Abstract
Rationale
The endogenous cannabinoid system plays a vital role in the control of nausea and emesis. Because of the rapid breakdown and hydrolysis of endocannabinoids, such as anandamide, the therapeutic effects may be enhanced by prolonging their duration of action.
Objective
The present experiment evaluated the potential of various doses of URB597, a fatty acid amide hydrolase (FAAH) inhibitor, alone and in combination with systemic administration of anandamide to modulate the establishment of lithium-induced conditioned taste reactivity responses in rats.
Materials and methods
In experiment 1, on the conditioning day, rats first received an injection of 0.3 mg/kg URB597, 0.15 mg/kg URB597, or vehicle and then received a second injection of anandamide (5 mg/kg) or vehicle, before a 3-min exposure of 0.1% saccharin by intraoral infusion. Immediately after the saccharin exposure, the rats were injected with lithium chloride. On each of three test days, rats received a 3-min intraoral infusion of saccharin solution, and the taste reactivity responses were videotaped and monitored. In experiment 2, the effects of pretreatment with the CB1 antagonist, AM-251, on URB597 and anandamide-induced suppressed aversion was evaluated.
Results
Administration of URB597 alone and in combination with anandamide reduced active rejection reactions elicited by a LiCl-paired saccharin solution; both effects were reversed by pretreatment with AM-251, suggesting that they were CB1 receptor mediated.
Conclusions
The results suggest that prolonging the action of anandamide by pretreatment with the FAAH inhibitor, URB597, suppresses lithium-induced nausea in the rat.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There is increasing evidence that the endogenous cannabinoids, anandamide and 2-arachidonolylglyceral (2-AG), play vital roles in the control of nausea and vomiting. Anandamide has weak antiemetic properties in both shrews (Darmani 2002) and ferrets (Van Sickle et al. 2001), but 2-AG induces vomiting in shrews (Darmani 2002). The cannabinoid agonist, Δ9-THC, has potent antiemetic actions to prevent cisplatin and lithium-induced vomiting in ferrets (Van Sickle et al. 2003) and shrews (Darmani 2001; Kwiatkowska et al. 2004; Parker et al. 2004) via its action on CB1 receptors. The selective CB1 receptor antagonist, SR-141716A, produces vomiting on its own at higher doses but significantly attenuates the antiemetic effects of Δ9-THC at lower doses in the least shrew (Darmani 2001).
There has been considerable investigation of the antinausea effects of cannabinoid drugs in rats, a non-emetic species. Although rats are incapable of vomiting (Hatcher 1924), they do display conditioned rejection reactions upon reexposure to a flavor previously paired with treatments that produce vomiting in emetic species (Parker 1998). The taste reactivity test developed by Grill and Norgren (1978), in which fluids are infused directly into the oral cavity by means of a surgically implanted intraoral cannula, is uniquely designed to assess these conditioned rejection reactions. Depending on the taste characteristics of the infused fluid, the animal may display ingestive reactions (such as tongue protrusions or mouth movements) that facilitate in the ingestion of the fluid, or it may actively reject the fluid by producing oral rejection responses such as gapes and chin rubs. Lithium chloride (LiCl), an emetic agent, produces vomiting and retching in emetic species such as the house musk shrew (Parker and Kemp 2001; Parker et al. 2003), but decreases positive ingestive responding and increases oral rejection reactions to a sucrose-paired flavor in the rat (e.g., Ossenkopp and Eckel 1995; Breslin et al. 1992; Eckel and Ossenkopp 1996). Indeed, only agents that produce vomiting in emetic species are capable of establishing conditioned rejection reactions in rats (Parker 1998) and classical antiemetic agents, such as the 5-HT3 antagonist ondansetron, prevent the establishment of conditioned rejection reactions produced by LiCl (Limebeer and Parker 2000). Thus, current research indicates that conditioned rejection reactions reflect nausea in the rat, a species that is incapable of vomiting (Parker 2003).
