Abstract
Rationale
Enhancement of the endocannabinoid (EC) system may reduce anticipatory nausea (AN).
Objectives
The experiments evaluated the potential of the dual fatty acid amide hydrolase (FAAH)/monoacylglycerol lipase (MAGL) inhibitor, JZL195, on its own and combined with anandamide (AEA) and 2-arachidonoyl glycerol (2-AG) to reduce contextually elicited gaping, a measure of AN in rats.
Methods
Following four context lithium chloride (LiCl) pairings, rats were injected with vehicle (VEH) or JZL195 (10 mg kg–1, intraperitoneally) 105 min before an injection of VEH, 2-AG (1.25 mg kg–1), or AEA (5.0 mg kg–1). Fifteen minutes later, all rats were placed in the LiCl-paired context for 5 min and in a different context for a 15-min locomotor test. Whole brains were extracted for EC analysis. The potential of the CB1 antagonist, SR141716, to reverse the suppression of AN by both JZL195 and AEA and of the CB2 antagonist, AM630, to reverse the suppression of AN by JZL195 was then evaluated.
Results
JZL195 suppressed gaping and elevated AEA, palmitoylethanolamine, and oleoylethanolamide. As the suppression of gaping was reversed by SR141716, but not by AM630, the effect was CB1 mediated. The suppressive effect of JZL195 on gaping, as well as elevation of AEA and 2-AG, was amplified by pretreatment with either AEA or 2-AG. On its own, AEA, but not 2-AG, also suppressed gaping—an effect that was also prevented by CB1 antagonism.
Conclusions
JZL195 reduces AN primarily by acting as a FAAH inhibitor, but MAGL inhibition is also indicated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Anticipatory nausea (AN) develops as a conditional association between the contextual cues of the clinic and nausea-inducing chemotherapy treatment (Neese et al. 1980). Once AN develops, classical anti-emetic treatments, such as the 5-HT3 antagonist ondansetron, have little effect, thus underscoring the challenge in treating this condition (Morrow et al. 1998; Foubert and Vaessen 2005). AN is reported as one of the most distressing factors of chemotherapy treatment and significantly decreases the patient's quality of life (Akechi et al. 2010).
The search for an effective treatment for AN has been limited by the lack of selective rodent preclinical models of nausea because rats and mice do not vomit in response to toxins. Recently, a rat model of AN, contextually elicited conditioned gaping reactions, has been identified (Limebeer et al. 2008). Gaping is a disgust reaction in rats that is elicited unconditionally by bitter tasting quinine (Garcia et al. 1974; Grill and Norgren 1978). Gaping consists of rapid, large amplitude openings of the mouth with simultaneous retraction at the corners of the mouth (Grill and Norgren 1978) requiring the same orofacial musculature used by other species for vomiting (Travers and Norgren 1986). Following pairings with emetic drugs, rats display conditioned gaping reactions to a sweet solution (Grill and Norgren 1978). Unlike traditional intake measures of flavor–drug associations in rats, conditioned gaping reactions are exclusively produced by treatments that produce vomiting in other species and are consistently prevented by anti-emetic treatments, including 5-HT3 antagonists and cannabinoids (see Parker et al. 2009a for review).
Rats not only display conditioned gaping to an illness paired flavor, but they also display conditioned gaping to an illness paired context, in a dose-dependent relation (Limebeer et al. 2008). Contextually elicited conditioned gaping is analogous to the phenomenon of AN in human chemotherapy patients. In this model, rats are injected with an emetic drug, such as lithium chloride (LiCl) before they are placed in a distinctive conditioning chamber. Following three to four pairings, rats repeatedly gape when reintroduced to the context in the absence of LiCl. Interestingly, shrews which vomit in response to an emetic treatment also display a conditioned retching reaction when reintroduced to the context in which they previously vomited (Parker and Kemp 2001; Parker et al. 2006). With the development of animal models of AN, it is possible to evaluate the potential of drug treatments to reduce AN.
Exogenous cannabinoids, such as ∆9-tetrahydrocannabinol (THC) found in the marijuana plant, are effective in suppressing AN in both the rat conditioned gaping model (Limebeer et al. 2006) and in a shrew conditioned retching model (Parker and Kemp 2001; Parker et al. 2006). On the other hand, just as is seen with human chemotherapy patients, ondansetron is ineffective in reducing AN once it has developed (Limebeer et al. 2006; Parker et al. 2006). Currently, AN is most likely treated with nonspecific anti-anxiety drugs (benzodiazepines, such as lorazepam, Malik et al. 1995; Razavi et al. 1993). However, both benzodiazepines and THC have sedating side effects, which may diminish their therapeutic effectiveness.
The endocannabinoid (EC) system has been implicated in control of nausea and vomiting (Parker et al. 2011). Anandamide (AEA) and 2-arachidonyl glycerol (2-AG) are endogenous agonists for cannabinoid G protein-coupled receptors CB1 and CB2 (Devane et al. 1992; Mechoulam et al. 1995), which are rapidly degraded by fatty acid amide hydrolase (FAAH) (Deutsch and Chin 1993) and monoacylglycerol lipase (MAGL) (Dinh et al. 2002; Blankman et al. 2007), respectively. Inhibitors of AEA and 2-AG degradation offer a potentially effective alternative treatment strategy for stimulating the endocannabinoid system (Long et al. 2009a). These selective FAAH and MAGL inhibitors produce a subset of the behavioral responses seen with direct CB1 agonists, including analgesia (Long et al. 2009b; Lichtman et al. 2004; Kinsey et al 2009). Moreover, inhibition of MAGL, but not FAAH, produces hypomotility in mice (Long et al. 2009a). Neither FAAH nor MAGL inhibitors induce the cataleptic behavioral reactions evident with direct CB1 agonists (Kathuria et al. 2003; Long et al. 2009b), and FAAH inhibitors are inactive in animal models of drug abuse (Gobbi et al. 2005).
