Abstract
Summary
In biologic-naïve female RA patients, switching oral BPs to DMAb significantly reduced radiographic joint destruction compared to continuing oral BPs or switching to TPTD at 12 months, which were significantly associated with a decrease of a bone resorption marker at 6 months.
Introduction
The aim of this study was to clarify the effects of switching oral bisphosphonates (BPs) to denosumab (DMAb) or daily teriparatide (TPTD) on the progression of radiographic joint destruction in patients with biologic-naïve rheumatoid arthritis (RA).
Methods
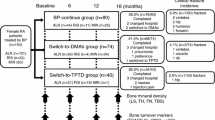
A retrospective, case-controlled study involving 90 female RA patients (mean age 68.2 years, 96.7% postmenopausal, disease activity score assessing 28 joints with CRP (DAS28-CRP) 2.4, methotrexate treatment 81.1%, prednisolone treatment 68.9%, and prior BP treatment 44.8 months), who were allocated depending on each patient’s and physician’s wishes, to (1) the BP-continue group (n = 30), (2) the switch-to-DMAb group (n = 30), or (3) the switch-to-TPTD group (n = 30), was conducted. Patients were retrospectively selected to minimize the difference of possible clinical backgrounds that may affect the joint destruction of RA. The primary endpoint was to clarify the change of the modified total Sharp score (mTSS) from baseline to 12 months.
Results
After 12 months, the mean changes of the modified Sharp erosion score were significantly lower in the switch-to-DMAb group (0.2 ± 0.1; mean ± standard error) than in the switch-to-TPTD group (1.3 ± 0.5; P < 0.05), and mTSS was significantly lower in the switch-to-DMAb group (0.3 ± 0.2) than in the BP-continue group (1.0 ± 0.3; P < 0.05) and the switch-to-TPTD group (1.7 ± 0.6; P < 0.05). The logistic regression analysis showed that mTSS changes were significantly associated with the percent changes of TRACP-5b at 6 months (β = 0.30, 95% CI = 0.002–0.016; P < 0.01).
Conclusions
Changes of systemic bone turnover induced by switching BPs to DMAb or TPTD may affect not only systemic bone mass, but also local joint destruction, and its clinical relevance should be considered comprehensively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rheumatoid arthritis (RA) is characterized by systemic inflammation, which is associated with increased osteoclast activity leading to bone erosion and joint destruction [1, 2]. Proinflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α), interleukin (IL)-1, IL-6, and IL-17, are strongly involved in receptor activator of nuclear factor kappa B (RANK) ligand (RANKL) induction, which is essential for osteoclast differentiation and activation [3]. Moreover, previous reports demonstrated that increased bone turnover [4, 5] and low bone mineral density (BMD) [6] are associated with future radiographic joint destruction in RA, suggesting the significance of inhibiting bone turnover and obtaining high BMD to protect against joint destruction.
Bisphosphonates (BPs), which induce apoptosis of osteoclasts by inhibiting farnesyl diphosphate synthase, play pivotal roles in the treatment of both primary and secondary osteoporosis [7]. However, the efficacy of switching BPs to denosumab (DMAb), an anti-RANKL antibody that strongly inhibits bone resorption [8], or daily teriparatide (TPTD), a bone anabolic agent that strongly induces bone formation [9], has been reported in primary osteoporosis. In addition, we have recently reported that switching BPs to DMAb significantly inhibited bone turnover [10], and Takeuchi et al. demonstrated that DMAb inhibited progression of the bone erosion of RA [11]. On the other hand, switching BPs to daily TPTD induced overshoot of the bone turnover of RA [10, 12].
Taken together, we hypothesized that the change of bone turnover induced by these osteoporosis agents may have some effects on the progression of joint destruction (especially on bone erosion) in RA. The aim of this retrospective, case-controlled study was to clarify the effects of switching BPs to DMAb or TPTD on radiographic joint destruction in biologic-naïve female patients with RA.