Cannabinoid agonists, including Δ9-THC and HU-210, have been shown to interfere with the establishment of lithium-induced conditioned rejection responses to a saccharin flavor in rats (Parker et al. 2003). Importantly, these antinausea effects were significantly attenuated by the administration of the selective CB1 antagonist, SR-141716A. Finally, it was found that administration of SR-141716A, on its own, actually potentiated lithium-induced conditioned rejection reactions (Parker et al. 2003). These findings suggest that the endogenous cannabinoid system modulates nausea in rats. The endogenous cannabinoid anandamide is synthesized on demand from phospholipid precursors in membranes (Piomelli 2003). Although anandamide is generated rapidly, it is also taken up by cells and rapidly metabolized to arachidonic acid and ethanolamine by the intracellular enzyme fatty acid amide hydrolase (FAAH). Therefore, it may be possible to amplify the actions of this endogenous cannabinoid through the inhibition of its breakdown. URB597 is a potent inhibitor of the FAAH enzyme, which has been shown to increase basal levels of anandamide in the brains of rats and mice (Kathuria et al. 2003; Fegley et al. 2005). Furthermore, it has been shown that systemic administration of URB597 in rats potentiates the hypothermic actions of anandamide (Fegley et al. 2005). However, very little research has been conducted on the antinausea effects of URB597.
The present study examined the effects of the FAAH inhibitor, URB597, on the establishment of conditioned oral reactions to a lithium chloride-paired taste in the rat. Using the taste reactivity test, the effect of pretreatment with URB597 alone and URB597 combined with exogenously administered anandamide on the association between a saccharin flavor paired with the illness-producing effects of lithium chloride were evaluated. URB597 alone may reduce lithium-induced nausea by inhibiting the breakdown of endogenous anandamide in response to the lithium challenge. Anandamide alone may prevent lithium-induced nausea by its action on the CB1 receptor. However, the effects of anandamide are short-lived; therefore, the combined effects of URB597 and anandamide may produce a greater effect than either agent alone in suppressing lithium-induced nausea, and thereby, preventing the establishment of conditioned rejection of saccharin solution. The role of activation of the CB1 receptor on the modulation of conditioned rejection reactions by URB597 and anandamide was also evaluated using the CB1 receptor antagonist, AM-251.
Materials and methods
Subjects
The subjects were naïve, male Long Evans rats (Charles River, Quebec), weighing between 225 and 275 g at the start of the experiment. All rats were housed individually in stainless steel wire mesh cages in a colony room maintained at 21±1°C and a 12:12 h light:dark schedule (with lights on at 07:00 h). All subjects had ad libitum access to both rat chow (ProLab) and tap water. All handling and procedures were carried out in accordance with the guidelines set forth by the Canadian Council for Animal Care (CCAC).
Intraoral cannulation
All rats underwent surgical implantation of intraoral cannulae according to the procedure of Parker (1980). One week after arriving in the laboratory, the rats were anesthetized with a mixture of xylazine [5 mg/kg, intraperitoneally (i.p.)] and ketamine (75 mg/kg, i.p.). A 15-gauge stainless steel needle was then inserted through the dorsal midneck region and was threaded subcutaneously below the ear and along the cheek where it exited the oral cavity just rostral to the first maxillary molar. A 10-cm piece of polyethylene tubing (PE 90) was then inserted through the barrel of the needle, and the needle was removed. The tubing was held in place by an intramedic adapter at the neck and in the mouth by a smooth plastic washer (5 mm in diameter) and heat flaring the end of the tubing. The skin around the puncture sites was swabbed with alcohol and rats were given a minimum of 3 days of recovery before the testing procedure occurred. The cannulae were flushed daily with water to prevent blockage.
Drugs
All drugs were injected i.p. URB597 (provided by Kadmus Pharmaceuticals), anandamide (arachidonylethanolamide: NIDA Lot No: 10258-194A), and AM-251 (Cayman Chemicals) were prepared in a solution of 1 ml ethanol/1 ml Cremaphor (Sigma)/18 ml saline. Both doses of URB597 (0.3 and 0.15 mg/kg) were administered at 1 ml/kg, while anandamide (5 mg/kg) was administered at 4 ml/kg. AM-251 (1.25 mg/kg) was administered at a volume of 1 ml/kg. Lithium chloride was prepared in a 0.15-M solution with sterile water and was administered at a volume of 20 ml/kg.
