone
Context-independent fear conditioning was based on the procedures of Maren (1999). Naive rats received a single 10 min habituation session, followed 24 h later by a single conditioning trial. During conditioning, the rats received three tone (85 dB, 2,000 Hz, 10 s) - footshock (2 s, 0.8 mA) pairings with a 70 s intertrial interval that began 3 min after the rats were placed into the chambers. Twenty-four hours later, fear conditioning was assessed with a 10 min test that occurred in a novel context (context B). During this test, the tone occurred after 2 min and remained on for the remainder of the 10 min session. Forty-five minutes prior to the test, the rats were injected with the appropriate pretreatment (n = 8/group): VEH, 0.001 mg/kg CBDA, 0.01 mg/kg CBDA, 0.1 mg/kg CBDA, or 1.0 mg/kg CBDA.
Data analysis
In each experiment, the number of gapes and distance (cm) traveled in the locomotor activity test were entered into a one-way between groups analysis of variance (ANOVA), with subsequent Bonferroni pairwise post hoc comparisons of significant effects. For the expression of conditioned freezing, the mean number of seconds spent freezing every 2 min was entered into a 5 by 5 mixed factor ANOVA with the between group factors of pretreatment and the within groups factor of interval. Significance was defined as p < 0.05.
Results
Effects of OND, THC, CBDA, and CDP on contextually elicited conditioned gaping reactions and locomotor activity
OND and THC
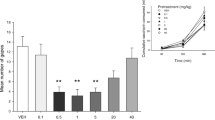
THC, but not OND, interfered with the expression of contextually elicited conditioned gaping reactions in rats. Neither pretreatment modified general activity level. Figure 1a(i) presents the mean number of gapes displayed during the test of AN for the rats pretreated with VEH, 0.1 mg/kg OND, 1.0 mg/kg OND, and 0.5 mg/kg THC. The single factor between group ANOVA revealed a significant main effect of pretreatment, F(3, 28) = 7.7; p < 0.001; subsequent Bonferroni pairwise comparison tests revealed that the group pretreated with THC displayed significantly (p < 0.01) less gaping than any other group. Figure 1b(ii) presents the mean distance (cm) traveled in the 15 min locomotor activity test. The one-way ANOVA was not significant, F(3, 28) = 0.6, p > 0.05.
a i Mean (±sem) number of gapes elicited during the AN test trial among rats pretreated (n = 8/group) with VEH, OND (0.1 or 1.0 mg/kg), or THC (0.5 mg/kg; **p < 0.01 relative to all other groups). iii Mean (±sem) number of gapes elicited during the AN test trial among rats pretreated (n = 6-10/group) with VEH or CBDA (0.0001, 0.001, 0.01 mg/kg, 0.1 or 1.0 mg/kg; ****p < 0.001 relative to groups VEH and 0.0001 mg/kg). v Mean (±sem) number of gapes elicited during the AN test trial among rats pretreated (n = 8/group) with VEH or CDP (1, 5, or 10 mg/kg; *p < 0.05, **p < 0.01 test relative to groups VEH or 1 mg/kg CDP). b ii Mean (±sem) distance (cm) traveled during the locomotor activity test among rats pretreated (n = 8/group) with VEH, OND (0.1 or 1.0 mg/kg), or THC (0.5 mg/kg). iv Mean (±sem) distance (cm) traveled during the locomotor activity test among rats pretreated (n = 6-10/group) with VEH or CBDA (0.0001, 0.001, 0.01 mg/kg, 0.1 or 1.0 mg/kg. vi Mean (±sem) distance (cm) traveled during the locomotor activity test among rats pretreated (n = 8/group) with VEH or CDP (1, 5, or 10 mg/kg; *p < 0.05, ***p < 0.001 relative to VEH or 1 mg/kg CDP)
CBDA
CBDA suppressed AN at doses of 0.001–0.1 mg/kg, but not 0.0001 or 1 mg/kg, suggesting a U-shaped dose–response function. However, CBDA did not modify locomotor activity at any dose tested. Figure 1a(iii) presents the mean number of contextually elicited gaping reactions displayed during the test trial among rats pretreated with various doses of CBDA. The single factor between group ANOVA revealed a significant main effect of pretreatment, F(5, 41) = 13.0; p < 0.001; subsequent Bonferroni pairwise comparison tests revealed that groups 0.001, 0.01, and 0.1 mg/kg CBDA displayed significantly (p’s < 0.001) less contextually elicited gaping reactions than Groups VEH or 0.0001 CBDA. Group 1 mg/kg CBDA, however, did not significantly differ from group VEH or any of the other groups. Figure 1b(iv) presents the mean distance (cm) traveled by the rats pretreated with the various doses of CBDA. The one-way ANOVA revealed no significant effect, F(5, 41) = 1.8, p > 0.05.
