Abstract
Rationale
Anticipatory nausea (AN) experienced by chemotherapy patients is resistant to current anti-nausea treatments. In this study, the effect of manipulation of the endocannabinoid (EC) system on a rat model of nausea (conditioned gaping) was determined.
Objective
The potential of cannabidiol (CBD) and the fatty acid amide hydrolase (FAAH) inhibitor, URB597 (URB) to reduce conditioned gaping in rats were evaluated.
Materials and methods
In each experiment, rats received four conditioning trials in which they were injected with lithium chloride immediately before placement in a distinctive odor-laced context. During testing, in experiment 1, rats were injected with vehicle (VEH), 1, 5 or 10 mg/kg CBD 30 min before placement in the context previously paired with nausea and in experiment 2, rats were injected with VEH, 0.1 or 0.3 mg/kg URB 2 h before placement in the context. Additional groups evaluated the ability of the CB1 antagonist/inverse agonist, SR141716A, to reverse the suppressive effects of URB. Experiment 3 measured the potential of URB to interfere with the establishment of conditioned gaping.
Results
When administered before testing, CBD (1 and 5, but not 10 mg/kg) and URB (0.3, but not 0.1 mg/kg) suppressed conditioned gaping. The effect of URB was reversed by pre-treatment with the CB1 antagonist/inverse agonist, SR141716A. When administered before conditioning, URB also interfered with the establishment of conditioned gaping.
Conclusions
Manipulations of the EC system may have therapeutic potential in the treatment of AN.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chemotherapy-induced nausea and vomiting can be classified into three categories: (1) acute onset, occurring within 24 h of the initial chemotherapy administration; (2) delayed onset, occurring 24 h to several days after the initial treatment; and (3) anticipatory nausea and vomiting (Jordan et al. 2005). Anticipatory nausea (AN) develops in response to chemotherapy treatments, in which cytotoxic drugs are delivered in the presence of a novel context (hospital sights, sounds, smells and tastes). Developing in approximately 30% of patients by their fourth treatment (Morrow et al. 1998; Aapro 2005), AN has traditionally been understood in terms of classical conditioning. After one or more treatment sessions, a conditional association develops between the distinctive contextual cues of the treatment environment (conditional stimuli; CS) and the unconditioned stimulus (US) of chemotherapy treatment that results in the unconditioned response (UR) of post-treatment illness experienced by the patient. Subsequent exposure to the treatment environment (CS) results in the patient experiencing a conditioned response (CR) of nausea and/or vomiting before initiation of chemotherapy treatment. Once it develops, AN has been reported to be especially refractive to anti-emetic treatment (Morrow et al. 1998; Foubert and Vaessen 2005).
The evaluation of potential treatments for AN would be accelerated by the establishment of a reliable rodent model of nausea. Although rats are incapable of vomiting, they display characteristic gaping reactions (which may reflect nausea) when exposed to a flavoured solution (see Parker 2003; Limebeer et al. 2006) previously paired with lithium-induced nausea. In fact, this gaping reaction in the rat requires the same orofacial musculature as that required for vomiting in other species (Travers and Norgren 1986). Only drugs that produce emesis in species capable of vomiting produce conditioned gaping in rats, although many non-emetic drugs produce conditioned taste avoidance (see Parker 2003 for review). Furthermore, anti-emetic drugs interfere with the establishment of conditioned gaping reactions elicited by a nausea-paired flavor, presumably by interfering with the nausea (Limebeer and Parker 2000; Parker et al. 2002, 2003; Parker and Limebeer 2006). Conditioned gaping in rats appears to be a selective index of conditioned nausea.