Rock et al (2008) recently demonstrated that the FAAH inhibitor, URB597 (Fegley et al. 2005), dose dependently suppressed the expression of AN elicited by a LiCl-paired chamber. The suppression of AN was reversed by the CB1 antagonist/inverse agonist, SR141716, which on its own did not modify the strength of the contextually elicited gaping reactions (Rock et al. 2008). Inhibition of FAAH not only elevates AEA, but also other fatty acids including N-palmitoylethanolamine (PEA) and oleoylethanolamide (OEA), which act as agonists of the peroxisome proliferator-activated receptors (PPAR; e.g., Gaetani et al. 2008). However, the finding that SR141716 reversed the suppression indicates a CB1 mechanism of action, most likely due to an elevation of AEA. The MAGL inhibitor, JZL184, which selectively elevated 2-AG, has also been shown to reduce LiCl-induced vomiting in the Suncus murinus in a CB1-dependent manner. Although JZL184 is much less effective in suppressing MAGL in rats (Long et al. 2009b) than in mice or S. murinus (Sticht et al. 2012), exogenous 2-AG interfered with the establishment of LiCl-induced gaping elicited by a flavor in rats, due to the action of downstream metabolites (Sticht et al. 2012). Therefore, both elevated AEA and 2-AG have been shown to reduce conditioned nausea in rats.
Recently, the dual FAAH and MAGL inhibitor, JZL195, has been reported to reduce both enzymes in mouse and rat brains (Long et al. 2009a); albeit, it is equipotent in inhibiting both enzymes in mouse brain tissue, but eightfold more potent in inhibiting FAAH than MAGL in rat brain. Recently, in vivo microdialysis of interstitial AEA and 2-AG levels from the rat nucleus accumbens revealed that JZL195 increased 2-AG and AEA levels approximately 50 and 100 %, respectively, from basal levels (requiring an ionic pulse; Wiskerke et al 2012). In mice, in vivo studies demonstrated that, when compared with selective inhibitors of FAAH or MAGL per se, the dual inhibitor produced a dramatic CB1 receptor-mediated antinociceptive effect, as well as generalized to the THC discriminative cue in the drug discrimination paradigm and elicited catalepsy (Long et al 2009a). The following experiments evaluated the potential of JZL195 alone and in combination with AEA and 2-AG to interfere with AN in the contextually elicited conditioned gaping model in rats and to modify activity level per se. The potential of the CB1 inverse agonist/antagonist SR141716 to reverse the suppression of AN was evaluated as well. Finally, whole brains were removed following the behavioral testing and analyzed for levels of AEA, 2-AG, PEA, OEA, and the metabolic product, arachidonic acid (AA).
Materials and methods
Animals
Animal procedures complied with the Canadian Council on Animal Care, and the protocols were approved by the Institutional Animal Care Committee at University of Guelph. A total of 73 näive male Sprague–Dawley rats, obtained from Charles River Laboratories (St. Constant, Quebec), were used for assessment of AN. They were individually housed in shoebox cages, subjected to an ambient temperature of 21 °C and a 12/12-h light–dark schedule (lights off at 7 am), and maintained on food (Highland Rat Chow [8640]) and water ad libitum. Their body weights ranged from 300 to 440 g on the day of testing for AN.
Drugs
All injections were administered intraperitoneally (ip). Lithium chloride (Sigma-Aldrich) was prepared in a 0.15 M solution with sterile water and was administered at a volume of 20 ml kg–1 (127.2 mg kg–1 dose). The drugs JZL195, AEA, 2-AG (Cayman Chemicals), SR141716 (Sequoia Chemicals, UK), and AM630 (Tocris) were prepared in a 1:1:18 solution of ethanol/Tween 80/physiological saline. The drugs were first dissolved in ethanol and then Tween 80 was added to the solution, and the ethanol was evaporated off with a nitrogen stream after which the saline was added. The final vehicle (VEH) consisted of 1:9 (Tween/saline). At test, all drugs were administered at a volume of 1 ml kg–1. The concentration of JZL195 was 10 mg ml–1, the concentration of AEA was 5 mg ml–1, the concentration of 2-AG was 1.25 mg ml–1, the concentration of SR141716 was 2.5 mg ml–1, and the concentration of AM630 was 3.0 mg ml–1. The dose of JZL195 was selected on the basis of a body surface area dose translation (Reagan-Shaw et al. 2007) from effective mouse doses (Long et al. 2009b). The doses of AEA (Cross-Mellor et al. 2007) and 2-AG (Sticht et al 2012) were selected on the basis of their potential to interfere with the acute nausea produced by LiCl in rats. The dose of SR141716 was selected on the basis of its potential to reverse the suppression of AN by URB597, without producing an effect on AN on its own (Rock et al. 2008). The dose of AM630 was selected on the basis of its potential to block the anti-allodynic effects of FAAH and MAGL inhibition (Guindon et al. 2013).