Materials and methods
Study design and subjects
This 12-month retrospective, case-controlled study was conducted based on a two-center, open-label design. A total of 155 biologic-naïve female (96.7% postmenopausal) patients with RA, who were treated with an oral BP according to the Japanese guidelines for prevention and treatment of osteoporosis 2011 [13] or the guidelines on the management and treatment of glucocorticoid-induced osteoporosis of the Japanese Society for Bone and Mineral Research 2004 [14], were enrolled. RA was diagnosed based on the 1987 revised American College of Rheumatology (ACR) criteria [15]. Registered patients were allocated based on each physician’s discretion and patients’ preference to the “BP-continue” group (n = 63), the “switch-to-DMAb” group (n = 61), or the “switch-to-TPTD” group (n = 31). Calcium (50–610 mg/day) and vitamin D (0.25–10 μg/day) supplements were provided, and dosing was adjusted by the attending physician. Patients who completed 12 months of osteoporosis treatment without biologic disease-modifying antirheumatic drugs (bDMARDs) of the three groups were matched with the following parameters, including baseline age, disease duration, rheumatoid factor (RF) and anticyclic citrullinated peptide antibody (ACPA) positivity, serum levels of bone turnover markers (BTMs), C-reactive protein (CRP), Disease Activity Score assessing 28 joints with CRP (DAS28-CRP), and the modified Total Sharp Score (mTSS), which may affect the progression of joint destruction as previously described [16]. BP-continue group (n = 63) and switch-to-DMAb group (n = 61) were independently matched with these parameters to switch-to-TPTD group (n = 31) with propensity score matching, using 1:1 optimal matching without replacement as previously described [17]. Finally, the “BP-continue” group (n = 30), the “switch-to-DMAb” group (n = 30), and the “switch-to-TPTD” group (n = 30) were evaluated.
This study was conducted in accordance with the ethical standards of the Declaration of Helsinki and was approved by the ethical review board at the clinical center (approval number 13231-2; Osaka University, Graduate School of Medicine). Written informed consent was obtained from each individual patient included in the study.
Radiographic assessment of the modified Sharp Score
The hand and foot radiographs were taken at baseline and at 12 months when switching osteoporosis therapies or starting observation. Two rheumatologists independently assessed the images blinded to patients’ clinical information, and the average scores of the two were used in the analysis, as previously described [18]. The primary endpoint was the change from baseline in the modified Sharp Erosion (ERO) Score, the modified Sharp Joint Space Narrowing (JSN) score, and the mTSS at 12 months [11]. The cumulative probability of the progression of mTSS per year (ΔmTSS/year) and the clinically relevant radiological progression rate (CRRP; ΔmTSS/year ≥ 3) were evaluated [19].
BMD and trabecular bone score assessment
Areal BMDs in the lumbar spine (LS; L2-L4), total hip (TH), and femoral neck (FN) were assessed by dual-energy X-ray absorptiometry (Discovery, Hologic, Inc., Waltham, MA, USA) at baseline and after 12 months of treatment. Regions of severe sclerosis, vertebral fractures, and operated sites were excluded from BMD measurements, as previously described [20]. The trabecular bone score (TBS) was assessed at the same regions used for LS DXA scans, using the TBS iNsight Software v1.7 (Med-Imaps, Bordeaux, France), as previously described [21].
Biochemical markers of bone turnover
BTMs were measured in serum obtained from each patient in the morning after overnight fasting. As for bone formation marker, N-terminal type I procollagen propeptide (PINP) (inter-assay coefficient of variation (CV), 3.2–5.2%; Intact UniQ assay; Orion Diagnostica, Espoo, Finland), and as for bone resorption marker, tartrate-resistant acid phosphatase (TRACP)-5b (inter-assay CV, 5.0–9.0%; Immunodiagnostic Systems Ltd., Boldon, UK), were measured by ELISA, as previously described [12]. Previous report demonstrated that TRACP-5b is a useful marker which shows higher clinical sensitivity and signal-to-noise ratio compared to serum collagen type 1 cross-linked C-telopeptide (CTX) [22]. Serum intact parathyroid hormone (PTH) levels were measured using a two-site immunoradiometric assay (inter-assay CV, 8.4%; Quest Diagnostics Nichols Institute, California, USA).
