Abstract
The aim of this observational, non-randomized study was to clarify the unknown effects of switching oral bisphosphonates (BPs) to denosumab (DMAb) or daily teriparatide (TPTD) in patients with rheumatoid arthritis (RA). The characteristics of the 194 female patients included in the study were 183 postmenopausal, age 65.9 years, lumbar spine (LS) T score −1.8, femoral neck (FN) T score −2.3, dose and rate of taking oral prednisolone (3.6 mg/day) 75.8%, and prior BP treatment duration 40.0 months. The patients were allocated to (1) the BP-continue group (n = 80), (2) the switch-to-DMAb group (n = 74), or (3) the switch-to-TPTD group (n = 40). After 18 months, the increase in bone mineral density (BMD) was significantly greater in the switch-to-DMAb group than in the BP-continue group (LS 5.2 vs 2.3%, P < 0.01; FN 3.8 vs 0.0%, P < 0.01) and in the switch-to-TPTD group than in the BP-continue group (LS 9.0 vs 2.3%, P < 0.001; FN 4.9 vs 0.0%, P < 0.01). Moreover, the switch-to-TPTD group showed a higher LS BMD (P < 0.05) and trabecular bone score (TBS) (2.1 vs −0.7%; P < 0.05) increase than the switch-to-DMAb group. Clinical fracture incidence during this period was 8.8% in the BP-continue group, 4.1% in the switch-to-DMAb group, and 2.5% in the switch-to-TPTD group. Both the switch-to-DMAb group and the switch-to-TPTD group showed significant increases in LS and FN BMD, and the switch-to-TPTD group showed a higher increase in TBS compared to the BP-continue group at 18 months. Switching BPs to DMAb or TPTD in female RA may provide some useful osteoporosis treatment options.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Patients with rheumatoid arthritis (RA) suffer reduced bone mineral density (BMD) with disease progression [1] and a higher risk of fractures than patients without RA [2,3,4]. Pro-inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α), interleukin (IL)-1, IL-6, and IL-17, are strongly involved in RANKL induction, which leads to osteoclastogenesis and bone loss in RA [5,6,7,8]. Wnt signaling is essential in bone formation [9], although circulating levels of Wnt signaling inhibitors, Dickkopf-1 (Dkk-1) and sclerostin, were both higher in patients with RA than in controls [10]. Moreover, TNF and glucocorticoids, which are deeply associated with the pathology of RA, also suppress Wnt signaling in osteoblasts [11], and glucocorticoids also induce apoptosis of osteoblasts and osteocytes and increase fracture risk [12, 13]. Taken together, inhibited bone formation may also play a role in the pathogenesis of osteoporosis in RA.
Bisphosphonates (BPs) play pivotal roles in the treatment of both primary and secondary osteoporosis, although prolonged BP treatment may attenuate the increase of BMD [14]. Recently, the efficacy of switching BPs to denosumab (DMAb), an anti-RANKL antibody that strongly inhibits bone resorption [15], or daily teriparatide (TPTD), a bone anabolic agent that strongly induces bone formation [16], has been reported in primary osteoporosis. Moreover, we have recently reported that switching daily TPTD to DMAb showed stronger bone turnover inhibition and higher BMD increase compared to switching to oral BPs in primary osteoporosis [17]. Another study also demonstrated that, in long-term prednisolone (PSL) users, switching oral BPs to DMAb significantly reduced bone turnover markers and increased BMD compared to continuing BPs [18]. In addition, Takeuchi et al. demonstrated that DMAb not only increased areal BMD, but also inhibited the progression of bone erosion in RA [19]. On the other hand, TPTD showed a greater increase in areal BMD than alendronate (ALN) in glucocorticoid-induced osteoporosis (GIO) [14], and we have previously shown that TPTD significantly increased femoral BMD in patients with RA compared to that in postmenopausal osteoporosis [20], indicating the efficacy of TPTD in both GIO and RA. Taken together, it has been assumed that switching BPs to DMAb or TPTD may have some advantages over continuing BPs in patients with RA, although no previous studies have compared these two switching therapies. The aim of this study was to clarify the unknown effects of switching BPs to DMAb or TPTD in female patients with RA.