CDP
At doses of 5 or 10 mg/kg, CDP suppressed contextually elicited gaping, but these doses also reduced activity level. Figure 1a(v) presents the mean number of gaping reactions among the rats pretreated with CDP prior to the test for AN. The one-way ANOVA revealed a significant effect of pretreatment dose, F(3, 28) = 8.6; p < 0.001; subsequent Bonferroni pairwise comparison tests revealed that groups 5 (p < 0.05) and 10 (p < 0.01) mg/kg CDP displayed less gaping than groups VEH or 1 mg/kg CDP.
Figure 1b(vi) presents the mean distance traveled in the 15 min locomotor test. The one-way ANOVA revealed a significant effect of dose of CDP, F(3, 28) = 32.3; p < 0.001; subsequent Bonferroni pairwise comparison tests indicated that rats pretreated with 10 mg/kg CDP displayed suppressed locomotor activity relative to all groups (p’s < 0.001). As well, rats pretreated with 5 mg/kg CDP were less active than rats pretreated with VEH or 1 mg/kg (p < 0.05).
Effect of co-administration of subthreshold doses of CBDA and THCA on contextually elicited conditioned gaping and locomotor activity
Co-administration of 0.1 μg/kg CBDA with 5 μg/kg THCA reduced contextually elicited conditioned gaping in rats and administration of either SR or WAY blocked this effect. A one-way ANOVA of the mean number of gapes elicited by the LiCl-paired chamber among the pretreatment groups revealed a significant main effect of group, F(5,42) = 3.8; p < 0.01. Subsequent post hoc comparisons revealed that the group pretreated with CBDA-THCA gaped significantly less than the VEH-VEH-treated controls (p < 0.003). In addition, those rats pretreated with SR or WAY prior to CBDA and THCA gaped significantly more than group CBDA-THCA (Figure 2a).
a Mean (±sem) number of gapes elicited during the AN test trial (n = 8/group) among rats pretreated with VEH or CBDA (0.1 μg/kg), followed by VEH or THCA (5 μg/kg; **p < 0.003 relative to VEH-VEH). Additional groups were also pretreated with either SR or WAY to determine the mechanism of action for co-administered CBDA and THCA (# p < 0.05, ## p < 0.004 relative to group CBDA-THCA). b Mean (±sem) distance (cm) traveled during the locomotor activity test (n = 8/group) among rats pretreated with VEH or CBDA (0.1 μg/kg), followed by VEH or THCA (5 μg/kg). Additional groups were also pretreated with either SR or WAY to determine the mechanism of action for co-administered CBDA and THCA
No differences in activity were seen between groups. A one-way ANOVA of the distance traveled in the chamber revealed no significant effects, p > 0.05 (Figure 2b).
Effects of CBDA on expression of conditioned freezing
CBDA did not modify the expression of conditioned freezing to a shock-paired tone. Figure 3 presents the mean number of seconds that each group spent freezing during each interval of testing. The tone was turned on during min 3. The 5 by 5 mixed factors ANOVA revealed only a significant effect of interval, F(4, 140) = 28.2; p < 0.001. Overall, the rats spent more time freezing with the onset of the tone during min 3–4 than during any other interval (p’s < 0.001), showing the establishment of conditioned fear to the tone. The fear gradually extinguished across the testing; during min 7–10, the rats no longer significantly differed in time spent freezing from min 1–2 prior to the onset of the tone. However, CBDA pretreatment did not interact with the effect of interval.