Statistical analysis
Differences among study groups were tested using analysis of variance for normally distributed data, and the nonparametric Kruskal-Wallis test was used for non-normally distributed data. Changes in BMD and ranked bone turnover marker data from baseline to specified time points were compared within each study group using the nonparametric Wilcoxon signed-rank test. Patients’ clinical background characteristics that showed significant correlations with 12-month mTSS change as evaluated by Spearman correlation coefficients were selected as predictor variables, and multivariate logistic regression analysis with a forward stepwise procedure was performed to identify significant indicators of 12-month mTSS change. The 95% confidence intervals (CIs) for correlation coefficients were calculated based on Fisher’s z-transformation. Results are expressed as means ± standard error. A P value < 0.05 was considered significant. All tests were performed using IBM SPSS Statistics version 22 software (IBM, Armonk, NY, USA).
Results
The patients’ baseline characteristics and changes after 12 months are shown in Table 1. No significant differences were observed in baseline age, body mass index, disease duration of RA, RF and ACPA positivity, mTSS, CRP, swollen/tender joint count, and DAS28-CRP. In addition, no significant changes and no differences between the groups were observed in the swollen/tender joint count and DAS28-CRP after 12 months.
The patients’ medications and bone metabolism-related parameters are shown in Table 2. No significant differences were observed in combined prednisolone (PSL) or methotrexate (MTX) doses and usage rates, areal BMD (T-scores), trabecular bone score (TBS), serum intact-PTH levels (which increase in response to a low-serum 25-hydroxycholecalciferol [25(OH)D)] level and low-calcium intake [23]), and BTMs at baseline. On the other hand, the switch-to-TPTD group showed longer prior BP therapy duration and a lower rate of combined vitamin D use compared to the BP-continue group and the switch-to-DMAb group at baseline. The switch-to-DMAb group had a higher rate and dose of calcium and native vitamin D (cholecalciferol; VD3) administration compared to both the BP-continue group and the switch-to-TPTD group throughout the study. There was no significant difference in the prescription rate of each active vitamin D (alfacalcidol [ALF] and eldecalcitol [ELD]) between the BP-continue group and the switch-to-TPTD group, respectively.
Bone turnover markers
Percent changes in BTMs from baseline are shown in Fig. 1a and b. The switch-to-DMAb group showed a significantly greater decrease compared to the BP-continue group in both PINP levels (− 28.7 vs. 0.9%; P < 0.05) and TRACP-5b levels (− 29.0 vs. − 4.6%; P < 0.01) at 6 months. On the other hand, the switch-to-TPTD group showed a significantly greater increase compared to the BP-continue group in PINP levels from 6 months (218.6 vs. 0.9%; P < 0.001) to 12 months (165.5 vs. 5.8%; P < 0.001), and in TRACP-5b levels from 6 months (64.9 vs − 4.6%; P < 0.001) to 12 months (63.5 vs. − 6.4%; P < 0.001).
Mean changes in serum concentrations of bone turnover markers, PINP (a) and TRAP-5b (b). BP, bisphosphonate; DMAb, denosumab; TPTD, teriparatide; PINP, type I collagen N-terminal propeptide; TRACP-5b, isoform 5b of tartrate-resistant acid phosphatase. Bars indicate standard errors. ##P < 0.01, ###P < 0.001 BP-continue group versus switch-to-TPTD group. *P < 0.05, **P < 0.01 BP-continue group versus switch-to-DMAb group. †††P < 0.001 switch-to-DMAb group versus switch-to-TPTD group
Changes in BMD and TBS
Changes in BMD and TBS are shown in Table 2. The switch-to-TPTD group showed the highest increases in LS BMD, TBS, and BTMs. On the other hand, the switch-to-DMAb group tended to show the highest increases in FN and TH BMD compared to the other two groups.