Materials and methods
Study design and subjects
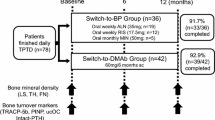
This 18-month observational, non-randomized study was conducted based on a two-center, open-label design. A total of 194 female (183 postmenopausal) patients with RA who were treated with oral BPs according to the Japanese 2011 guidelines for prevention and treatment of osteoporosis [21] and the 2004 guidelines on the management and treatment of GIO of the Japanese Society for Bone and Mineral Research [22] were enrolled in the study (Fig. 1). RA was diagnosed based on the 1987 revised American College of Rheumatology (ACR) criteria [23]. C-reactive protein (CRP), matrix metalloproteinase-3 (MMP-3), and the Disease Activity Score assessing 28 joints with CRP (DAS28-CRP) were evaluated as parameters reflecting inflammation, as well as the disease activity of RA [24, 25]. Registered patients were allocated based on each physician’s discretion and patients preference to the ‘BP-continue’ group (n = 80), the ‘switch-to-DMAb’ group (n = 74), or the ‘switch-to-TPTD’ group (n = 40). Calcium (50–610 mg/day) and vitamin D (0.25–10 μg/day) supplements were provided and dosing adjusted according to the attending physician’s decision.
Study design, schedule, patient flow, and clinical fracture incidence. Registered patients were allocated based on each physician’s discretion to the ‘BP-continue’ group (n = 80), consisting of patients who wanted to continue their current therapies, the ‘switch-to-DMAb’ group (n = 74), or the ‘switch-to-TPTD’ group (n = 40). Bone mineral density and bone turnover markers were evaluated every 6 months. Reasons for discontinuation and clinical fracture incidence during the period were evaluated at 18 months. BP bisphosphonate, ALN alendronate, RIS risedronate, MIN minodronate, DMAb denosumab, TPTD teriparatide, LS lumbar spine, TH total hip, FN femoral neck, TBS trabecular bone score, TRAP-5b isoform 5b of tartrate-resistant acid phosphatase, PINP type I collagen N-terminal propeptide, ucOC undercarboxylated osteocalcin
This study was conducted in accordance with the ethical standards of the Declaration of Helsinki and was approved by the ethical review board at the clinical center (approval number 13231-2; Osaka University, Graduate School of Medicine). Written informed consent was obtained from each individual patient included in the study.
BMD and trabecular bone score (TBS) assessment
Areal BMD in the lumbar spine (LS; L2–L4), total hip (TH), and femoral neck (FN) were assessed by dual-energy X-ray absorptiometry (Discovery, Hologic, Inc., Waltham, MA, USA) at baseline and after 6, 12, and 18 months of treatment. Regions of severe sclerosis, vertebral fracture, and operated sites were excluded from BMD measurements, as previously described [26]. The TBS consists of experimental variograms of two-dimensional projection images, and low TBS is indicative of degraded microarchitecture, such as low connectivity, reduced trabecular number, and high trabecular spacing [27]. The TBS was assessed at the same regions used for LS DXA scans, using TBS iNsight Software v1.7 (Med-Imaps, Bordeaux, France), as previously described [28]. LS TBS was calculated as the mean value of individual measurements for vertebrae L2–L4. Fractured vertebrae were excluded from the analysis.
Biochemical markers of bone turnover
Bone turnover markers were measured in serum obtained from each patient on the morning after overnight fasting. The bone formation marker, N-terminal type I procollagen propeptide (PINP) [inter-assay coefficient of variation (CV) 3.2–5.2%; Intact UniQ assay; Orion Diagnostica, Espoo, Finland] and bone resorption marker, isoform 5b of tartrate-resistant acid phosphatase (TRACP-5b) (inter-assay CV 5.0–9.0%; Immunodiagnostic Systems Ltd., Boldon, UK) were measured by ELISA, as previously described [20]. Levels of undercarboxylated osteocalcin (ucOC) were measured by a solid-phase enzyme immunoassay kit (inter-assay CV 5.2–8.3%; Takara Bio, Shiga, Japan), with a sensitivity of 0.25 ng/mL. UcOC reflects not only vitamin K deficiency, but also total bone turnover, because it is released from both osteoblasts and absorbed bone extracellular matrix by osteoclasts, as previously described [20, 29]. Intact parathyroid hormone (PTH) was measured using a two-site immunoradiometric assay (inter-assay CV 8.4%; Quest Diagnostics Nichols Institute, CA, USA). Serum PTH levels increase in response to a low serum 25-hydroxycholecalciferol level and low calcium intake [30], which promote bone resorption and consequent bone loss [31].