Not only are flavor cues capable of eliciting conditioned gaping reactions when paired with lithium chloride (LiCl)-induced nausea in rats, but recently Limebeer et al. (2006, 2007) have demonstrated that re-exposure to LiCl-paired contextual cues also elicit conditioned gaping reactions in rats. This paradigm more closely resembles that reported to produce AN in chemotherapy patients. Rats were injected with LiCl (group paired) or saline (group unpaired) before placement in a vanilla-odor laced chamber with lights and texture different than their home cage on each of four trials, separated by 72 h. To equate both groups for experience with illness, the rats in group unpaired were injected with LiCl and those in group paired were injected with saline 24 h after each conditioning trial but were then simply returned to their home cage. When the rats were returned to the conditioning context, 72 h after the final conditioning trial, rats in group paired showed the conditioned gaping reaction, as a measure of AN.
Although classical anti-emetic treatments such as the 5-hydroxytryptamine-3 (5-HT3) antagonist, Ondansetron (OND), effectively reduce unconditioned nausea and vomiting, they are ineffective in the alleviation of conditioned nausea once it develops in humans (Morrow et al. 1998). Indeed, OND also did not suppress the conditioned gaping reactions displayed during re-exposure to the LiCl-paired context (Limebeer et al. 2006). Furthermore, using the emetic species, Suncus murinus (house musk shrew) as an animal model for AN, pre-treatment with a dose of OND that was shown to alleviate acute vomiting (Kwiatkowska et al. 2004; Parker et al. 2004b), did not reduce the display of conditioned retching reactions during re-exposure to a nausea-paired context (Parker et al. 2006). Thus, although OND has proven effective in the reduction of acute post-treatment nausea and vomiting, it does not appear to relieve conditioned nausea when it does develop.
The endocannabinoid (EC) system has been implicated in control of nausea and vomiting (Kwiatkowska and Parker 2005). The psychoactive component in marijuana—delta-9-tetrahydrocannabinol (Δ9-THC)—has been shown to interfere with the expression of vomiting in shrews and ferrets (Darmani 2001; Parker et al. 2004b; Van Sickle et al. 2001) and conditioned gaping reactions elicited by a lithium-paired flavor in rats (Limebeer and Parker 1999). The Δ9-THC-induced suppression of conditioned nausea could be reversed by a CB1 receptor antagonist/reverse agonist (SR141716A), implicating the CB1 receptor in this effect (Parker et al. 2003). Limebeer et al. (2006) demonstrated that, unlike OND, Δ9-THC also interfered with the expression of conditioned gaping elicited by the LiCl paired contextual cues in rats. This finding was consistent with the demonstration that Δ9-THC, unlike OND, also suppressed the expression of conditioned retching in shrews when returned to a LiCl-paired context (Parker et al. 2006).
The primary non-psychoactive compound found in marijuana, cannabidiol (CBD), has also been shown to suppress nausea and vomiting. In shrews, vomiting elicited by LiCl is suppressed by low doses (5–10 mg/kg) of CBD, while higher doses (20–40 mg/kg) were found to facilitate vomiting, rather than reducing its expression (Parker et al. 2004b). Additionally, CBD reduced conditioned retching in shrews elicited by a lithium-paired context (Parker et al. 2006). In rats, a dose of 5 mg/kg CBD interfered with the establishment of conditioned gaping elicited by a LiCl-paired flavor, as well as its expression (Parker et al. 2002; Parker and Mechoulam 2003). Because CBD has not been shown to bind with known CB receptors, this suppression of nausea and vomiting does not appear to be linked to activity of the CB1 or CB2 receptors (Kwiatkowska et al. 2004). These results suggest that the primary nonpsychoactive compound found in marijuana may be a useful treatment for conditioned nausea.
Arachidonylethanolamide or anandamide (AEA) is an endogenous agonist for cannabinoid receptors (Devane et al. 1992) which is rapidly degraded by the fatty acid amide hydrolase (FAAH) (Deutsch and Chin 1993) that is distributed throughout the brain and periphery (Piomelli 2003). The action of AEA can be prolonged by inhibiting its degradation, through the use of URB597 (URB), an FAAH enzyme inhibitor, that can increase basal levels of AEA in the rat brain (Fegley et al. 2005). URB administration has been shown to reduce the establishment of conditioned gaping elicited by LiCl-paired saccharin solution in rats (Cross-Mellor et al. 2007). Thus, prolonging the action of AEA with the FAAH inhibitor URB has been shown to suppress the establishment of conditioned nausea in rats.