Effects of switching osteoporosis therapy on joint space narrowing and bone erosion
The mean changes from baseline at 12 months in the radiographic modified Sharp Erosion Score are shown in Fig. 2. The changes from baseline in the modified Sharp JSN Score at 12 months showed no significant difference among the three groups (Fig. 2a). On the other hand, as shown in Fig. 2b, the change from baseline in the modified Sharp Erosion Score at 12 months was significantly lower in the switch-to-DMAb group than in the switch-to-TPTD group (0.2 ± 0.1 vs. 1.3 ± 0.5; P < 0.05). Consequently, the changes from baseline in the mTSS at 12 months were significantly lower in the switch-to-DMAb group than in the BP-continue group (0.3 ± 0.2 vs. 1.0 ± 0.3; P < 0.05) and the switch-to-TPTD group (0.3 ± 0.2 vs. 1.7 ± 0.6; P < 0.05) (Fig. 2c).
Mean changes in the radiographic score evaluated by the van der Heijde-modified Sharp method at 12 months. Modified Sharp Joint Space Narrowing (JSN) score (a), modified Sharp Erosion (ERO) Score (b), and modified total Sharp Score (mTSS) (c). BP, bisphosphonate; DMAb, denosumab; TPTD, teriparatide. Bars indicate standard errors. N.S. not significant, *P < 0.05
Cumulative probability plots for changes in the modified Sharp JSN Score (Fig. 3a), the modified Sharp ERO Score (Fig. 3b), and mTSS (Fig. 3c) at 12 months are shown. The clinically relevant radiological progression rate (CRRP; ΔmTSS/year ≥ 3) [19] was significantly lower in the switch-to-DMAb group than in the switch-to-TPTD group (3.3 vs. 20.0%; P < 0.05). In addition, the structural remission rate (ΔmTSS/year ≤ 0.5) [18] tended to be higher in the switch-to-DMAb group than in the BP-continue group (76.7 vs. 53.3%; P = 0.06) and the switch-to-TPTD group (76.7 vs. 56.7%; P = 0.10).
Significant predictor variables of 12-month mTSS progression on multivariate linear regression analysis
Spearman correlation coefficients of possible clinical background characteristics (including baseline age, disease duration, modified Sharp Score, DAS28-CRP, combined PSL and MTX dose, prior BP therapy duration, RF and ACPA titers, areal BMD, TBS, and baseline and change of BTMs) with 12-month mTSS progression were investigated for all patients (Table 3), and all significant (P < 0.05) predictors (DAS28-CRP, ACPA positivity, and Δ 6-month TRACP-5b (%)) were identified and subjected to stepwise multivariable linear regression analysis to investigate significant predictors of 12-month mTSS progression. The significant predictor of 12-month mTSS progression was Δ 6-month TRACP-5b (%).
Discussion
To the best of our knowledge, this is the first report demonstrating the effect of switching oral BPs to DMAb or daily TPTD on the progression of radiographic joint destruction in biologic-naïve patients with RA. Previous reports showed that increased bone turnover is associated with future radiographic joint destruction in RA [4, 5], suggesting the critical role of bone turnover in joint destruction, especially in osteoclast-induced periarticular bone erosion.