Biochemical analyses
Extraction and quantification of endocannabinoids by liquid chromatography-tandem mass spectrometry
Rats were sacrificed 2.5 h following vehicle or JZL195 (10, 20, or 40 mg kg–1 ip). Additionally, subjects were given an injection of VEH, AEA (5 mg kg–1 ip), or 2-AG (1.25 mg kg–1 ip) 105 min after vehicle or JZL195. Rats were euthanized by rapid decapitation (restrained in a decapicone—Braintree Scientific, MA, USA), and their brains were harvested, snap frozen in dry ice-cooled isopentane, and stored in a −80 °C freezer until being shipped to Virginia Commonwealth University under dry ice and stored at −80 °C until the time of processing. Tissues were further processed according to methods described previously (Ramesh et al. 2011; Kinsey et al. 2013)
On the day of processing, the pre-weighed rat brains were homogenized with 5 ml chloroform/methanol (2:1 v/v containing 0.0348 g phenylmethylsulfonyl fluoride/ml). One quarter of the homogenate (1.25 ml) was taken and diluted to 1.4 ml with the chloroform/methanol used for homogenization. Internal standards (2 pmol AEA-d8, 1 nmol 2-AG-d8, 3.3 nmol PEA-d4, 3 nmol OEA-d4, and 1 nmol AA-d8) were added to each sample. Homogenates were mixed with 0.3 ml of 0.73 % w/v NaCl, vortexed, and centrifuged for 10 min at 3,220×g (4 °C). The aqueous phase and debris were collected and extracted again twice with 0.8 ml chloroform. The organic phases from the three extractions were pooled, and the organic solvents were evaporated under nitrogen gas. Dried samples were reconstituted with 0.1 ml chloroform and mixed with 1 ml cold acetone. The mixtures were centrifuged for 5 min at 1,811×g (4 °C) to precipitate protein. The upper layer of each sample was collected and evaporated under nitrogen. Dried samples were reconstituted with 0.1 ml methanol and placed in autosample vials for analysis.
LC/MS/MS was used to quantify AEA, 2-AG, PEA, OEA, and AA. The mobile phase consisted of methanol (90:10)/0.1 % ammonium acetate and 0.1 % formic acid. The column used was a Discovery® HS C18, 4.6 × 15 cm, 3 μm (Supelco, USA). Ions were analyzed in multiple reaction monitoring mode and the following transitions were monitored in positive mode: (348 > 62) and (348 > 91) for AEA; (356 > 62) for AEA-d8; (379 > 287) and(279 > 269) for 2-AG; (387 > 96) for 2-AG-d8; (300 > 62) and (300 > 283) for PEA; (304 > 62) for PEA-d4; (326 > 62) and (326 > 309) for OEA; and (330 > 66) for OEA-d4; in negative mode: (303 > 259) and (303 > 59) for AA and (311 > 267) for AA-d8.
A calibration curve was constructed for each assay based on linear regression using the peak area ratios of the calibrators. The extracted standard curves ranged from 1.25 to 400 pmol for AEA, from 1 to 64 nmol for 2-AG, from 0.078 to 1.25 nmol for PEA and OEA, and from 2 to 32 nmol for AA.
Apparatus
The distinctive context utilized for conditioning varied the dimensions of location, visual, and tactile cues from the home cage environment. The room was dark with two 40-W lights on either side of the conditioning chamber. The conditioning chamber was made of black Plexiglas sides (22.5 × 26 × 20 cm) with an opaque lid. The chamber was placed on a table with a clear Plexiglas top. A mirror beneath the chamber on a 45° angle facilitated viewing of the ventral surface of the rat. A Sony videocamera (Handycam, Henry's Camera, Waterloo, ON, Canada) was used to videotape the rats from the mirror beneath the chamber. The videotapes were later scored using “The Observer” event recording software (Noldus, Inc., Netherlands).
The activity chamber was constructed of white Plexiglas with the dimensions of 60 × 25 × 25 cm and located in a different room than the AN chamber illuminated with a red light. A videocamera mounted on an extension pole above the chamber captured the activity of the rat which was sent to a computer for analysis of distance (centimeter) traveled using the Ethovision software program (Noldus, Inc., Netherlands).
Behavioral procedures
Effect of JZL195 alone and co-administered with AEA and 2-AG on AN, locomotor activity, and EC levels
All rats received four conditioning trials, with 72 h between trials. On each conditioning trial, each rat was injected with LiCl and immediately placed in the distinctive context for 30 min. The AN test trial occurred 72 h after the final conditioning trial. On the test trial, the rats were randomly assigned to one of six groups on the basis of treatment 1 (VEH or JZL195) and treatment 2 (VEH, AEA, or 2-AG), with n = 8–9/group. The rats received treatment 1 (VEH or JZL195) 105 min prior to receiving treatment 2 (VEH, AEA, or 2-AG) 15 min prior to placement in the context. The rats remained in the context for 5 min and their orofacial reactions were videotaped from the mirror beneath the chamber. VEH-injected rats display the most contextually elicited gaping reactions during the first 5 min of a longer test trial (Limebeer et al 2008; Rock et al 2008). The videotapes were later scored for the number of gapes (large amplitude openings of the mouth with simultaneous retractions of the corners of the mouth exposing incisors). Immediately following the AN test trial, the rats were placed in the novel activity chamber for 15 min, and their locomotor activity was automatically videotracked. Immediately following the activity trial, the rat brains were removed as previously described.
Role of CB1 receptor: reversal by SR141716
In order to determine the role of the CB1 receptor in the suppression of conditioned gaping, three additional groups were conditioned and tested. Groups JZL195-SR (n = 8) and VEH-SR (n = 8) were injected with JZL195 (10 mg/kg) or VEH 90 min before receiving SR141716 (2.5 mg/kg) and 30 min later were placed in the AN chamber for 5 min. Group SR-AEA (n = 8) was injected with SR141716 (2.5 mg/kg) 15 min prior to receiving AEA (5 mg/kg) and 15 min later was placed in the AN chamber for 5 min. Immediately following the AN test, the rats were placed in the novel activity chamber for 15 min and their locomotor activity was videotracked. For statistical comparison, the number of gapes and distance (centimeter) traveled displayed by each of these groups was compared with groups VEH-VEH, VEH-AEA, and JZL195-VEH.
In experiment 2b, the potential of CB2 antagonism to reverse the suppressive effect of JZL195 on contextually elicited gaping was assessed in a separate group that was administered with JZL195 (10 mg kg–1) 90 min before AM630 (3 mg kg–1) and was placed in the AN chamber 30 min later for 5 min. Immediately following the AN test, they were given the 15-min locomotor test. This group was compared with groups VEH-VEH and JZL195-VEH.