Radiographs
Spinal radiographs were obtained at baseline and at unscheduled times if subjects had symptoms suggestive of clinical vertebral fractures. For incidental non-vertebral fractures, radiographs were assessed by the investigator if subjects had symptoms.
Statistical analysis
Differences between each study group were tested using analysis of variance for normally distributed data, and the nonparametric Kruskal–Wallis test was used for non-normally distributed data. Changes in BMD and ranked bone turnover marker data from baseline to specified time points within each study group were compared using the nonparametric Wilcoxon signed-rank test. Results are expressed as mean ± standard error. A P value <0.05 was considered significant. All tests were performed using IBM SPSS Statistics version 22 software (IBM, Armonk, NY, USA).
Results
Patient baseline characteristics are shown in Table 1. Of the 194 study patients, 114 (58.8%) had their BPs switched. No significant differences were observed in baseline age, body mass index, estimated glomerular filtration rate, disease duration of RA, rheumatoid factor (RF) and anti-cyclic citrullinated peptide antibody (ACPA) positivity, and the prednisolone (PSL)/methotrexate/biologics usage rates among the three groups. The switch-to-TPTD group showed longer prior BP therapy duration, higher prior vertebral fracture rate, lower LS/TH/FN T score, and higher serum PINP and TRACP-5b levels, DAS28-CRP, and PSL dose, compared to the BP-continue group, suggesting that patients with severe osteoporosis and high disease activity were preferentially switched to TPTD. The switch-to-DMAb group tended to show a higher prior vertebral fracture rate, lower LS T score, higher serum PINP and TRACP-5b levels, and PSL dose, compared to the BP-continue group. The switch-to-DMAb group had a higher rate of vitamin D and calcium administration compared to both the BP-continue group and the switch-to-TPTD group. Eventually, 95.0% (76/80) of patients in the BP-continue group, 91.9% (68/74) of patients in the switch-to-DMAb group, and 85.0% (34/40) of patients in the switch-to-TPTD group completed the 18-month trial (Fig. 1).
Rate of clinical fragility fracture
During the 18-month period, the BP-continue group patients experienced 2 clinical vertebral, 2 hip, and 3 other non-vertebral fragility fractures (8.8%). The switch-to-DMAb group experienced 1 clinical vertebral and 2 non-vertebral fragility fractures (4.1%), and the switch-to-TPTD group experienced 1 hip fragility fracture (2.5%). No significant difference in the total fracture rate was observed among the groups (Fig. 1).
Bone turnover markers
Percent changes in bone turnover markers from baseline are shown in Fig. 2. The switch-to-DMAb group showed a significantly greater decrease compared to the BP-continue group in TRACP-5b levels from 6 months (−28.5 vs −3.5%; P < 0.001) to 18 months (−20.7 vs −1.7%; P < 0.05), in PINP levels at 18 months (−17.8 vs 19.9%; P < 0.05), and in ucOC levels at 12 months (−28.7 vs 21.7%; P < 0.01). On the other hand, the switch-to-TPTD group showed a significantly greater increase compared to the BP-continue group in TRACP-5b levels from 6 months (77.1 vs −3.5%; P < 0.001) to 18 months (44.9 vs −1.7%; P < 0.001), in PINP levels from 6 months (296.5 vs −9.6%; P < 0.001) to 18 months (156.8 vs 19.9%; P < 0.001), and in ucOC levels from 6 months (476.9 vs 13.5%; P < 0.001) to 18 months (219.4 vs 44.1%; P < 0.001). The absolute values of bone turnover markers are shown in Fig. 3. The switch-to-DMAb group showed significantly lower values compared to the BP-continue group in TRACP-5b levels from 12 months (153.7 vs 226.3 mU/dL; P < 0.001) to 18 months (152.2 vs 203.6 mU/dL; P < 0.01), in PINP levels from 12 months (18.1 vs 25.8 μg/L; P < 0.01) to 18 months (17.0 vs 31.6 μg/L; P < 0.01), and in ucOC levels from 6 months (0.9 vs 2.2 ng/mL; P < 0.001) to 18 months (1.0 vs 2.1 ng/mL; P < 0.001). On the other hand, the switch-to-TPTD group showed significantly higher values compared to the BP-continue group in TRACP-5b levels from 6 months (573.0 vs 220.1 mU/dL; P < 0.001) to 18 months (412.6 vs 203.6 mU/dL; P < 0.001), in PINP levels from 6 months (144.4 vs 26.8 μg/L; P < 0.001) to 18 months (85.7 vs 31.6 μg/L; P < 0.001), and in ucOC levels from 6 months (12.4 vs 2.2 ng/mL; P < 0.001) to 18 months (7.1 vs 2.1 ng/mL; P < 0.001). In the BP-continue group and the switch-to-DMAb group, the mean values of bone turnover markers were all within the reference values (TRACP-5b 120–420 mU/dL, PINP 14.9–68.8 μg/L, and ucOC <4.5 ng/mL).