Discussion
The present results extend the findings of Bolognini et al. (2013) showing that CBDA is a highly potent anti-nausea compound when evaluated in the preclinical model of AN, contextually elicited conditioned gaping in rats. Indeed, a systemic dose as low as 1 μg/kg potently suppressed AN in this model. This is in contrast with CBD, which requires a dose of 1–5 mg/kg to attenuate contextually elicited conditioned gaping reactions in rats (Rock et al. 2008). Therefore, the acidic precursor of CBD is more potent than CBD in reducing both AN and acute nausea (conditioned gaping to a LiCl-paired flavor) (Rock and Parker 2013; Bolognini et al. 2013). Neither CBD (Parker et al. 2004) nor CBDA (Bolognini et al. 2013) produces their anti-nausea effect by activation of the CB1 receptor; instead, both act at the 5-HT1A receptor (like 8-OH-DPAT, Limebeer and Parker 2003) because their anti-nausea (both acute, conditioned gaping to a LiCl-paired flavor, and AN) effects are reversed by the 5-HT1A antagonist, WAY (Rock et al. 2012; Bolognini et al. 2013).
In line with these findings, we also demonstrated that co-administration of subthreshold doses of CBDA and THCA reduced contextually elicited gaping. Our group has previously shown that THCA potently reduces AN (0.5–0.05 mg/kg, ip), as well as acute nausea (conditioned gaping to a LiCl-paired flavor) in rats, and toxin-induced vomiting in shrews (Rock et al. 2013). Furthermore, these effects seem to be CB1 receptor mediated, as SR, but not WAY, blocks THCA’s anti-nausea effects in AN. This CB1 receptor-mediated mechanism of action indicates that THCA could exhibit CB1 receptor-mediated abuse liability problems, perhaps making CBDA a preferred therapeutic agent over that of THCA.
It has been repeatedly reported with human chemotherapy patients, when AN and vomiting occur, OND is completely ineffective in reducing these symptoms (Morrow et al. 1995; Hsu 2010). Consistently, OND at doses of 0.1 and 1 mg/kg did not reduce contextually elicited AN in rats, providing further face validity for the model. As well, OND did not reduce the expression of previously established conditioned retching in shrews when reexposed to a context in which they previously vomited (Parker and Kemp 2001; Parker et al. 2006). As with human chemotherapy patients, however, OND does prevent acute nausea in rats when administered prior to LiCl-induced malaise as measured by the behavior of lying on belly (Parker 1984) and the establishment of conditioned gaping (Limebeer and Parker 2000; Tuerke et al. 2012). Indeed, when delivered directly to the interoceptive region of the insular cortex, the visceral insular cortex, OND suppresses the nausea produced by LiCl. Therefore, as is also the case with chemotherapy patients, OND reduces acute nausea (conditioned gaping to a LiCl-paired flavor) but not AN in rats. Interestingly, a subthreshold dose of CBDA potentiates the effectiveness of OND in reducing acute nausea in rats (Rock and Parker 2013).
CBDA, THC, CDP, as well as CBDA and THCA co-administered at subthreshold doses; all suppressed contextually elicited conditioned gaping, suggesting that they may all be potential treatments for AN in chemotherapy patients. Indeed, THC also suppressed contextually elicited conditioned retching in shrews (Parker and Kemp 2001; Parker et al. 2006). In addition, CDP is the treatment most likely to be prescribed for AN and vomiting in chemotherapy patients (Razavi et al. 1993; Malik et al. 1995). Both THC and CDP, however, are psychoactive, and at the doses that produce anxiolytic-like effects, CDP has been shown to exhibit sedative properties. These sedative properties were again demonstrated here, as CDP (5 and 10 mg/kg; doses producing anxiolytic-like effects) reduced locomotor activity. CBDA (as well as co-administration of CBDA with THCA), however, did not modify activity levels at any dose tested, and its lack of effect on CB1 receptors, in vitro and in vivo, indicates that it would not trigger any CB1 receptor-mediated abuse liability problems.