Data analyses
In experiment 1, to determine the potential of the treatments to modify AN, activity, and EC levels, the number of gapes, distance (centimeter) traveled in activity chambers, and amount of whole brain 2-AG, AEA, PEA, OEA, and AA was entered into a 2 (treatment 1: VEH, JZL195) × 3 (treatment 2: VEH, 2-AG, AEA) between-groups analysis of variance (ANOVA). Significant interactions were analyzed: (1) as one-way ANOVAs for each of VEH and JZL195 (treatment 1) across VEH, 2-AG, and AEA (treatment 2) with subsequent least significant difference (LSD) pairwise comparisons indicated by *p < 0.05, **p < 0.01, and ***p < 0.001 and (2) as planned pairwise comparisons of VEH and JZL195 (treatment 1) for each of VEH, 2-AG, and AEA (treatment 2) with significant effects depicted as # p < 0.05, ## p < 0.01, and ### p < 0.001. In experiment 2a, to determine the role the CB1 receptor antagonism, the number of gapes and distance traveled were entered into a single factor ANOVA (groups: VEH-VEH, VEH-SR, VEH-AEA, JZL195-VEH, SR-AEA, and JZL195-SR) with subsequent LSD pairwise comparison tests. As well, in experiment 2b, to determine the effect of CB2 antagonism, the number of gapes and distance traveled were entered into a single factor ANOVA (groups: VEH-VEH, JZL-VEH, and JZL-195-VEH).
Results
Effect of JZL195, alone and co-administered with AEA and 2-AG, on AN, locomotor activity, and EC levels
AN test
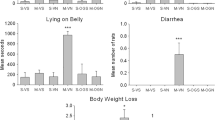
Systemic administration of JZL195 suppressed conditioned gaping elicited by a LiCl-paired context, an effect that was enhanced by co-administration of AEA or 2-AG. Systemic administration of AEA alone, but not 2-AG alone, also suppressed conditioned gaping. Figure 1 presents the mean (±SEM) number of gapes elicited by the LiCl-paired context for each group. The upper section represents the rats given VEH as treatment 1, the lower section represents the rats given JZL195 as the treatment 1, and the abscissa represents the rats given VEH, 2-AG, or AEA as treatment 2. The analysis revealed main effects of treatment 1 (F(1, 43) = 17.96, p < 0.001), treatment 2 (F(2, 43) = 8.19, p = 0.001), and a treatment 1 × treatment 2 interaction (F(2, 43) = 3.29; p = 0.047). Separate one-way ANOVAs revealed significant effects for both the VEH-treated groups (F(2, 22) = 4.83; p = 0.018) and the JZL195-treated groups (F(2, 22) = 4.83; p = 0.012). Among the VEH-treated groups, the rats in group VEH-AEA, but not VEH-2-AG, gaped less than rats in group VEH-VEH (**p < 0.01). Among the JZL195-treated groups, rats in both groups JZL195-AEA (**p = 0.008) and JZL195-2-AG (*p = 0.032) gaped less than group JZL195-VEH. Additionally, group JZL195-VEH showed suppressed gaping relative to VEH-VEH (# p = 0.016), and group JZL195-2-AG showed less gaping than VEH-2-AG (## p = 0.002).
Mean (±SEM) number of gapes elicited by the LiCl-paired context during the 5-min test by rats in the various groups. The top half of the figure presents the groups treated with VEH 2 h prior to the AN test, and the bottom half of the figure presents the groups treated with JZL195 2 h prior to the AN test. The abscissa depicts the groups treated with VEH, AEA, or 2-AG 15 min prior to the AN test. Asterisks (*) signify significant differences when the VEH pretreatment groups and the JZL195 pretreatment groups are analyzed separately. The number signs (#) signify significant differences between VEH and JZL195 when each treatment 2 group (VEH, AEA, and 2-AG) is analyzed separately *, # p < 0.05; **, ## p < 0.01; ***, ### p < 0.001
Locomotor test
Anandamide increased locomotor activity in the 15-min test that followed the AN test. Figure 2 presents the mean distance (centimeter) traveled during the 15-min locomotor activity test that immediately followed the AN test. The overall analysis revealed a significant effect of treatment 2 (F(2, 43) = 15.23; p < 0.001) and a treatment 1 × treatment 2 interaction (F(2, 43) = 3.29; p = 0.047). Separate one-way ANOVAS revealed significant effects for both the VEH-treated groups (F(2, 21) = 8.59; p = 0.002) and the JZL195-treated groups (F(2, 22) = 9.86; p < 0.001). Among both the VEH- (*p < 0.05) and JZL195- (**p < 0.01) treated groups, rats subsequently treated with AEA were more active than any other group. The rats in group JZL195-VEH displayed reduced locomotion relative to rats in group VEH-VEH (# p = 0.046) in agreement with Long et al. (2009a, b) as well.
Mean (±SEM) distance (centimeter) traveled during the 15-min locomotor activity test that immediately followed the AN test. The top half of the figure presents the groups treated with VEH 2 h prior to the AN test, and the bottom half of the figure presents the groups treated with JZL195 2 h prior to the AN test. The abscissa depicts the groups treated with VEH, AEA, or 2-AG 15 min prior to the AN test. Asterisks (*) signify significant differences when the VEH pretreatment groups and the JZL195 pretreatment groups are analyzed separately. The number signs (#) signify significant differences between VEH and JZL195 when each treatment 2 group (VEH, AEA, and 2-AG) is analyzed separately. *, # p < 0.05; **, ## p < 0.01; ***, ### p < 0.001
Biochemical analysis
The results of the analyses of whole brains collected immediately after the 20-min behavioral testing for 2-AG, AEA, PEA, OEA, and AA are presented in Table 1.