Taste reactivity test chambers
At the start of each testing session, rats were placed individually in the test chamber (29×25×29 cm) made of clear Plexiglas that sat upon a glass plate. A mirror was mounted at a 45° angle beneath the glass floor and aided in viewing the ventral surface of the rat. All intraoral infusions were delivered at a constant rate (0.78 ml/min) through an infusion hose (PE 90, approximately 1 m in length) that was attached to the rat’s cannula and an infusion pump (model 341-A; Sage Instruments, Cambridge, MA). The behavioral responses produced by the rat during intraoral infusions were videotaped with a video camera (Sony DCR-DVD201; London, Ontario) located approximately 1 m from the mirror, which was attached directly to a computer where the video files were stored.
Procedure
Experiment 1: Effect of URB597 and anandamide on conditioned aversion
All rats were habituated to the general testing procedure for three consecutive days before the conditioning day. During habituation, the rats were placed individually into the test chamber for 10 min where they received a 3-min infusion of distilled water at the end of each session. The timeline for the conditioning day of experiment 1 is presented in the upper section of Table 1. On the conditioning day, the rats received an i.p. injection of 0.3 mg/kg URB597, 0.15 mg/kg URB597, or vehicle (VEH) (see Fegley et al. 2005). One hour and 45 min later, the rats received a second injection of either 5 mg/kg anandamide (ANA) or vehicle (VEH) and were placed individually in the taste reactivity chambers. Fifteen minutes after the second injection, rats were infused with 0.1% saccharin solution for 3 min. The behaviors displayed during the intraoral infusions were videorecorded. Immediately after the saccharin infusion, all rats were injected with 20 ml/kg LiCl (0.15 M). Thus, the six experimental groups included 0.3 mg/kg URB–ANA (n = 10), 0.3 mg/kg URB–Veh (n = 9), 0.15 mg/kg URB–ANA (n = 8), 0.15 mg/kg URB–Veh (n = 7), Veh–ANA (n = 10), and Veh–Veh (n = 9) with group designation denoted by the first and second injections, respectively, that rats received before the pairing of the saccharin infusion with LiCl.
To observe the conditioned effects, each rat received three taste reactivity test trials 72 h after the conditioning trial. On each of these test trials (days 4, 5 and 6), the rats were placed individually in the test chambers for 10 min, and in the absence of any drug injections, received a 3-min infusion of the saccharin solution at the end of the session. Similarly, orofacial and somatic behaviors elicited during the intraoral infusions of the taste were videorecorded and later analyzed for response frequency.
Experiment 2: Effect of AM-251 on URB597 and anandamide modulation of conditioned aversion
Experiment 2 evaluated the role of the CB1 receptor in the suppression of lithium-induced aversions by URB and anandamide using the CB1 receptor antagonist, AM-251. Rats were treated exactly as in experiment 1 except as indicated. The timeline for the conditioning day of experiment 2 is presented in the bottom half of Table 1. On the conditioning day, rats received an i.p. injection of 0.3 mg/kg URB597 or Veh. One hour and 15 min later, the rats received a second injection of either 1.25 mg/kg AM-251 or Veh. Thirty minutes later, they received 5 mg/kg anandamide (ANA) or Veh and were placed individually in the taste reactivity chambers. Fifteen minutes after the second injection, rats were infused with 0.1% saccharin solution for 3 min. The behaviors displayed during the intraoral infusions were videorecorded. Immediately after the saccharin infusion, the rats were injected with 20 ml/kg LiCl (0.15 M) or saline. There were five groups (URB–ANA–LiCl, URB–Veh–LiCl, Veh–ANA–LiCl, Veh–Veh–LiCl, and Veh–Veh–saline) that were either pretreated with AM-251 (AM) or vehicle. The experimental groups were: URB–Veh–ANA–LiCl (n = 10), URB–AM–ANA–LiCl (n = 8), URB–Veh–ANA–LiCl (n = 10), URB–AM–ANA–LiCl (n = 8), URB–Veh–Veh–LiCl (n = 9), URB–AM–Veh–LiCl (n = 8), Veh–Veh–ANA–LiCl (n = 10), Veh–AM–ANA–LiCl (n = 8), Veh–Veh–Veh–LiCl (n = 9), Veh–AM–Veh–LiCl (n = 6), Veh–Am–Veh–saline (n = 8), Veh–Veh–Veh–saline (n = 7).