The experiments described below evaluated the potential of the non-psychoactive component of marijuana, CBD, and the FAAH inhibitor, URB, to interfere with conditioned gaping elicited by a LiCl-paired chamber in rats. On each of four conditioning trials, rats were injected with LiCl-paired (127 mg/kg, intraperitoneally (ip)) immediately before placement in a distinctive context laced with the odor of vanilla extract. After the conditioning trials, the rats were injected with CBD (experiment 1) or URB (experiment 2) before placement in the distinctive CS context. Additionally, experiment 2 evaluated the potential of the CB1 antagonist/inverse agonist, SR141716A (SR), to reverse the suppression of conditioned gaping produced by URB. Finally, in experiment 3, the ability of URB to interfere with the establishment of conditioned gaping was also assessed. In each experiment, to ensure that the suppression of conditioned gaping was not merely an artefact of drug-induced suppression of general activity, the number of seconds that the rats remained immobile (no body movement) was recorded as a measure of activity.
The doses of CBD (1, 5 and 10 mg/kg) were selected on the basis of our previous work (Parker et al. 2004b) demonstrating that these lower doses suppressed lithium-induced vomiting in the shrew, but higher doses (20–40 mg/kg) potentiated vomiting. Furthermore, a dose of 5 mg/kg of CBD interfered with the establishment and the expression of conditioned gaping elicited by a lithium-paired flavour (Parker et al. 2002). The doses of URB (0.1 and 0.3 mg/kg) were chosen based upon results showing that in vivo FAAH activity is blocked with a half-maximal inhibitory dose (ID50) of 0.15 mg/kg ip with concurrent increase in brain AEA (Kathuria et al. 2003; Fegley et al. 2005). Additionally, a dose of 0.3 mg/kg has been shown to attenuate the establishment of conditioned gaping elicited by a flavored stimulus (Cross-Mellor et al. 2007).
Materials and methods
Subjects
The subjects were male Sprague-Dawley rats (experiment 1) and Long-Evans rats (experiments 2 and 3; Charles River Lab, St Constant, Quebec). The change in strain across experiments coincided with a change in laboratory locations. There were no strain differences in baseline measures. The animals were group-housed in Plexiglas cages in the colony room at an ambient temperature of 21°C with a 12/12 light dark schedule (lights off at 8 AM) and were maintained on an ad libitum schedule of food and water. All procedures adhered to the guidelines of the Canadian Council of Animal Care and were approved by the Animal Care Committee of University of Guelph.
Drugs
Lithium chloride (LiCl) was prepared in a 0.15-M solution with sterile water and was administered intraperitoneally (ip) at a volume of 20 ml/kg (127.2 mg/kg). CBD, URB and SR were prepared in a vehicle of 45% 2-hydroxypropyl-β-cyclodextrin with sterile water. CBD was prepared at concentrations of 1 mg/2 ml, 5 mg/2 ml and 10 mg/4 ml which were each administered at a volume of 4 ml/kg. SR was prepared at a concentration of 1.25 mg/ml and was injected at a volume of 2 ml/kg (2.5 mg/kg). URB was prepared at a concentration of 0.1 and 0.3 mg/ml and was administered at a volume of 1 ml/kg (0.1 and 0.3 mg/kg).
Conditioning context
The distinctive context utilized for conditioning varied the dimensions of location, visual, tactile and olfactory cues from the home cage environment. The room was dark with two 50-Watt red lights on either side of the conditioning chamber. The conditioning chamber was made of opaque Plexiglas sides (22.5 × 26 × 20 cm) with an opaque lid. The chamber was placed on a table with a clear Plexiglas top. A mirror beneath the chamber on a 45° angle facilitated viewing of the ventral surface of the rat. Four plastic containers were permanently attached to holes on each side of the chamber in which a cotton dental roll saturated with vanilla flavour extract (Clubhouse; 35% alcohol) was placed to create the olfactory cue in the chamber. The cotton roll was inaccessible to the rat, with a newly saturated cotton roll used for each rat placed in the context.