In addition, here we present further evidence for CBDA and THCA’s mechanisms of action in reducing contextually elicited conditioned gaping. Administration of either SR or WAY equivalently blocked the enhanced suppression of contextually elicited gaping by subthreshold co-administered CBDA and THCA. This finding would suggest that this effect is mediated by activation of both the 5-HT1A and CB1 receptors. This is in line with previous reports from our laboratory indicating that CBDA acts as an indirect 5-HT1A receptor agonist, and that THCA’s effects are blocked by administration of the CB1 receptor antagonist SR (Bolognini et al. 2013; Rock et al. 2013).
CBDA, unlike CDP (e.g., Merlo Pich and Samanin 1989; Ramos et al. 2008), did not produce an anxiolytic-like behavioral profile when evaluated in the expression of conditioned freezing to a shock-paired tone. Indeed, the only behavioral effect of CBDA was the suppression of contextually elicited conditioned gaping, highlighting its selectivity in reducing AN. This finding suggests that CBDA may be a highly specialized treatment to attenuate nausea and vomiting and may be particularly useful in the attenuation of AN for which there is no currently available specific treatment. In fact, recently, Takeda et al. (2012) reported that CBDA (5, 10, 25 mM) actually inhibits cancer cell migration in highly aggressive human breast cancer, an important factor in preventing cancer metastasis. These results argue strongly for the need for clinical trials with CBDA for the treatment of AN in human chemotherapy patients.
References
Bolognini D, Rock EM, Cluny NL, Cascio MG, Limebeer CL, Duncan M et al (2013) Cannabidiolic acid prevents vomiting in Suncus murinus and nausea-induced behaviour in rats by enhancing 5-HT1A receptor activation. Br J Pharmacol 168:1456–1470
Chaouloff F, Durand M, Mormede P (1997) Anxiety- and activity-related effects of diazepam and chlordiazepoxide in the rat light/dark and dark/light tests. Behav Brain Res 85:27–35
Cross-Mellor SK, Ossenkopp KP, Piomelli D, Parker LA (2007) Effects of the FAAH inhibitor, URB597, and anandamide on lithium-induced taste reactivity responses: a measure of nausea in the rat. Psychopharmacol (Berl) 190:135–143
Fegley D, Gaetani S, Duranti A, Tontini A, Mor M, Tarzia G et al (2005) Characterization of the fatty acid amide hydrolase inhibitor cyclohexyl carbamic acid 3′-carbamoyl-biphenyl-3-yl ester (URB597): effects on anandamide and oleoylethanolamide deactivation. J Pharmacol Exp Ther 313:352–358
Gaoni Y, Mechoulam R (1964) Isolation, structure, and partial synthesis of an active constituent in hashish. J Am Chem Soc 86:1646–1647
Grill HJ, Norgren R (1978) The taste reactivity test. I. Mimetic responses to gustatory stimuli in neurologically normal rats. Brain Res 143:263–279
Haller J, Hohmann J, Freund TF (2010) The effect of Echinacea preparations in three laboratory tests of anxiety: comparison with chlordiazepoxide. Phythother Res 24:1605–1613
Hsu ES (2010) A review of granisetron, 5-hydroxytryptamine3 receptor antagonists, and other antiemetics. Am J Ther 17:476–486
Kamen C, Tejani MA, Chandwani K, Janelsins M, Peoples AR, Roscoe JA et al (2014) Anticipatory nausea and vomiting due to chemotherapy. Eur J Pharmacol 722:172–179
Kwiatkowska M, Parker LA, Burton P, Mechoulam R (2004) A comparative analysis of the potential of cannabinoids and ondansetron to suppress cisplatin-induced emesis in the Suncus murinus (house musk shrew). Psychopharmacol (Berl) 174:254–259