Taste reactivity scoring and data analysis
In both experiments, the videotapes were scored using the Observer (Noldus Information Technology, Sterling, VA) event-recording program. The behaviors scored included the frequency of 2-s bouts of ingestion reactions (sum of tongue protrusions, mouth movements, and paw licks). Tongue protrusions included both midline and lateral extensions of the tongue while mouth movements consisted of small amplitude movements of the mouth without extensions of the tongue. Paw licks consisted of licking the flavored solution from the forepaws. Active aversive responses included the frequency of gaping (large triangular opening of the mouth), chin rubs (chin or mouth of rat in direct contact with the floor or wall of the chamber while the body is projected forward), head shakes with fluid expulsion and paw treads (sequential extension of one forelimb against the floor or wall of the chamber while the other forepaw is being retracted). These aversive reactions were summed to provide an aversive rejection reaction score.
In experiment 1, data from the conditioning day were analyzed using a one-way analysis of variance (ANOVA) with group as the between-subjects factor (at six levels: 0.3 mg/kg URB–ANA, 0.3 mg/kg URB–Veh, 0.15 mg/kg URB–ANA, 0.15 mg/kg URB–Veh, Veh–ANA and Veh–Veh). Data from the test days were analyzed using mixed factor ANOVAs with group (at six levels) as the between subjects factor and day (at three levels: test days 1, 2, and 3) as the within subjects factor. Post hoc examinations of significant main effects and interactions were done with Tukey HSD tests using α = 0.05 as the criterion for significance. In experiment 2, data from the test days were analyzed using 5 (groups) by 2 (AM-251 or vehicle) by 3 (day) mixed factor ANOVAs with groups (at five levels) and antagonist treatment (at two levels: AM-251 or vehicle) as between subject factors and test days (at three levels) as a within subjects factor.
Results
Experiment 1: Effect of URB597 and anandamide on conditioned aversion
Conditioning day
Analysis of the conditioning day data yielded no significant group differences on either the ingestive reaction score or on the aversive rejection reaction score. Thus, URB597 alone and in combination with anandamide did not produce baseline taste reactivity differences to the saccharin flavor when compared to vehicle-treated controls (Veh–Veh).
Taste reactivity test days
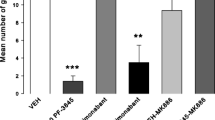
As is seen in Fig. 1, administration of the high dose of URB597 alone or in conjunction with anandamide significantly reduced active rejection reactions to the saccharin previously paired with lithium collapsed across test days. Statistical analysis revealed a significant main effect of group, F(5, 47) = 5.5; p < 0.001, but not day nor a significant interaction between the two factors. Subsequent Tukey HSD post hoc comparison tests revealed that groups 0.3 mg/kg URB+ANA (p < 0.01) and 0.3 mg/kg URB+Veh (p < 0.05) displayed fewer conditioned rejection reactions than group Veh–Veh; additionally, group 0.3 URB+ANA displayed fewer conditioned rejection reactions than group 0.15 URB+Veh (p < 0.01).
Group mean frequencies (+SEM) of total active aversive responses (sum of gaping, chin rubs, head shakes with fluid expulsion, and paw treads) elicited by a 3-min intraoral infusion of saccharin collapsed across the three test days in the absence of any drug injections. Groups were pre-injected on the conditioning day first with either 0.3 mg/kg URB597, 0.15 mg/kg URB597, or vehicle and then with either 5 mg/kg anandamide or vehicle before a saccharin–LiCl association. Asterisks indicate a significant difference (**p < 0.01; * p < 0.05) from group Veh–Veh
Figure 2 depicts the group mean frequency of 2-s bouts of ingestion reactions produced by the rats during the 3-min intraoral infusion of the saccharin solution that had previously been paired with LiCl, pooled across the three test days. Statistical analysis revealed significant main effects of day, F(2, 94) = 9.9, p < 0.001 and of group, F(5, 47) = 3.6, p < 0.01, but the interaction was not significant. As can be seen in Fig. 2, rats that received a high dose of URB597 in combination with anandamide (0.3 mg/kg URB+ANA) displayed significantly more ingestive reactions than group Veh–Veh (p < 0.01).