2-AG
2-AG levels were not elevated by JZL195 alone, but were elevated when JZL was combined with either 2-AG or AEA. Neither exogenous 2-AG nor AEA alone produced elevated 2-AG levels. The overall analysis revealed a significant main effect of treatment 1 (F(1, 43) = 34.47; p < 0.001), treatment 2 (F(2, 43) = 4.45; p = 0.18), and a treatment 1 × treatment 2 interaction (F(2, 43) = 6.15; p = 0.004). Separate one-way ANOVAs revealed that only the JZL195-treated groups differed significantly (F(2, 22) = 5.71; p = 0.01), which was the result of group JZL195-AEA showing enhanced 2-AG relative to JZL195-VEH (***p < 0.001) or JZL195-2-AG (*p = 0.018). Groups JZL195-2-AG and JZL195-AEA had higher levels of 2-AG than groups VEH-2-AG (## p < 0.010) and VEH-AEA (### p < 0.001), respectively. However, the 2-AG levels of group JZL195-VEH did not significantly differ from group VEH-VEH (p = 0.093). This latter finding prompted us to evaluate the potential of higher doses (20 and 40 mg kg–1) of JZL195 to elevate 2-AG levels under similar temporal conditions as the experiments above. With an n = 2/group, the mean level of 2-AG (nanomoles per gram) produced by the higher doses of JZL195 was VEH (7.322), 20 mg kg–1 JZL195 (8.112), and 40 mg kg–1 (9.135), indicating that even these higher doses administered in vivo to rats did not elevate 2-AG whole brain levels.
AEA
JZL195 alone elevated the AEA levels, and this effect was dramatically enhanced by its combination with exogenous AEA. The overall analysis revealed a significant main effect of treatment 1 (F(1, 43) = 23.78; p < 0.001), treatment 2 (F(2, 43) = 16.30; p < 0.001), and a treatment 1 × treatment 2 interaction (F(2, 43) = 16.06; p < 0.001). Separate one-way ANOVAs revealed a significant effect among VEH (F(2, 21) = 4.36; p = 0.026) and JZL195 (F(2, 22) = 16.73; p < 0.001) treatment 1 groups; exogenous administration of AEA elevated brain AEA in both VEH-AEA (*p < 0.05) and JZL195-AEA (***p < 0.001) relative to other groups. As well, comparison tests of VEH and JZL195 treated rats showed that JZL195 significantly elevated AEA levels in rats subsequently treated with VEH, 2-AG, or AEA (###ps < 0.001).
PEA and OEA
JZL195 elevated levels of both PEA and OEA overall, an effect that did not vary by treatment 2. The overall analysis revealed only a significant main effect of treatment 1 for levels of both PEA (F(1, 43) = 219.23; p < 0.001) and OEA (F(1, 43) = 204.3; p < 0.001).
AA
Among the JZL195-treated animals, but not the VEH-treated animals, subsequent treatment with either AEA or 2-AG reduced levels of AA, but JZL195 treatment alone did not modify levels of AA. The overall analysis revealed a significant main effect of treatment 2 (F(2, 43) = 10.7; p < 0.001) and an interaction of treatment 1 × treatment 2 that approached statistical significance (F(2, 43) = 2.9; p = 0.06). Separate one-way ANOVAS revealed that the JZL195-treated groups differed (F(2, 22) = 9.3; p < 0.001), following pretreatment with JZL195, exogenous administration of AEA (p = 0.001), or 2-AG (p = 0.003) reduced AA levels relative to VEH. As well, groups JZL195-AEA (p = 0.011) and JZL195-2-AG (p = 0.022) had lower levels of AA than groups VEH-AEA and VEH-2-AG, respectively.
Role of CB1 receptor: reversal by SR141716
The CB1 inverse agonist/antagonist, SR141716, reversed the suppressive effect of both JZL 195 and AEA on AN in the gaping model and the enhancement of locomotor activity produced by AEA in the activity test. A single factor ANOVA for the gaping measure revealed a significant main effect of treatment (F(5, 43) = 3.6; p = 0.009). As is evident in Fig. 3, groups VEH-AEA and JZL195-VEH (*ps < 0.025) displayed significantly less gaping than any other group. A single factor ANOVA for the distance traveled measure also revealed a significant main effect of treatment (F(5, 43) = 4.6; p = 0.002); as shown in Fig. 4, group VEH-AEA was more active than all other groups (ps < 0.05), including SR-AEA (p < 0.001).
CB2 antagonism did not reverse the suppressive effect of JZL195. The single factor ANOVA for gaping revealed a significant main effect of treatment (F(2, 22) = 6.7; p = 0.005; Group VEH-VEH (mean = 22.7 ± 6.6) displayed significantly (p < 0.01) more gaping than either group JZL195-VEH (mean = 5.3 ± 1.5) or JZL195-AM630 (mean = 4.6 ± 1.8). There were no significant differences in the locomotor test.
Discussion
As has previously been demonstrated with the FAAH inhibitor, URB597 (Rock et al 2008), pretreatment with the dual FAAH/MAGL inhibitor, JZL195 (10 mg kg–1), reduced the contextually elicited LiCl-induced conditioned gaping in rats. This effect was CB1 dependent because pretreatment with the CB1 antagonist, SR141716, but not the CB2 antagonist, AM630, reversed the suppressive effect of JZL195. Pretreatment with exogenous doses of AEA (5 mg kg–1) and 2-AG (1.25 mg kg–1), which have previously been shown to interfere with the establishment of nausea-induced gaping to a flavor cue (Cross-Mellor et al. 2007; Sticht et al. 2012), revealed that only AEA reduced AN. This effect was also reversed by pretreatment with SR141716. This finding suggests that the primary suppressive effect on contextually elicited gaping may be mediated by the inhibition of FAAH, rather than MAGL. However, the action of MAGL is also indicated because when combined with either AEA or 2-AG, the suppressive effect of JZL195 on AN was enhanced.