Lau AH, Rudd JA, Yew DT (2005) Action of ondansetron and CP-99,994 on cisplatin-induced emesis and locomotor activity in Suncus murinus (house musk shrew). Behav Pharmacol 16:605–612
Limebeer CL, Parker LA (1999) Delta-9-tetrahydrocannabinol interferes with the establishment and the expression of conditioned rejection reactions produced by cyclophosphamide: a rat model of nausea. Neuroreport 10:3769–3772
Limebeer CL, Parker LA (2000) The anti-emetic drug, ondansetron, interferes with lithium-induced conditioned rejection reactions, but not lithium-induced conditioned taste avoidance. J Exp Psych: Animal Behav Proc 26:371–384
Limebeer CL, Parker LA (2003) The 5-HT1A agonist 8-OH-DPAT dose-dependently interferes with the establishment and the expression of lithium-induced conditioned rejection reactions in rats. Psychopharmacol (Berl) 166:120–126
Limebeer CL, Hall G, Parker LA (2006) Exposure to a lithium-paired context elicits gaping in rats: a model of anticipatory nausea. Physiol Behav 88:398–403
Limebeer CL, Krohn JP, Cross-Mellor S, Litt DE, Ossenkopp KP, Parker LA (2008) Exposure to a context previously associated with nausea elicits conditioned gaping in rats: a model of anticipatory nausea. Behav Brain Res 187:33–40
Limebeer CL, Abdullah RA, Rock EM, Imhof E, Wang K, Lichtman AH, Parker LA (2014) Attenuation of anticipatory nausea in a rat model of contextually elicited conditioned gaping by enhancement of the endocannabinoid system. Psychopharmacol (Berl) 231:603–612
Malik IA, Khan WA, Qazilbash M, Ata E, Butt A, Khan MA (1995) Clinical efficacy of lorazepam in prophylaxis of anticipatory, acute, and delayed nausea and vomiting induced by high doses of cisplatin. A prospective randomized trial. Am J Clin Oncol 18:170–175
Maren S (1999) Neurotoxic basolateral amygdala lesions impair learning and memory but not the performance of conditional fear in rats. J Neurosci 19:8696–8703
Mechoulam R, Gaoni Y (1965) Hashish. IV. The isolation and structure of cannabinolic cannabidiolic and cannabigerolic acids. Tetrahedron 21:1223–1229
Merlo Pich E, Samanin R (1989) A two-compartment exploratory model to study anxiolytic/anxiogenic effects of drugs in the rat. Pharmacol Res 21:595–602
Morrow GR, Hickok JT, Rosenthal SN (1995) Progress in reducing nausea and emesis. Comparisons of ondansetron (Zofran), granisetron (Kytril), and tropisetron (Navoban). Cancer 76:343–357
Morrow GR, Roscoe JA, Kirshner JJ, Hynes HE, Rosenbluth RJ (1998) Anticipatory nausea and vomiting in the era of 5-HT3 antiemetics. Support Care Cancer 6:244–247
Parker LA (1984) Behavioral conditioned responses across multiple conditioning/testing trials elicited by lithium- and amphetamine-paired flavors. Behav Neural Biol 41:190–199
Parker LA, Kemp SW (2001) Tetrahydrocannabinol (THC) interferes with conditioned retching in Suncus murinus: an animal model of anticipatory nausea and vomiting (ANV). Neuroreport 12:749–751
Parker LA, Kwiatkowska M, Burton P, Mechoulam R (2004) Effect of cannabinoids on lithium-induced vomiting in the Suncus murinus (house musk shrew). Psychopharmacol (Berl) 171:156–161
Parker LA, Kwiatkowska M, Mechoulam R (2006) Delta-9-tetrahydrocannabinol and cannabidiol, but not ondansetron, interfere with conditioned retching reactions elicited by a lithium-paired context in Suncus murinus: an animal model of anticipatory nausea and vomiting. Physiol Behav 87:66–71