Factors affecting the progression of joint destruction (especially bone erosion) in RA have been reported. Syversen et al. demonstrated that baseline RF and ACPA positivity, high disease activity, and female sex were independent predictors of progression of mTSS in a 10-year prospective study [24]. Another cross-sectional study showed that the presence of bone erosions in RA correlates with low-BMD levels [25]. In the present study, to investigate the effects of osteoporosis treatments, these factors affecting the progression of joint destruction were controlled between the groups. In addition, 12-month mTSS progression was significantly associated with baseline DAS28-CRP, ACPA positivity, and Δ6-month TRACP-5b (%), in accordance with previous reports. Finally, multivariate linear regression analysis showed that Δ6-month TRACP-5b (%) was the significant factor associated with 12-month mTSS progression.
Concerning BPs, zoledronate is one of the BPs that most strongly induces apoptosis of osteoclasts [26], and a previous animal study showed that the combination of zoledronate and MTX prevented bone erosion in collagen-induced arthritis of rats [27]. On the other hand, human prospective, randomized trials failed to show the positive effects of zoledronate monotherapy on bone erosion in patients with psoriatic arthritis [28] and tophaceous gout [29]. Taken together, BP monotherapy may be insufficient, but its combination with MTX may have some positive effects on inhibition of bone erosion in arthritis.
Takeuchi et al. reported that DMAb significantly inhibited the progression of bone erosion compared with placebo in Japanese RA patients who had bone erosions or C-reactive protein (CRP) ≥ 1.0 mg/dL and who were also never treated by BPs or biologics at baseline [11]. This population may be relatively rare compared to the real-world use of DMAb, since most patients are considered to be treated by BPs at first line according to the osteoporosis guidelines [13, 14]. Moreover, the placebo group was not treated by any bone resorption inhibitors such as BPs in this study. So the effects of switching BPs to DMAb on bone erosion of RA still remained unclear.
Recently, Solomon et al. demonstrated that 1-year daily TPTD treatment failed to show significant effects on bone erosion of the hands or wrists compared to a control group in RA, who were all strictly controlled by TNF inhibitors and not taking osteoporosis treatment [30]. Taken together, TPTD may not reduce or enhance bone erosion compared to a non-osteoporosis treatment group, but its effects on bone erosion compared to BPs or DMAb still remained unclear.
The present study demonstrated for the first time that switching oral BPs to DMAb significantly reduced Δ12-month mTSS compared to continuing oral BPs or switching to TPTD, which were significantly associated with a decrease of a bone resorption marker. It has been reported that low BMD and thinning at the cortical site were significantly associated with bone erosions of RA [31]. DMAb showed positive effects in improving cortical porosity compared to BPs [32], while TPTD failed to show positive effects on cortical sites in the short-term treatment [33, 34]. Taken together, the differential effects of each agent on both cortical bone and bone turnover may affect the results.
There are several limitations to this study. First, since this was a small cohort, retrospective study, we could not completely match all the clinical backgrounds between the groups, and a large, prospective study is required to confirm the results. Second, as the treatment assignment was dependent on each patient’s and physician’s wishes, the initial treatment selection may affect the results. Third, since TPTD is recommended to patients at high-fracture risk, the switch-to-TPTD group showed a tendency of higher rate and dose of PSL, with a longer duration of prior BP prescription than other groups. Fourth, there was significant difference in the form of vitamin D among the groups, because only active vitamin D combination is allowed in the treatment of BP or TPTD in our country. Fifth, the switch-to-TPTD group was treated with a lower rate of calcium and vitamin D supplementation compared to other groups, because of the recommendation of careful consideration in calcium and active vitamin D supplementation due to the risk of hypercalcemia in our country. Sixth, although mean serum intact-PTH levels of the three groups at baseline were all within the reference range (< 65 pg/ml), we did not monitor serum 25OH(D) levels and other standard bone turnover markers.
In conclusion, the changes of systemic bone turnover induced by switching BPs to DMAb or TPTD may affect not only systemic bone mass, but also local joint destruction, and its clinical relevance should be comprehensively considered by factors such as RA disease activity and fracture risk.