Mean changes from baseline in serum concentrations of bone turnover markers TRAP-5b (a), PINP (b), and ucOC (c). BP bisphosphonate, DMAb denosumab, TPTD teriparatide, TRAP-5b isoform 5b of tartrate-resistant acid phosphatase, PINP type I collagen N-terminal propeptide, ucOC undercarboxylated osteocalcin. Bars indicate standard errors. ## P < 0.01, ### P < 0.001 BP-continue group versus switch-to-TPTD group. *P < 0.05, **P < 0.01, ***P < 0.001 BP-continue group versus switch-to-DMAb group. ††† P < 0.001 switch-to-DMAb group versus switch-to-TPTD group
Mean absolute values of bone turnover markers TRAP-5b (a), PINP (b), and ucOC (c). BP bisphosphonate, DMAb denosumab, TPTD teriparatide, TRAP-5b isoform 5b of tartrate-resistant acid phosphatase, PINP type I collagen N-terminal propeptide, ucOC undercarboxylated osteocalcin. Bars indicate standard errors. ## P < 0.01, ### P < 0.001 BP-continue group versus switch-to-TPTD group. **P < 0.01, ***P < 0.001 BP-continue group versus switch-to-DMAb group. †† P < 0.01, ††† P < 0.001 switch-to-DMAb group versus switch-to-TPTD group
Changes in BMD and TBS
Changes in LS BMD and TBS are shown in Fig. 4. In all groups, LS BMD showed significant increases compared to baseline from 6 to 18 months. The switch-to-DMAb group showed a significant increase in LS BMD from 6 months (2.8 vs 0.9%; P < 0.05) to 18 months (5.2 vs 2.3%; P < 0.01) compared to the BP-continue group. On the other hand, the switch-to-TPTD group showed significant increases in LS BMD from 6 months (4.2 vs 0.9%; P < 0.01) to 18 months (9.0 vs 2.3%; P < 0.001), and in TBS (2.1 vs −1.8%; P < 0.001) at 18 months compared to the BP-continue group. Moreover, the switch-to-TPTD group showed significantly greater increases in LS BMD (9.0 vs 5.2%; P < 0.05) and TBS (2.1 vs −0.7%; P < 0.05) compared to the switch-to-DMAb group at 18 months.