Group mean frequencies (+ SEM) of total ingestive reactions (sum of 2-s bouts of tongue protrusions, mouth movements and paw licks) elicited by a 3-min intraoral infusion of saccharin collapsed across the three test days in the absence of any drug injections. Groups were pre-injected on the conditioning day first with either 0.3 mg/kg URB597, 0.15 mg/kg URB597, or vehicle and then with either 5 mg/kg anandamide or vehicle before a saccharin–LiCl association. Asterisks indicate a significant difference from group Veh–Veh (**p < 0.01)
Experiment 2: Effect of AM-251 on URB-597 and anandamide modulation of conditioned aversion
Figure 3 presents the mean frequency of aversive reactions displayed pooled across the three test days of experiment 2. The analysis of the aversive reactions revealed significant effects of group, F(4, 74) = 5.6; p < 0.001, antagonist, F(1, 74) = 9.0; p < 0.005; and a group by antagonist interaction, F(4, 74) = 3.4; p < 0.025. To evaluate the interaction for each group, the frequency of aversive reactions displayed by AM-251 pretreated rats was compared with that displayed by vehicle pretreated rats using t-tests corrected for experimentwise error with the criterion for significance set at p < 0.01. Pretreatment with AM-251 reversed the suppressed aversive reactions displayed by groups URB–ANA–LiCl and URB–Veh–LiCl.
Group mean frequencies (+SEM) of total active aversive responses (sum of gaping, chin rubs, head shakes with fluid expulsion, and paw treads) elicited by a 3-min intraoral infusion of saccharin collapsed across the three test days in the absence of any drug injections. The black bars are rats pretreated with vehicle and the gray bars are rats pretreated with AM-251 (1.25 mg/kg). Groups are depicted on the abscissa on the basis of whether they received an injection of 0.3 mg/kg URB, 5 mg/kg ANA or LiCl (+) or vehicle or saline (−). Asterisks indicate a significant difference (**p < 0.01) between AM-251 and vehicle for the indicated group
Figure 4 presents the mean frequency of ingestive reactions displayed pooled across the three test days of experiment 2. The analysis of the ingestive reactions revealed significant effects of days, F(2, 148) = 8.3; p < 0.001; group, F(4, 74) = 5.1; p < 0.001, antagonist, F(1, 74) = 39.1; p < 0.01, and a group by antagonist interaction, F(4, 74) = 3.5; p < 0.025. Pretreatment with AM-251 reversed the enhanced ingestion reactions displayed by groups URB–ANA–LiCl and URB–VEH–LiCl.
Group mean frequencies (+SEM) of total ingestive reactions (sum of 2-s bouts of tongue protrusions, mouth movements, and paw licks) elicited by a 3-min intraoral infusion of saccharin collapsed across the three test days in the absence of any drug injections. The black bars depict rats pretreated with vehicle and the gray bars depict rats pretreated with AM-251 (1.25 mg/kg). Groups are depicted on the abscissa on the basis of whether they received an injection of 0.3 mg/kg URB, 5 mg/kg ANA or LiCl (+) or vehicle or saline (−). Asterisks indicate a significant difference (**p < 0.01) between AM-251 and vehicle for the indicated group
Discussion
When the FAAH inhibitor, URB597, was administered to rats before a saccharin-illness pairing, there was a clear reduction in the conditionally aversive properties of saccharin. Relative to vehicle-treated rats, rats treated with the high dose of URB597 showed fewer conditioned rejection reactions. Furthermore, when URB597 was administered in conjunction with endogenous anandamide, this effect was even greater than that seen in rats treated with URB597 alone. In experiment 2, the suppressed aversion to the lithium-paired saccharin in both groups URB+ANA and URB–Veh, was reversed by pretreatment with the CB1 antagonist, AM-251, suggesting that this receptor plays a role in the suppressed nausea by these pretreatments. Anandamide and URB597 did not affect the palatability of saccharin solution on their own because the groups did not differ in oral reactions on the conditioning day of experiment 1. As conditioned rejection responding in the taste reactivity test is exclusively produced by emetic agents (Parker 2003), these results suggest that the FAAH inhibitor is able to interfere with the nausea induced by LiCl, possibly by preventing the rapid breakdown of anandamide, and thus, increasing the endogenous level of anandamide (e.g., Fegley et al. 2005).