Evaluation of whole brain endocannabinoid levels collected following the AN and activity tests revealed that JZL195 on its own elevated the fatty acids, AEA, OEA, and PEA, but not 2-AG. The effect was most likely the result of AEA and not OEA or PEA which act as agonists of the α-PPAR receptor (e.g., Gaetani et al. 2008) because the suppressive effect of JZL195 on AN was reversed by CB1 antagonism. Even when the dose of JZL195 was increased to 40 mg kg–1, it did not elevate 2-AG relative to VEH. However, whole brain levels of AEA and 2-AG measured in the present study may not reflect the concentrations of these endogenous cannabinoids at key CB1 receptors that mediate the blockade of conditional gaping produced by JZL195. For instance, Wiskerke et al (2012) found that JZL195 elevated 2-AG and AEA levels in the nucleus accumbens about 50 and 100 %, respectively.
When co-administered with either AEA or 2-AG, JZL195 elevated the respective EC. The suppression of LiCl-induced conditioned gaping by JZL195 was enhanced when it was combined with either AEA or 2-AG as well. Indeed, group JZL195-AEA displayed approximately a 75-fold increase in AEA relative to group VEH-AEA. Curiously, administration of exogenous anandamide to JZL195-treated rats also resulted in about a twofold increase in 2-AG brain levels. These findings suggest that JZL195 prevented the catabolism of the exogenously delivered AEA and concomitantly elevated 2-AG levels by possibly enhancing synthesis or interfering with catabolism of this endogenous cannabinoid. Similarly, group JZL195-2-AG elevated 2-AG by threefold relative to group VEH-2-AG; however, group JZL195-VEH did not differ from JZL195-2-AG. Collectively, this pattern of findings suggests that JZL195 may be more effective in inhibiting FAAH in the whole rat brain, but MAGL inhibition is also indicated. High bulk levels of 2-AG could mask detection of local changes in 2-AG when endocannabinods are measured in whole brain samples. Therefore, a role for MAGL cannot be discounted on the basis of measurement of endocannabinoid content alone.
The locomotor activity test revealed that exogenously administered AEA produced slightly elevated activity level relative to VEH or 2-AG, regardless of JZL195 or VEH pretreatment. Since the effects of AEA on motor activity are biphasic, with low doses elevating activity and high doses suppressing activity (e.g., Sulcova et al. 1998), these results suggest that at least by the time of the locomotor test (approximately 20 min post-AEA injection), the brain concentration of AEA was similar to that of rats experiencing a low dose of AEA. These results may also reflect an anxiolytic effect of anandamide, indicated by increased exploration of an open field. The suppressed gaping produced by AEA was therefore not merely a function of decreased sensorimotor responding. The elevated locomotor activity by AEA was reversed by SR141716, indicating that it was a CB1-mediated effect. As well, in experiment 1, group JZL195-VEH displayed slightly suppressed locomotor activity relative to VEH-VEH consistent with the findings of Long et al. (2009a).
The present findings confirm previous results indicting that FAAH inhibition reduces contextually elicited conditioned gaping (Rock et al. 2008) in rats as it also reduces acute nausea (Cross-Mellor et al. 2007) and vomiting in shrews (Parker et al. 2009b) and ferrets (van Sickle et al. 2005; Sharkey et al. 2007). Although inhibition of FAAH elevates multiple endocannabinoid-like molecules that show activity at multiple target receptors, the anti-emetic effects (similar to the anti-nausea effects reported here) were reversed by pretreatment with SR141716, indicating a CB1 mechanism of action (e.g., see Parker et al. 2011).
The suppression of contextually elicited conditioned gaping by JZL195 is not likely simply the result of impaired retention of a previously learned response. FAAH inhibition by OL-135 and genetic deletion of FAAH in mice enhanced both acquisition and extinction of spatial learning in a fixed platform water maze task, and this enhancement was blocked by SR141716 (Varvel et al. 2007). The FAAH inhibitor URB597 also enhanced passive avoidance learning (Mazzola et al. 2009) when given prior to conditioning, but not when given prior to testing (as is the case in the present studies). The enhancement of learning by FAAH deletion appears to be selective to aversive tasks; in rewarding tasks, no effects on learning are detected (Wise et al. 2009). Although Wise et al (2012) found that JZL195 disrupted short-term spatial memory in a water maze in mice, they also demonstrated elevation of both 2-AG and AEA, unlike our findings in rats. Thus, it is unlikely that the suppression of gaping elicited by the LiCl-paired context among the JZL195-treated animals was the result of a drug-induced deficit in reference memory.
As new inhibitors are developed that are more effective in suppression of MAGL in rats, there may be the opportunity to better evaluate the role of 2-AG in regulation of nausea in these rat models. We have previously shown that exogenous 2-AG suppresses acute nausea in rats (Sticht et al. 2012), but the effect was mediated by downstream metabolites of 2-AG, because (1) the suppression of LiCl-induced gaping was not reversed by the CB1 antagonist AM251, and (2) the effect was reversed by the COX inhibitor, indomethacin. The MAGL inhibitor, JZL184, which is relatively ineffective in suppressing MAGL in rat tissue (Long et al. 2009b), was highly effective in suppressing vomiting by a CB1 mechanism of action and in suppressing MAGL in the tissue of the S. murinus (Sticht et al. 2012). Indeed, when the catabolism of exogenous 2-AG was prevented by co-administration of the MAGL inhibitor, JZL184, the suppression of acute nausea in rats by 2-AG was reversed by a CB1 antagonist. Although 2-AG has anti-emetic effects in ferrets (Van Sickle et al 2005) and house musk shrews (Sticht et al. 2012, 2013), it appears to be pro-emetic in the least shrew (Darmani 2002; Darmani et al. 2005), indicating species differences in the role of the endocannabinoid system in the regulation of nausea and vomiting.
The development of treatments which boost the activity of the “on demand” endocannabinoid system may have promise in the regulation of both acute and anticipatory nausea in chemotherapy treatment of cancer. The treatment of AN is of particular interest, given that no selective treatments are currently available for this debilitating side effect of cancer treatment.