References
Schett G, Hayer S, Zwerina J, Redlich K, Smolen JS (2005) Mechanisms of disease: the link between RANKL and arthritic bone disease. Nat Clin Pract Rheumatol 1:47–54
Scott DL, Wolfe F, Huizinga TW (2010) Rheumatoid arthritis. Lancet 376:1094–1108
Braun T, Schett G (2012) Pathways for bone loss in inflammatory disease. Curr Osteoporos Rep 10:101–108
Jansen LM, van der Horst-Bruinsma I, Lems WF, van Schaardenburg D, van de Stadt R, de Koning M, Dijkmans BA (2004) Serological bone markers and joint damage in early polyarthritis. J Rheumatol 31:1491–1496
Syversen SW, Goll GL, van der Heijde D, Landewe R, Gaarder PI, Odegard S, Haavardsholm EA, Kvien TK (2009) Cartilage and bone biomarkers in rheumatoid arthritis: prediction of 10-year radiographic progression. J Rheumatol 36:266–272
Rossini M, Adami G, Viapiana O, Idolazzi L, Orsolini G, Fassio A, Giollo A, Gatti D (2017) Osteoporosis: an independent determinant of bone erosions in rheumatoid arthritis? J Bone Miner Res 32:2142–2143
Russell RG, Watts NB, Ebetino FH, Rogers MJ (2008) Mechanisms of action of bisphosphonates: similarities and differences and their potential influence on clinical efficacy. Osteoporos Int 19:733–759
Kendler DL, Roux C, Benhamou CL, Brown JP, Lillestol M, Siddhanti S, Man HS, San Martin J, Bone HG (2010) Effects of denosumab on bone mineral density and bone turnover in postmenopausal women transitioning from alendronate therapy. J Bone Miner Res 25:72–81
Finkelstein JS, Wyland JJ, Lee H, Neer RM (2010) Effects of teriparatide, alendronate, or both in women with postmenopausal osteoporosis. J Clin Endocrinol Metab 95:1838–1845
Ebina K, Hirao M, Hashimoto J, Hagihara K, Kashii M, Kitaguchi K, Matsuoka H, Iwahashi T, Chijimatsu R, Yoshikawa H (2017) Assessment of the effects of switching oral bisphosphonates to denosumab or daily teriparatide in patients with rheumatoid arthritis. J Bone Miner Metab
Takeuchi T, Tanaka Y, Ishiguro N, Yamanaka H, Yoneda T, Ohira T, Okubo N, Genant HK, van der Heijde D (2016) Effect of denosumab on Japanese patients with rheumatoid arthritis: a dose-response study of AMG 162 (Denosumab) in patients with RheumatoId arthritis on methotrexate to validate inhibitory effect on bone Erosion (DRIVE)-a 12-month, multicentre, randomised, double-blind, placebo-controlled, phase II clinical trial. Ann Rheum Dis 75:983–990
Ebina K, Hashimoto J, Shi K, Kashii M, Hirao M, Yoshikawa H (2014) Comparison of the effect of 18-month daily teriparatide administration on patients with rheumatoid arthritis and postmenopausal osteoporosis patients. Osteoporos Int 25:2755–2765
Orimo H, Nakamura T, Hosoi T, Iki M, Uenishi K, Endo N, Ohta H, Shiraki M, Sugimoto T, Suzuki T, Soen S, Nishizawa Y, Hagino H, Fukunaga M, Fujiwara S (2012) Japanese 2011 guidelines for prevention and treatment of osteoporosis—executive summary. Arch Osteoporos 7:3–20
Nawata H, Soen S, Takayanagi R, Tanaka I, Takaoka K, Fukunaga M, Matsumoto T, Suzuki Y, Tanaka H, Fujiwara S, Miki T, Sagawa A, Nishizawa Y, Seino Y, The Subcommittee to Study Diagnostic Criteria for Glucocorticoid-Induced Osteoporosis (2005) Guidelines on the management and treatment of glucocorticoid-induced osteoporosis of the Japanese Society for Bone and Mineral Research (2004). J Bone Miner Metab 23:105–109