Similarly, other FAAH inhibitors have been shown to potentiate various effects of exogenous anandamide administration. For instance, palmitylsuphonyl fluoride (AM374) was found to significantly enhance the effect of anandamide on electrically-evoked [3H]acetylcholine release (Gifford et al. 1999). AM374 administered in conjunction with exogenous anandamide led to a significant decrease in lever pressing for food reinforcement compared to either agent administered alone (Arizzi et al. 2004).
Although URB597 has been shown to increase brain anandamide levels (Fegley et al. 2005; Kathuria et al. 2003), very little research has evaluated the antinausea effects of this agent. Recently Darmani et al. (2005) showed that very high doses of URB597 (5–10 mg/kg) administered 10 min before 2-AG or cisplatin did not prevent emesis in the least shrew. However, in the present experiment, when much lower doses of URB597 were administered 2 h before LiCl (e.g., Fegley et al. 2005), URB597 facilitated the effect of anandamide in suppressing nausea in rats as measured by suppressed conditioned rejection reactions and enhanced conditioned ingestion reactions in rats. It is, therefore, likely that the interval between URB597 injection and the emesis induced by the toxin was too short in the prior study to facilitate suppression of the emetic response in the shrew. Future studies will evaluate the potential of URB597 administered 2 h before cisplatin to modify emesis in the Suncus murinus (house musk shrew). Furthermore, it is known that the efficacy of various emetic stimuli can be species dependent (see Darmani et al. 2005). Nevertheless, it has been shown previously that URB597 can magnify the hypothermic response produced by anandamide (Fegley et al. 2005) and produce anxiolytic-like responses in both the zero-maze test and the isolation-induced ultrasonic emission test when administered 2 h before the behavioral test (Kathuria et al. 2003). These anxiolytic type responses were significantly attenuated with an injection of the CB1 antagonist, rimonabant, suggesting URB597 produces its effects through the CB1 receptor (Kathuria et al. 2003).
It is clear from the present results and with past research that the cannabinoid system plays a vital role in nausea in the rat. Although rats are incapable of vomiting (Hatcher 1924), they show a pattern of taste reactivity responses consisting of decreased hedonic responding and increased active rejection upon reexposure to flavors previously paired with toxic substances (Parker 1998). Furthermore, these responses are exclusively produced by treatments that produce emesis in emetic species (Parker 1998). It is important to note that treatment with antiemetic drugs known to alleviate vomiting in emetic species also attenuate LiCl-induced conditioned rejection reactions in rats (e.g. Limebeer and Parker 2000; Limebeer et al. 2004). Parker and colleagues demonstrated that the cannabinoid agonist, Δ9-THC, interfered with not only the unconditioned nausea elicited by LiCl in the Suncus murinus (Parker et al. 2004), but also with conditioned nausea elicited by a flavor paired with LiCl in the rat (Parker et al. 2003). Recently, it was shown that the CB1 antagonist, AM251, administered systemically to rats produced conditioned rejection reactions in the taste reactivity test at doses greater than 4 mg/kg (McLaughlin et al. 2005). Additionally, another CB1 antagonist, SR-141716, was found to potentiate lithium-induced conditioned gaping in the rat (Parker et al. 2003). Therefore, it was not surprising that URB597 also reduced conditioned rejection reactions to the LiCl-paired saccharin flavor. These results suggest that that the endogenous cannabinoid system plays a role in the control of nausea.
The finding that anandamide alone was not effective in suppressing conditioned nausea was not unexpected. Although anandamide binds to and activates cannabinoid receptors with high affinity (Devane et al. 1992), it is rapidly eliminated through a two-step process which includes a carrier-mediated transport followed by hydrolysis catalyzed by the enzyme FAAH (Di Marzo et al. 1994; Piomelli 2003). Exogenous administration of the high dose of URB597 in combination with anandamide may have inhibited the hydrolysis of anandamide and allowed for higher levels of anandamide to remain present, thus, interfering with the LiCl-induced nausea. However, we can only speculate that this mechanism underlies the suppressed conditioned aversive reactions because anandamide was not specifically measured after the injection of LiCl. URB597 not only blocks the degradation of anandamide, but also other fatty acid amides, such as oleoylethanolamine (Piomelli 2003).