Procedure
Experiment 1: effect of CBD on the expression of conditioned gaping
The rats received four conditioning trials, during which the contextual chamber was paired with 127 mg/kg LiCl. On each conditioning trial, each rat was injected with LiCl and immediately placed in the distinctive context for a 30-min period. This procedure was followed for a total of four conditioning trials, with 72 h between each trial.
On the test trial, the rats were randomly assigned into four pre-treatment groups (with n = 7/group): VEH, 1 mg/kg CBD, 5 mg/kg CBD and 10 mg/kg CBD 30 min before placement in the chamber. The rats received the appropriate pre-treatment injection 30 min before placement in the chamber. The doses and time of CBD pre-treatment were selected on the basis of previous studies (Parker et al. 2002, 2004b). Each rat was taken individually to the conditioning context and was placed in the chamber for 15 min while the orofacial responses were video-recorded from a mirror beneath the chamber.
Experiment 2: effect of URB on the expression of conditioned gaping
The rats received four conditioning trials, during which the chamber was paired with LiCl as in experiment 1. On the test trial, the rats were randomly assigned into five groups: VEH (n = 9), 0.1 URB (n = 9), 0.3 URB (n = 9), VEH-SR (n = 7) and URB-SR (n = 9). The URB or VEH pre-treatment was injected 2 h (Fegley et al. 2005) before placement in the chamber and SR (in the groups given SR) was injected 30 min before placement in the chamber. As in experiment 1, the rats remained individually in the conditioning chamber for 15 min while the orofacial responses were video-recorded.
Experiment 3: effect of URB on the establishment/expression of conditioned gaping
The rats received four conditioning trials as in experiment 1. However, 2 h before each conditioning trial, approximately half of the rats were injected with VEH or 0.3 mg/kg URB on each trial separated by 72 h. Seventy-two hours later, on the test trial, the rats were injected with VEH or 0.3 mg/kg URB 2 h before placement in the chamber that had previously been paired with LiCl. The groups were (conditioning pre-treatment–test pre-treatment): URB-URB (n = 7), URB-VEH (n = 7), VEH-URB (n = 8), VEH-VEH (n = 9). Because the results of experiments 1 and 2 demonstrated that the strongest conditioned response was evident during the first 5 min of testing, the duration of the test trial was reduced to 5 min while the rats’ orofacial responses were video-recorded.
Behavioural measures
The videotapes were scored by an observer, blind to the experimental condition using the Observer (Noldus Information Technology, Sterling, VA, USA) event-recording program. The behaviours measured included gaping reactions (wide opening of the mouth, exposing the teeth) and the amount of time (seconds) the rat remained immobile (no body movement) as a measure of inactivity.
Results
Experiment 1: CBD on the expression of conditioned gaping
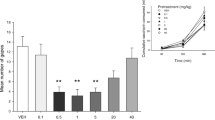
At doses of 1 and 5 mg/kg, CBD suppressed the expression of conditioned gaping. Figure 1 presents the mean frequency of gaping responses expressed in discrete 5-min intervals for the various pre-treatment groups. A four (pre-treatment) by three (time) repeated measures analysis of variance (ANOVA) for the gaping reactions revealed significant main effects of pre-treatment, F(1, 24) = 3.3; p < 0.05 and time F(2, 48) = 28.4; p < 0.01. Overall, rats displayed fewer gaping responses after pre-treatment with 1 mg/kg (p < 0.05) and 5 mg/kg (p < 0.01), but not 10 mg/kg, CBD than after pre-treatment with VEH. Furthermore, overall rats displayed more gaping reactions during the first interval than during the second and third intervals, ps < 0.01.