Potter DJ, Clark P, Brown MB (2008) Potency of delta 9-THC and other cannabinoids in cannabis in England in 2005: implications for psychoactivity and pharmacology. J Forensic Sci 53:90–94
Ramos A, Pereira E, Martins GC, Wehrmeister TD, Izidio GS (2008) Integrating the open field, elevated plus maze and light/dark box to assess different types of emotional behaviors in one single trial. Behav Brain Res 193:277–288
Razavi D, Delvaux N, Farvacques C, De Brier F, Van Heer C, Kaufman L et al (1993) Prevention of adjustment disorders and anticipatory nausea secondary to adjuvant chemotherapy: a double-blind, placebo-controlled study assessing the usefulness of alprazolam. J Clin Oncol 11:1384–1390
Rock EM, Parker LA (2013) Effect of low doses of cannabidiolic acid and ondansetron on LiCl-induced conditioned gaping (a model of nausea-induced behaviour) in rats. Br J Pharmacol 169:685–692
Rock EM, Limebeer CL, Mechoulam R, Piomelli D, Parker LA (2008) The effect of cannabidiol and URB597 on conditioned gaping (a model of nausea) elicited by a lithium-paired context in the rat. Psychopharmacol (Berl) 196:389–395
Rock EM, Bolognini D, Limebeer C, Cascio M, Anavi-Goffer S, Fletcher P et al (2012) Cannabidiol, a non-psychotropic component of cannabis, attenuates vomiting and nausea-like behaviour via indirect agonism of 5-HT(1A) somatodendritic autoreceptors in the dorsal raphe nucleus. Br J Pharmacol 165:2620–2634
Rock EM, Kopstick RL, Limebeer CL, Parker LA (2013) Tetrahydrocannabinolic acid reduces nausea-induced conditioned gaping in rats and vomiting in Suncus murinus. Br J Pharmacol 170:641–648
Rudd JA, Naylor RJ (1994) Effects of 5-HT3 receptor antagonists on models of acute and delayed emesis induced by cisplatin in the ferret. Neuropharmacology 33:1607–1608
Rudd JA, Naylor RJ (1996) An interaction of ondansetron and dexamethasone antagonizing cisplatin-induced acute and delayed emesis in the ferret. Br J Pharmacol 118:209–214
Sam TS, Cheng JT, Johnston KD, Kan KK, Ngan MP, Rudd JA et al (2003) Action of 5-HT3 receptor antagonists and dexamethasone to modify cisplatin-induced emesis in Suncus murinus (house musk shrew). Eur J Pharmacol 472:135–145
Takeda S, Okajima S, Miyoshi H, Yoshida K, Okamoto Y, Okada T et al (2012) Cannabidiolic acid, a major cannabinoid in fiber-type cannabis, is an inhibitor of MDA-MB-231 breast cancer cell migration. Toxicol Lett 214:314–319
Tuerke KJ, Winters BD, Parker LA (2012) Ondansetron interferes with unconditioned lying-on belly and acquisition of conditioned gaping induced by LiCl as models of nausea-induced behaviors in rats. Physiol Behav 105:856–860
Zuardi AW, Cosme RA, Graeff FG, Guimarães FS (1993) Effects of ipsapirone and cannabidiol on human experimental anxiety. J Psychopharmacol 7:82–88
Acknowledgment
The funding for these experiments was provided by G.W. Pharmaceuticals, UK and the Natural Sciences and Engineering Research Council of Canada to LAP.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rock, E.M., Limebeer, C.L., Navaratnam, R. et al. A comparison of cannabidiolic acid with other treatments for anticipatory nausea using a rat model of contextually elicited conditioned gaping. Psychopharmacology 231, 3207–3215 (2014). https://doi.org/10.1007/s00213-014-3498-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-014-3498-1