Arnett FC, Edworthy SM, Bloch DA, Mcshane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS, Medsger TA, Mitchell DM, Neustadt DH, Pinals RS, Schaller JG, Sharp JT, Wilder RL, Hunder GG (1988) The American rheumatism association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 31:315–324
Matsui T, Kuga Y, Kaneko A, Nishino J, Eto Y, Chiba N, Yasuda M, Saisho K, Shimada K, Tohma S (2007) Disease activity score 28 (DAS28) using C-reactive protein underestimates disease activity and overestimates EULAR response criteria compared with DAS28 using erythrocyte sedimentation rate in a large observational cohort of rheumatoid arthritis patients in Japan. Ann Rheum Dis 66:1221–1226
Chieffo A, Meliga E, Latib A, Park SJ, Onuma Y, Capranzano P, Valgimigli M, Jegere S, Makkar RR, Palacios IF, Kim YH, Buszman PE, Chakravarty T, Sheiban I, Mehran R, Naber C, Margey R, Agnihotri A, Marra S, Capodanno D, Leon MB, Moses JW, Fajadet J, Lefevre T, Morice MC, Erglis A, Tamburino C, Alfieri O, Serruys PW, Colombo A (2012) Drug-eluting stent for left main coronary artery disease. The DELTA registry: a multicenter registry evaluating percutaneous coronary intervention versus coronary artery bypass grafting for left main treatment. JACC Cardiovasc Interv 5:718–727
Hasegawa T, Kaneko Y, Izumi K, Takeuchi T (2017) Efficacy of denosumab combined with bDMARDs on radiographic progression in rheumatoid arthritis. Joint Bone Spine 84:379–380
Bruynesteyn K, Van Der Heijde D, Boers M et al (2002) Detecting radiological changes in rheumatoid arthritis that are considered important by clinical experts: influence of reading with or without known sequence. J Rheumatol 29:2306–2312
Ebina K, Hashimoto J, Shi K, Kashii M, Hirao M, Yoshikawa H (2015) Undercarboxylated osteocalcin may be an attractive marker of teriparatide treatment in RA patients: response to Mokuda. Osteoporos Int 26:1445
Boutroy S, Hans D, Sornay-Rendu E, Vilayphiou N, Winzenrieth R, Chapurlat R (2013) Trabecular bone score improves fracture risk prediction in non-osteoporotic women: the OFELY study. Osteoporos Int 24:77–85
Nenonen A, Cheng S, Ivaska KK, Alatalo SL, Lehtimäki T, Schmidt-Gayk H, Uusi-Rasi K, Heinonen A, Kannus P, Sievänen H, Vuori I, Väänänen HK, Halleen JM (2005) Serum TRACP 5b is a useful marker for monitoring alendronate treatment: comparison with other markers of bone turnover. J Bone Miner Res 20:1804–1812
Aloia J, Bojadzievski T, Yusupov E, Shahzad G, Pollack S, Mikhail M, Yeh J (2010) The relative influence of calcium intake and vitamin D status on serum parathyroid hormone and bone turnover biomarkers in a double-blind, placebo-controlled parallel group, longitudinal factorial design. J Clin Endocrinol Metab 95:3216–3224
Syversen SW, Gaarder PI, Goll GL, Odegard S, Haavardsholm EA, Mowinckel P, van der Heijde D, Landewe R, Kvien TK (2008) High anti-cyclic citrullinated peptide levels and an algorithm of four variables predict radiographic progression in patients with rheumatoid arthritis: results from a 10-year longitudinal study. Ann Rheum Dis 67:212–217
Rossini M, Bagnato G, Frediani B, Iagnocco A, LAM G, Minisola G, Caminiti M, Varenna M, Adami S (2011) Relationship of focal erosions, bone mineral density, and parathyroid hormone in rheumatoid arthritis. J Rheumatol 38:997–1002