Mean (+SEM) frequency of gaping responses expressed in 5-min intervals for group vehicle, 1, 5 and 10 mg/kg CBD, respectively, during the 15-min test trial of experiment 1. Asterisks indicate significant (*p < 0.05; **p < 0.01) differences from group vehicle during the first 5-min interval. There were no other significant differences among groups during any other interval
Analysis of the pre-treatment effect during the first 5 min interval of testing produced the identical pattern of results as the overall main effect.
The pre-treatment drug did not modify overall activity levels during the test. A four by three mixed factors ANOVA of the null activity data revealed only a significant main effect of intervals, F(2, 48) = 7.3; p < 0.01. Rats were more active during the first 5 min interval than any of the remaining intervals (ps < 0.01).
Experiment 2: URB on the expression of conditioned gaping
At a dose of 0.3 mg/kg URB suppressed the expression of conditioned gaping and SR reversed this effect. Figure 2 presents the mean frequency of gapes expressed in discrete 5-min intervals for the various groups during the 15-min test trial in experiment 2. A five (pre-treatment) by three (time) repeated measures ANOVA revealed significant main effects of pre-treatment, F(4, 37) = 3.4, p < 0.02 and time F(2, 74) = 28.9, p < 0.01, as well as a pre-treatment by time interaction that approached statistical significance F(8, 74) = 1.9, p = 0.07. A simple main effects analysis of the frequency of gaping at each interval of testing revealed that in the first 5 min interval, group 0.3 mg/kg URB gaped significantly less than group VEH, group VEH-SR and group URB-SR (ps < 0.01), with no other significant group differences. The groups did not significantly differ at any other interval.
Mean (+SEM) frequency of gaping responses expressed in 5-min intervals for group vehicle, 0.1 mg/kg URB, 0.3 mg/kg URB, SR-vehicle and SR-0.3 mg/kg URB, respectively, during the 15-min test trial of experiment 2. Asterisks indicate significant (**p < 0.01) difference from group vehicle, SR-vehicle and SR-0.3 mg/kg URB during the first 5-min interval. There were no other significant differences among groups during any other interval
The pre-treatment drugs did not modify overall activity level during the test. The five by three mixed factors ANOVA for the null activity data revealed only a significant effect of intervals, F(2,74) = 17.1; p < 0.01. The rats were more active during the first 5 min (ps < 0.01) than during the remaining intervals.
Experiment 3: URB on the establishment/expression of conditioned gaping
URB interfered with both the establishment and the expression of conditioned gaping. Figure 3 presents the mean frequency of gaping responses displayed by the rats pre-treated with VEH or URB during testing that had been pre-treated with VEH or URB before conditioning. A two (conditioning pre-treatment) by two (test pre-treatment) repeated measures ANOVA revealed a significant main effect of test pre-treatment, F(1, 27) = 6.0, p < 0.025 and a significant conditioning pre-treatment by test pre-treatment interaction ""F(1, 27) = 9.7, p < 0.01. A simple main effects analysis of the interaction revealed that among the rats pre-treated during conditioning with VEH, but not URB, those tested with URB displayed fewer gapes than those tested with VEH, F(1, 15) = 14.7, p < 0.01. Additionally, among the rats tested with VEH, but not URB, those pre-treated with URB during conditioning displayed fewer gapes than those pre-treated with VEH during conditioning, F(1, 14) = 10.1, p < 0.01. Therefore, URB interfered with both the establishment and the expression of conditioned gaping elicited by a lithium-paired context. The suppression of gaping observed is not simply the result of state-dependent memory, as those rats conditioned and tested in the URB-induced state displayed the same suppression of gaping as those receiving URB during only conditioning or testing. There were no significant differences in activity between the groups during the test trial.