In the present experiments, rats were administered treatments before a saccharin–LiCl pairing. We have assumed that the reduced aversion was the result of suppressed nausea; however, it is also possible that the treatments modified the establishment of the association, itself, rather than modifying nausea. Although the design of the present experiment cannot rule out this explanation, previous work by our group (Parker et al. 2003) provides evidence that the cannabinoid agonist, Δ9-tetrahydrocannabinol (Δ9-THC), does not suppress conditioned rejection reactions by interfering with the establishment of a saccharin–LiCl association. Rats were injected with Δ9-THC, 30 min before a saccharin–LiCl pairing. In a subsequent test, the saccharin did not elicit conditioned rejection reactions, but the rats avoided drinking the saccharin. Limebeer and Parker (2000) reported the same pattern of results when rats were pretreated with the classic antiemetic agent, ondansetron, before a saccharin–LiCl pairing. That is, Δ9-THC and ondansetron, interfered with LiCl-induced nausea preventing the establishment of conditioned rejection of the saccharin but did not interfere with the association between saccharin and the novel change in state produced by lithium (even in the absence of nausea) that produces taste avoidance. Both Δ9-THC (Parker 2003) and ondansetron (Limebeer and Parker 2000) also interfered with the expression of previously established conditioned rejection reactions when given during the test trial rather than during conditioning. Future research will evaluate whether URB597 and anandamide also interfere with the expression of conditioned nausea.
Together with past research, the current findings provide support for a role of the endocannabinoid system in the protection against nausea in response to a toxin-induced challenge. When the rapid breakdown of anandamide is prevented via the administration of the FAAH inhibitor, URB597, there is a clear interference with the establishment of conditioned rejection responses produced by a flavor–illness association in the rat. This work has important therapeutic implications in the prevention of nausea in humans and should, therefore, prompt further investigation.
References
Arizzi MN, Cervone KM, Aberman JE, Betz A, Liu Q, Lin S, Makriyannis A, Salamone JD (2004) Behavioral effects of inhibition of cannabinoid metabolism: the amidase inhibitor AM374 enhances the suppression of lever pressing produced by exogenously administered anandamide. Life Sci 74:1001–1011
Breslin PAS, Grill HJ, Spector AC (1992) A quantitative comparison of taste reactivity behaviors to sucrose before and after lithium chloride pairings: a unidimensional account of palatability. Behav Neurosci 106:820–836
Darmani NA (2001) Delta(9)-tetrahydrocannabinol and synthetic cannabinoids prevent emesis produced by the cannabinoid CB(1) receptor antagonist/inverse agonist SR 141716A. Neuropsychopharmacology 24:198–203
Darmani NA (2002) The potent emetogenic effects of the endocannabinoid, 2-AG (2-arachidonoylglycerol) are blocked by delta (9)-tetrahydrocannabinol and other cannnabinoids. J Pharmacol Exp Ther 300:34–42
Darmani NA, McClanahan BA, Trinh C, Petrosino S, Valenti M, Di Marzo V (2005) Cisplatin increases brain 2-arachidonoylglycerol (2-AG) and concomitantly reduces intestinal 2-AG and anandamide levels in the least shrew. Neuropharmacology 48:1154–1163
Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R (1992) Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 258:1946–1949
Di Marzo V, Fontana A, Cadas H, Schinelli S, Cimino G, Schwartz JC, Piomelli D (1994) Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature 372:686–691
Eckel LA, Ossenkopp K-P (1996) Area postrema mediates the formation of rapid, conditioned palatability shifts in lithium-treated rats. Behav Neurosci 110:202–212
Fegley D, Gaetani S, Duranti A, Tontini A, Mor M, Tarzia G, Piomelli D (2005) Characterization of the fatty acid amide hydrolase inhibitor cyclohexyl carbamic acid 3′-carbamoyl-biphenyl-3-yl ester (URB597): effects on anandamide and oleoylethanolamide deactivation. J Pharmacol Exp Ther 313:352–358