Dunford JE, Thompson K, Coxon FP, Luckman SP, Hahn FM, Poulter CD, Ebetino FH, Rogers MJ (2001) Structure-activity relationships for inhibition of farnesyl diphosphate synthase in vitro and inhibition of bone resorption in vivo by nitrogen-containing bisphosphonates. J Pharmacol Exp Ther 296:235–242
Le Goff B, Soltner E, Charrier C, Maugars Y, Redini F, Heymann D, Berthelot JM (2009) A combination of methotrexate and zoledronic acid prevents bone erosions and systemic bone mass loss in collagen induced arthritis. Arthritis Res Ther 11:R185
McQueen F, Lloyd R, Doyle A, Robinson E, Lobo M, Exeter M, Taylor WJ, Jones P, Reid IR, Dalbeth N (2011) Zoledronic acid does not reduce MRI erosive progression in PsA but may suppress bone oedema: the Zoledronic acid in psoriatic arthritis (ZAPA) study. Ann Rheum Dis 70:1091–1094
Dalbeth N, Aati O, Gamble GD, Horne A, House ME, Roger M, Doyle AJ, Chhana A, McQueen FM, Reid IR (2014) Zoledronate for prevention of bone erosion in tophaceous gout: a randomised, double-blind, placebo-controlled trial. Ann Rheum Dis 73:1044–1051
Solomon DH, Kay J, Duryea J, Lu B, Bolster MB, Yood RA, Han R, Ball S, Coleman C, Lo E, Wohlfahrt A, Sury M, Yin M, Yu Z, Zak A, Gravallese EM (2017) Effects of teriparatide on joint erosions in rheumatoid arthritis: a randomized controlled trial. Arthritis Rheumatol 69:1741–1750
Rossini M, Adami G, Viapiana O, Idolazzi L, Gatti D (2016) Denosumab, cortical bone and bone erosions in rheumatoid arthritis. Ann Rheum Dis 75:e70
Zebaze RM, Libanati C, Austin M, Ghasem-Zadeh A, Hanley DA, Zanchetta JR, Thomas T, Boutroy S, Bogado CE, Bilezikian JP, Seeman E (2014) Differing effects of denosumab and alendronate on cortical and trabecular bone. Bone 59:173–179
Adami G, Rossini M, Viapiana O, Fassio A, Idolazzi L, Orsolini G, Gatti D (2017) No effects of teriparatide on joint erosions in rheumatoid arthritis: an expected result. Arthritis Rheumatol
Keaveny TM, McClung MR, Wan X, Kopperdahl DL, Mitlak BH, Krohn K (2012) Femoral strength in osteoporotic women treated with teriparatide or alendronate. Bone 50:165–170
Acknowledgments
The authors would like to thank Dr. Masao Yukioka and Dr. Kenrin Shi for their excellent cooperation in conducting the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
K Ebina, M Hirao, J Hashimoto, and H Yoshikawa have received research grants from Astellas Pharma and Eisai Co. Ltd. K Ebina, M Hirao, and H Yoshikawa have received research grants from Daiichi Sankyo. H Yoshikawa has received research grants from MSD. K Ebina has received payments for lectures from Astellas Pharma, Chugai Pharmaceutical, Eisai Co. Ltd., Ono Pharmaceutical, Daiichi Sankyo, and Eli Lily. H Matsuoka, T Iwahashi, R Chijimatsu, Y Etani, G Okamura, and A Miyama declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Ebina, K., Hirao, M., Hashimoto, J. et al. Impact of switching oral bisphosphonates to denosumab or daily teriparatide on the progression of radiographic joint destruction in patients with biologic-naïve rheumatoid arthritis. Osteoporos Int 29, 1627–1636 (2018). https://doi.org/10.1007/s00198-018-4492-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-018-4492-y