Discussion
When re-introduced to a context previously paired with LiCl, rats display conditioned gaping (Limebeer et al. 2006, 2007), a measure of conditioned nausea. The expression of this gaping reaction is not reduced by pre-treatment with a classic anti-emetic agent, OND, as has been reported by human chemotherapy patients; however, the psychoactive component in marijuana, Δ9-THC, did suppress conditioned gaping elicited by a LiCl-paired chamber (Limebeer et al. 2006). In agreement with the gaping data with rats, Parker et al. (2006) reported that shrews (which are capable of vomiting) display a conditioned retching reaction when re-introduced to a chamber previously paired with LiCl (which produced vomiting) and this conditioned retching reaction was suppressed by pre-treatment with Δ9-THC or CBD, but not OND. The present findings provide additional support for the potential of cannabinoid treatments to suppress AN on the basis of results with the rat gaping model.
Experiment 1 demonstrated that low doses of CBD (1 and 5 mg/kg) that suppressed LiCl-induced vomiting (Parker et al. 2004b) and conditioned retching in the shrew (Parker et al. 2006) as well as the establishment and the expression of gaping elicited by a LiCl-paired flavor in the rat (Parker et al. 2002), also suppressed the expression of gaping elicited by LiCl-paired context in the rat. A low dose of 5 mg/kg CBD has also been reported to serve as an effective anti-inflammatory agent (Malfait et al. 2000), as well as a neuroprotective antioxidant (Hampson et al. 1998). As CBD is a non-psychoactive component in marijuana, the ability of CBD to suppress conditioned gaping is promising for its use in the suppression of AN.
The effective anti-nausea range of CBD appears to be narrow because at the highest dose tested (10 mg/kg), CBD did not suppress the expression of gaping elicited by a LiCl-paired context. Our previous work with the Suncus murinus (Parker et al. 2004b) revealed that although doses of 1–10 mg/kg CBD reversed LiCl-induced vomiting, higher doses (20–40 mg/kg) potentiated LiCl-induced vomiting. It is possible, that CBD also produces a biphasic effect on the expression of conditioned gaping with lower doses reducing the expression of conditioned gaping, but higher doses enhancing the expression of gaping elicited by a LiCl-paired context.
Experiments 2 and 3 demonstrated that the FAAH inhibitor, URB, also suppressed the display of AN as measured by gaping in rats. Presumably, prolonging the action of endogenous AEA produced an anti-nausea effect when rats were re-exposed to the LiCl-paired context. The effect of URB on the expression of conditioned gaping was dose-dependent, with 0.3 mg/kg (but not 0.1 mg/kg) serving as the effective dose. The suppression of gaping by URB was reversed by pre-treatment with the CB1 antagonist/inverse agonist, SR141716A, suggesting a CB1 mechanism of action. URB also interfered with the establishment of conditioned gaping when administrated before each pairing of the contextual stimuli with LiCl. This finding was consistent with that recently reported by Cross-Mellor et al. (2007) demonstrating that URB interfered with the establishment of conditioned gaping elicited by exposure to a LiCl-paired flavor. Presumably, URB reduced the nausea produced by the LiCl resulting in weaker conditioning.
The potential of CBD or URB to suppress the gaping reactions during re-exposure to the context previously paired with LiCl-induced nausea might also be accounted for by interference with memory for the previously established association, rather than by interference with conditioned nausea per se. The design of the present experiments cannot discriminate between these two potential mechanisms; however, a memory deficit explanation is less tenable in light of the finding in experiment 1 that the highest dose of CBD (10 mg/kg) did not modify the expression of gaping. Furthermore, at a dose of 5 mg/kg CBD neither affected the acquisition nor retrieval of a floor-amphetamine association in a conditioned place preference task (Parker et al. 2004a) or of a flavor-lithium association in a conditioned taste avoidance task (Parker et al. 2002). Finally, Varvel et al. (2007) reported that mice pre-treated with the FAAH inhibitor, OL-135, as well as FAAH knockout mice with elevated central AEA levels, did not display memory impairment or motor disruption in a spatial memory task (Morris water maze); in fact, the FAAH knockouts displayed a significant increase in acquisition rate.