References
Akechi T, Okuyama T, Endo C, Sagawa R, Uchida M, Nakaguchi T et al (2010) Anticipatory nausea among ambulatory cancer patients undergoing chemotherapy: prevalence, associated factors, and impact on quality of life. Cancer Sci 101:2596–2600
Blankman JL, Simon GM, Cravatt BF (2007) A comprehensive profile of brain enzymes that hydrolyze the endocannabionoid 2-arachidonoylglycerol. Chem Biol 14:1347–1356
Cross-Mellor SK, Ossenkopp KP, Piomelli D, Parker LA (2007) Effects of the FAAH inhibitor, URB597, and anandamide on lithium-induced taste reactivity responses: a measure of nausea in the rat. Psychopharmacology (Berl) 190:135–143
Darmani NA (2002) The potent emetogenic effects of the endocannabinoid, 2-AG (2 arachidonoylglycerol) are blocked by delta (9)-tetrahydrocannabinol and other cannabinoids. J Pharmacol Exp Ther 300:34–42
Darmani NA, McClanahan BA, Trinh C, Petrosino S, Valenti M, DiMarzo V (2005) Cisplatin increases brain 2-arachidonoylglycerol (2-AG) and concomitantly reduces intestinal 2-AG and anandamide levels in the least shrew. Neuropharmacology 49:502–513
Deutsch DG, Chin SA (1993) Enzymatic synthesis and degradation of anandamide, a cannabinoid receptor agonist. Biochem Pharmacol 46:791–796
Devane WA, Hanus L, Bruer A, Pertwee RG, Stevenson LA, Griffin G et al (1992) Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 258:1946–1949
Dinh TP, Carpenter D, Leslie FM, Freund TF, Katona I, Sensi SL et al (2002) Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc Natl Acad Sci U S A 99:10819–10824
Fegley D, Gaetani S, Duranti A, Tontini A, Mor M, Tarzia G et al (2005) Characterization of the fatty acid amide hydrolase inhibitor cyclohexyl ceramic acid 3′-carbamolyl-biphenyl-3-yl ester (URB597): effects on anandamide and oleoylethanolamide deactivation. J Pharmcol exp Ther 313:352–358
Foubert J, Vaessen G (2005) Nausea: the neglected symptom? Eur J ONcol Nurs 9:21–32
Gaetani S, Kaye WH, Cuomo V, Piomelli D (2008) Role of endocannabinoids and their analogues in obesity and eating disorders. Eat Weight Disorders 13:e42–e48
Garcia J, Hankins WG, Rusiniak KW (1974) Behavioral regulation of the milieu interne in man and rat. Science 185:824–831
Gobbi G, Bambico FR, Mangieri R, Bortolato M, Campolongo P, Solinas M et al (2005) Antidepressant-like activity and modulation of brain monoaminergic transmission by blockade of anandamide hydrolysis. Proc Natl Acad Sci U S A 102:18620–18625
Grill HJ, Norgren R (1978) The taste reactivity test. I. mimetic responses to gustatory stimuli in neurologically normal rats. Brain Res 143:263–279
Guindon J, Lai Y, Takacs SM, Bradshaw HB, Hohmann AG (2013) Alterations in endocannabinoid tone following chemotherapy-induced peripheral neuropathy: effects of endocannabinoid deactivation inhibitors targeting fatty-acid amide hydrolase and monoacylglycerol lipase in comparison to reference analgesics following cisplatin treatment. Pharm Res 67:94–109
Kathuria S, Gaetani S, Fegley D, Valino F, Duranti A, Tontini A et al (2003) Modulation of anxiety through blockade of anandamide hydrolysis. Nat Med 9:76–81
Kinsey SG, Long JZ, O’Neal ST, Abdullah RA, Poklis JL, Boger DL, Cravatt BF, Lichtman AH (2009) Blockade of endocannabinoid-degrading enzymes attenuates neuropathic pain. J Pharmacol Exp Ther 330:902–910
Kinsey SG, Wise LE, Ramesh D, Abdullah RA, Selley DE, Cravatt BF, Lichtman AH (2013) Repeated low dose administration of the monoacylglycerol lipase inhibitor JZL184 retains CB1 receptor mediated antinociception and gastroprotective effects. J of Pharmacol Exp Ther 345:492–501
Lichtman AH, Leung D, Shelton CC, Saghatelian A, Hardouin C, Boger DL, Cravatt BF (2004) Reversible inhibitors of fatty acid amide hydrolase that promote analgesia: evidence for an unprecedented combination of potency and selectivity. J Pharmacol Exp Ther 311:441–448
Limebeer CL, Hall G, Parker LA (2006) Exposure to a lithium-paired context elicits gaping in rats: a model of anticipatory nausea. Physiol Behav 88:398–403
Limebeer CL, Krohn JP, Cross-Mellor S, Litt DE, Ossenkopp KP, Parker LA (2008) Exposure to a context previously associated with nausea elicits conditioned gaping in rats: a model of anticipatory nausea. Behav Brain Res 187:33–40
Long JZ, Nomura DK, Vann RE, Walentiny DM, Booker L, Jin X et al (2009a) Dual blockade of FAAH and MAGL identifies behavioral processes regulated by endocannabinoid crosstalk in vivo. Proc Natl Acad Sci U S A 106:20270–20275
Long JZ, Li W, Booker L, Burston JJ, Kinsey SG, Schlosburg JE et al (2009b) Selective blockade of 2-arachidonoylglycerol hydrolysis produces cannabinoid behavioral effects. Nat Chem Biol 5:37–44
Malik IA, Khan WA, Qazilbash M, Ata E, Butt A, Khan MA (1995) Clinical efficacy of lorazepam in prophylaxis of anticipatory, acute, and delayed nausea and vomiting induced by high doses of cisplatin. A prospective randomized trial. Am J Clin Oncol 18L:170–175
Mazzola D, Medalie J, Scherma M, Panlilio LV, Solinas M, Tanda G et al (2009) Fatty acid amide hydrolase (FAAH) inhibition enhances memory acquisition through activation of PPAR-alpha nuclear receptors. Learn Mem 16:332–337
Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR et al (1995) Identification of an endogenous 2-monoglyceride, present in canine gut that binds to cannabinoid receptors. Biochem Pharmacol 50:83–90
Morrow GR, Roscoe JA, Kirshner JJ, Hynes HE, Rosenbluth RJ (1998) Anticipatory nausea and vomiting in the era of 5-HT3 antiemetics. Support Care Cancer 6:244–247
Neese R, Carli T, Curtis GC, Kleinman PD (1980) Pretreatment nausea in cancer chemotherapy: a conditioned response? Psychosom Med 42:33–36
Parker LA, Kemp SW (2001) Tetrahydrocannabinol (THC) interferes with conditioned retching in Suncus murinus: an animal model of anticipatory nausea and vomiting (ANV). Neuroreport 12:749–751
Parker LA, Kwiatkowska M, Mechoulam R (2006) Delta-9-tetrahydrocannabinol and cannabidiol, but not ondansetron, interfere with conditioned retching reactions elicited by a lithium-paired context in Suncus murinus: an animal model of anticipatory nausea and vomiting. Physiol Behav 87:66–71
Parker LA, Limebeer CL, Rana SA (2009a) Conditioned disgust, but not conditioned taste avoidance, may reflect conditioned nausea in rats. In: Reilly S, Schachtman TR (eds) Conditioned taste aversions: behavioral and neural processes. Oxford University Press, New York
Parker LA, Limebeer CL, Rock EM, Litt DL, Kwiatkowska M, Piomelli D (2009b) The FAAH inhibitor URB-597 interferes with cisplatin- and nicotine- induced vomiting in the Suncus murinus (house musk shrew). Physiol Behav 97:121–124
Parker LA, Rock EM, Limebeer CL (2011) Regulation of nausea and vomiting by cannabinoids. Br J Pharmacol 163:1411–1422
Ramesh D, Ross G, Schlosburg JE, Owens RA, Abdullah RA, Kinsey SG, Long JZ, Nomura DK, Sim-Selley LJ, Cravatt BF, Akbarali HI, Lichtman AH (2011) Blockade of endocannabinoid hydrolytic enzymes attenuates precipitated opioid withdrawal symptoms in mice. J Pharmacol Exp Ther 339:173–185
Razavi D, Delvaux N, Farvacques C, DeBrier F, Van Heer C, Kaufman L et al (1993) Prevention of adjustment disorders and anticipatory nausea secondary to adjuvant chemotherapy: a double-blind, placebo-controlled study assessing the usefulness of alprazolam. J Clin Oncol 11:1384–1390
Reagan-Shaw S, Nihal M, Ahmad N (2007) Dose translation from animal to human studies revisited. FASEB 22:659–662
Rock EM, Limebeer CL, Mechoulam R, Piomelli D, Parker LA (2008) The effect of cannabidiol and URB597 on conditioned gaping (a model of nausea) elicited by a lithium-paired context in the rat. Psychopharmacology (Berl) 196:389–395
Sharkey KA, Cristino L, Oland LD, Van Sickle MD, Starowicz K, Pittman QJ et al (2007) Arvanil, anandamide and N-arachdonolyl-dopamine (NADA) inhbiti emesis through cannabinoid CB1 and vanilloid TRPV1 receptors in the ferret. Eur J Neurosci 25:2773–2782
Sticht MA, Rock EM, Parker LA (2013) 2-Arachidonoylglycerol (2-AG) interferes with Lithium Chloride induced vomiting in the house musk shrew, Suncus murinus. Physiol Behav, 120C:228-232
Sticht MA, Long JZ, Rock EM, Limebeer CL, Mechoulam R, Cravatt BF, Parker LA (2012) Inhibition of monoacylglycerol lipase attenuates vomiting in Suncus murinus and 2-arachidonoyl glycerol attenuates nausea in rats. Br J Pharmacol 165:2425–2435
Sulcova E, Mechoulam R, Fride E (1998) Biphasic effects of anandamide. Pharmacol Biochem Behav 59:347–352
Travers JB, Norgren R (1986) Electromyographic analysis of the ingestion and rejection of sapid stimuli in the rat. Behav Neurosci 100:544–555
Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K et al (2005) Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science 310:329–332
Varvel SA, Wise LE, Niyuhire F, Cravatt BF, Lichtman AH (2007) Inhibition of fatty-acid amide hydrolase accelerates acquisition and extinction rates in a spatial memory task. Neuropsychopharm 32:1032–1041
Wise LE, Harloe JP, Lichtman AH (2009) Fatty acid amide hydrolase (FAAH) knockout mice exhibit enhanced acquisition of an aversive, but not of an appetitive Barnes task. Neurobiol Learn Mem 92:597–601
Wise LE, Long KA, Abdullah RA, Long JZ, Cravatt BF, Lichtman AH (2012) Dual fatty acid amide hydrolase and monoacylglycerol lipase blockade produces THC-like Morris water maze deficits in mice. ACS Chem Neurosci 3:369–378
Wiskerke J, Irimia C, Cravatt BF, De Vries TJ, Schoffelmeer AN, Pattij T, Parsons LH (2012) Characterization of the effects of reuptake and hydrolysis inhibition on interstitial endocannabinoid levels in the brain: an in vivo microdialysis study. ACS Chem Neurosci 3:407–417
Acknowledgments
This study was supported by a research grant from the Natural Sciences and Engineering Council of Canada to L. Parker.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Limebeer, C.L., Abdullah, R.A., Rock, E.M. et al. Attenuation of anticipatory nausea in a rat model of contextually elicited conditioned gaping by enhancement of the endocannabinoid system. Psychopharmacology 231, 603–612 (2014). https://doi.org/10.1007/s00213-013-3282-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-013-3282-7