Gifford AN, Bruneus M, Lin S, Goutopoulos A, Makriyannis A, Volkow ND, Gatley SJ (1999) Potentiation of the action of anandamide on hippocampal slices by the fatty acid amide hydrolase inhibitor, palmitylsulphonyl fluoride (AM 374). Eur J Pharmacol 383:9–14
Grill HJ, Norgren R (1978) The taste reactivity test I. Mimetic responses to gustatory stimuli in neurologically normal rats. Brain Res 143:263–279
Hatcher RA (1924) The mechanism of vomiting. Physiol Rev 4:479–504
Kathuria S, Gaetani S, Fegley D, Valino F, Duranti A, Tontini A, Mor M, Tarzia G, La Rana G, Calignano A, Giustino A, Tattoli M, Palmery M, Cuomo V, Piomelli D (2003) Modulation of anxiety through blockade of anandamide hydrolysis. Nat Med 9:76–81
Kwiatkowska M, Parker LA, Burton P, Mechoulam R (2004) A comparative analysis of the potential of cannabinoids and ondansetron to suppress cisplatin-induced emesis in the Suncus murinus (house musk shrew). Psychopharmacology 174:254–259
Limebeer CL, Parker LA (2000) The antiemetic drug ondansetron interferes with lithium-induced conditioned rejection reactions, but not lithium-induced taste avoidance in rats. J Exp Psychol Anim Behav Process 26:371–384
Limebeer CL, Parker LA, Fletcher PJ (2004) 5,7-dihydroxytryptamine lesions of the dorsal and median raphe nuclei interfere with lithium-induced conditioned gaping, but not conditioned taste avoidance, in rats. Behav Neurosci 118:1391–1399
McLaughlin PJ, Winston KM, Limebeer CL, Parker LA, Makriyannis A, Salamone JD (2005) The cannabinoid CB1 antagonist AM 251 produces food avoidance and behaviors associated with nausea but does not impair feeding efficiency in rats. Psychopharmacology 180:286–293
Ossenkopp K-P, Eckel LA (1995) Toxin-induced conditioned changes in taste reactivity and the role of the chemosensitive area postrema. Neurosci Biobehav Rev 19:99–108
Parker LA (1980) Conditioned suppression of drinking: a measure of the CR elicited by a lithium-conditioned flavor. Learn Motiv 11:538–559
Parker LA (1998) Emetic drugs produce conditioned rejection reactions in the taste reactivity test. J Psychophysiol 12:3–13
Parker LA (2003) Taste avoidance and taste aversion: evidence for two different processes. Learn Behav 31:165–172
Parker LA, Kemp SW (2001) Tetrahydrocannabinol (THC) interferes with conditioned retching in Suncus murinus: an animal model of anticipatory nausea and vomiting (ANV). NeuroReport 12:749–751
Parker LA, Mechoulam R, Schlievert C, Abbott L, Fudge ML, Burton P (2003) Effects of cannabinoids on lithium-induced conditioned rejection reactions in a rat model of nausea. Psychopharmacology 166:156–162
Parker LA, Kwiatkowska M, Burton P, Mechoulam R (2004) Effect of cannabinoids on lithium-induced vomiting in the Suncus murinus (house musk shrew). Psychopharmacology 171:156–161
Piomelli D (2003) The molecular logic of endocannabinoid signalling. Nat Rev Neurosci 4:873–884
Van Sickle MD, Oland LD, Ho W, Hillard CJ, Mackie K, Davison JS, Sharkey KA (2001) Cannabinoids inhibit emesis through CB1 receptors in the brainstem of the ferret. Gastroenterology 121:767–774
Van Sickle MD, Oland LD, Mackie K, Davison JS, Sharkey KA (2003) Delta9-tetrahydrocannabinol selectively acts on CB1 receptors in specific regions of dorsal vagal complex to inhibit emesis in ferrets. Am J Physiol Gastrointest Liver Physiol 285:G566–G576
Acknowledgements
This research was supported by the Natural Sciences and Engineering Research Council of Canada (NSERC) operating grants awarded to L.A.P. and K. P.O. and an NSERC postdoctoral fellowship to S.K.C-M. D.P. is funded by grants from the National Institute on Drug Abuse, the University of California Discovery Program, the National Alliance for Research on Schizophrenia and Depression, and Kadmus Pharmaceuticals Inc.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cross-Mellor, S.K., Ossenkopp, KP., Piomelli, D. et al. Effects of the FAAH inhibitor, URB597, and anandamide on lithium-induced taste reactivity responses: a measure of nausea in the rat. Psychopharmacology 190, 135–143 (2007). https://doi.org/10.1007/s00213-006-0589-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-006-0589-7