The unpleasant experience of AN reported by chemotherapy patients may impact the patient’s resolve to continue treatment. Because classic anti-emetics such as OND have proven ineffective in the alleviation of AN, there is a need to develop effective pharmacotherapies. The current findings, along with past research provide support for the role of the EC system in the suppression of nausea to a context previously paired with illness. Through the use of the FAAH inhibitor URB, the action of AEA is prolonged, interfering with the expression of the conditioned gaping response to a context paired with illness, as a model for anticipatory nausea in rats. As URB administration modulates the EC system, it may be a preferred therapeutic over exogenously administered cannabinoids, in the alleviation of conditioned nausea.
References
Aapro M (2005) Optimising antiemetic therapy: what are the problems and how can they be overcome? Curr Med Res Opin 21:885–897
Cross-Mellor SK, Ossenkopp K-P, Piomelli D, Parker L (2007) Effects of the FAAH inhibitor, URB597, and anandamide on lithium-induced taste reactivity responses: a measure of nausea in the rat. Psychopharmacology 190:135–143
Darmani NA (2001) Delta-9-tetrahydrocannabinol and synthetic cannabinoids prevent emesis produced by the cannabinoid CB1 receptor antagonist/inverse agonist SR 141716A. Neuropsychopharmacology 24:198–203
Deutsch DG, Chin SA (1993) Enzymatic synthesis and degradation of anandamide, a cannabinoid receptor agonist. Biochem Pharmacol 46:791–796
Devane WA, Hanus L, Bruer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R (1992) Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 258:1946–1949
Fegley D, Gaetani S, Duranti A, Tontini A, Mor M, Tarzia G, Piomelli D (2005) Characterization of the fatty acid amide hydrolase inhibitor cyclohexyl carbamic acid 3′-carbamoyl-biphenyl-3-yl ester (URB597): effects on anandamide and oleoylethanolamide deactivation. J Pharmacol Exp Ther 313:352–358
Foubert J, Vaessen G (2005) Nausea: the neglected symptom? Eur J Oncol Nurs 9:21–32
Hampson AJ, Grimaldi M, Axelrod J, Wink D (1998) Cannabidiol and delta-9-tetrahydorcannabinol are neuroprotective antioxciants. Proc Natl Acad Sci USA 95:8268–8273
Jordan K, Kasper C, Schmoll H-J (2005) Chemotherapy-induced nausea and vomiting: current and new standards in the antiemetic prophylaxis and treatment. Eur J Cancer 41:199–205
Kathuria S, Gaetani S, Fegley D, Valino F, Duranti A, Tontini A, Mor M, Tarzia G, La Rana GL, Calignano A, Guistino A, Tattoli M, Palmery M, Cuomo V, Piomelli D (2003) Modulation of anxiety through blockade of anandamide hydrolysis. Nat Med 9:76–81
Kwiatkowska M, Parker LA (2005) Ondansetron and delta-9-tetrahydrocannabinol interfere with the establishment of lithium-induced conditioned taste avoidance in the house musk shrew (Suncus murinus) Behav Neurosci 119:974–982
Kwiatkowska M, Parker LA, Burton P, Mechoulam R (2004) A comparative analysis of the potential of cannabinoids and ondansetron to suppress cisplatin-induced emesis in the Suncus murinus (house musk shrew). Psychopharmacology 174:254–259
Limebeer CL, Parker LA (1999) Delta-9-tetrahydrocannabinol interferes with the establishment and the expression of conditioned rejection reactions produced by cyclophosphamide: a rat model of nausea. Neuroreport 10:3769–3772
Limebeer CL, Parker LA (2000) The antiemetic drug ondansetron interferes with lithium-induced conditioned rejection reactions, but not lithium-induced taste avoidance in rats. J Exp Psychol Anim Behav Processes 26:371–384
Limebeer CL, Hall G, Parker LA (2006) Exposure to a lithium-paired context elicits gaping in rats: a model of anticipatory nausea. Physiol Behav 88:398–403
Limebeer CL, Krohn JP, Cross-Mellor S, Litt, DE, Ossenkopp K-P, Parker LA (2007) Exposure to a context previously associated with toxin (LiCl)-or motion-induced sickness elicits conditioned gaping in rats: evidence in support of a model of anticipatory nausea. Behav Brain Res, in press
Malfait AM, Gallily R, Sumariwalla PF, Malik AS, Andreakos E, Mechoulam R, Feldmann M (2000) The nonpsychoactive cannabis constituent cannabidiol is an oral anti-arthritic therapeutic in murine collagen-induced arthritis. Proc Natl Acad Sci USA 97:9561–9566
Morrow GR, Roscoe JA, Hynes HE, Flynn PJ, Pierce HI, Burish T (1998) Progress in reducing anticipatory nausea and vomiting: a study of community practice. Support Care Cancer 6:46–50
Parker LA (2003) Taste avoidance and taste aversion: evidence for two different processes. Learn Behav 31:165–172
Parker LA, Limebeer CL (2006) Conditioned gaping in rats: a selective measure of nausea. Auton Neurosci 129:36–41
Parker LA, Mechoulam R (2003) Cannabinoid agonists and antagonist modulate lithium-induced conditioned gaping in rats. Integr Physiol Behav Sci 38:133–145
Parker LA, Mechoulam R, Schlievert C (2002) Cannabidiol, a non-psychoactive component of cannabis and its synthetic dimethylheptyl homolog suppress nausea in an experimental model with rats. Neuroreport 13:567–570
Parker LA, Mechoulam R, Schlievert C, Abbott L, Fudge ML, Burton P (2003) Effects of cannabinoids on lithium-induced conditioned rejection reactions in a rat model of nausea. Psychopharmacology 166:156–162
Parker LA, Burton P, Sorge RE, Yakiwchuk C, Mechoulam R (2004a) Effect of low doses of Δ9-tetrahydrocannabinol and cannabidiol on the extinction of cocaine-induced and amphetamine-induced conditioned place preference learning in rats. Psychopharmacology 175:360–366
Parker LA, Kwiatkowska M, Burton P, Mechoulam R (2004b) Effect of cannabinoids on lithium-induced vomiting in the Suncus murinus (house musk shrew). Psychopharmacology 171:156–161
Parker LA, Kwiatkowska M, Mechoulam R (2006) Delta-9-Tetrahydrocannabinol and cannabidiol, but not ondansetron, interfere with conditioned retching reactions elicited by a lithium-paired context in Suncus murinus: an animal model of anticipatory nausea and vomiting. Physiol Behav 87:66–71
Piomelli D (2003) The molecular logic of endocannabinoid signalling. Nat Rev Neurosci 4:873–884
Travers JB, Norgren R (1986) Electromyographic analysis of the ingestion and rejection of sapid stimuli in the rat. Behav Neurosci 100:544–555
Van Sickle MD, Oland LD, Ho W, Hillard CJ, Mackie K, Davison JS, Sharkey KA (2001) Cannabinoids inhibit emesis through CB1 receptors in the brainstem of the ferret. Gastroenterology 121:767–774
Varvel SA, Wise LE, Niyuhire F, Cravatt BF, Lichtman AH (2007) Inhibition of fatty-acid amide hydrolase accelerates acquisition and extinction rates in a spatial memory task. Neuropsychopharmacology 32:1032–1041
Acknowledgments
This research was supported by a grant from the Natural Sciences and Engineering Research Council of Canada to LAP, the Israel Science Foundation and NIH to RM, NIH to DP and by a grant from Wilfrid Laurier University’s Student Technology Endowment Program and an Ontario Graduate Scholarship to EMR.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article is available at http://dx.doi.org/10.1007/s00213-011-2332-2.
Rights and permissions
About this article
Cite this article
Rock, E.M., Limebeer, C.L., Mechoulam, R. et al. The effect of cannabidiol and URB597 on conditioned gaping (a model of nausea) elicited by a lithium-paired context in the rat. Psychopharmacology 196, 389–395 (2008). https://doi.org/10.1007/s00213-007-0970-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-007-0970-1