Mean changes from baseline in bone mineral density (BMD) at the lumbar spine (a) and trabecular bone score (b). ∫ P < 0.05, ∫∫∫ P < 0.001 change from baseline within each treatment group. BP bisphosphonate, DMAb denosumab, TPTD teriparatide. Bars indicate standard errors. ## P < 0.01, ### P < 0.001 BP-continue group versus switch-to-TPTD group. *P < 0.05, **P < 0.01 BP-continue group versus switch-to-DMAb group. † P < 0.05, †† P < 0.01 switch-to-DMAb group versus switch-to-TPTD group
Changes in TH and FN BMD are shown in Fig. 5. The switch-to-DMAb group showed significant increases in TH and FN BMD compared to the BP-continue group from 12 months (TH 3.1 vs 0.5%; P < 0.001/FN 3.9 vs 0.3%; P < 0.001) to 18 months (TH 3.9 vs 1.4%; P < 0.01/FN 3.8 vs 0.0%; P < 0.01). On the other hand, the switch-to-TPTD group showed significant decreases in TH and FN BMD compared to the switch-to-DMAb group at 6 months (TH −1.4 vs 1.5%; P < 0.05/FN −1.1 vs 2.0%; P < 0.01), although a linear increase was seen from 12 months. Finally, the switch-to-TPTD group showed equivalent increase in TH and FN BMD compared to the switch-to-DMAb group, and also showed significant increases in TH and FN BMD compared to the BP-continue group at 18 months (TH 4.0 vs 1.4%; P < 0.05/FN 4.9 vs 0.0%; P < 0.01).
Mean changes from baseline in bone mineral density (BMD) at the total hip (a) and femoral neck (b). BP bisphosphonate, DMAb denosumab, TPTD teriparatide. Bars indicate standard errors. ∫ P < 0.05, ∫∫ P < 0.01, ∫∫∫ P < 0.001 change from baseline within each treatment group. # P < 0.05, ## P < 0.01 BP-continue group versus switch-to-TPTD group. **P < 0.01, ***P < 0.001 BP-continue group versus switch-to-DMAb group. † P < 0.05, †† P < 0.01 switch-to-DMAb group versus switch-to-TPTD group
Discussion
Previous studies showed that systemic glucocorticoid therapy in RA is associated with enhanced endosteal bone resorption [32], and female patients with RA had a higher rate of cortical porosity compared to age-matched controls [33], suggesting cortical bone fragility in RA. DMAb showed greater inhibition of intracortical remodeling compared to ALN in humans [34], and switching ALN to DMAb showed reduced bone resorption and cortical porosity in ovariectomized monkeys [35]. Moreover, DMAb sustained modeling-based bone formation in endocortical and periosteal surfaces [36]. Taken together, switching BPs to DMAb may be effective, especially in inhibiting cortical remodeling and porosity with sustained modeling-based bone formation, which may be reflected as rapid and linear increases of TH and FN BMD, where cortical bone is relatively abundant.
PTH promotes bone formation not only by directly promoting Wnt signaling [9], but also by inhibiting Dkk-1 and sclerostin expression from osteocytes and osteoblasts [37]. Indeed, TPTD administration decreased serum sclerostin levels of postmenopausal women [38] and also showed greater induction of bone formation markers in RA compared to postmenopausal women [20]. In the present study, switching BPs to TPTD showed rapid induction of bone formation markers and linear increases of LS BMD and TBS, suggesting the efficacy of TPTD in restoring bone formation of RA.
Concerning hip BMD, a previous report showed that, in TPTD treatment, an initial hip BMD decrease was observed in anti-resorptive (mainly BP)-pretreated patients at 6 months, although a significant increase was seen from 12 months [39], and the same tendency was observed in the present study. This may be explained by endosteal cortical bone resorption in the initial phase, with simultaneous periosteal bone apposition and late mineralization of newly formed bone induced by TPTD administration [39].
Another interesting point is that in the prolonged BP-continue group, the TBS showed a significant decrease despite a significant increase of LS BMD. Previous reports showed that the change in TBS was relatively small compared to that of BMD [40, 41], and 24-month treatment with resedronate (RIS) significantly increased LS BMD, but didn’t increase TBS [40]. In addition, a low TBS was independently associated with daily glucocorticoid dose in patients with rheumatic diseases [42]. Taken together, prolonged BP treatment may not always assure the preservation of bone microarchitecture in patients with rheumatic diseases treated by glucocorticoids.
There are several limitations to this study. Baseline calcium intake and serum 25-hydroxy vitamin D levels could not be monitored. As TPTD is recommended to patients at high fracture risk, and also with careful administration in combination with calcium and vitamin D in Japan, the switch-to-TPTD group showed more severe osteoporosis, higher RA disease activity, longer duration of prior BPs prescription with a lower rate of minodronate (MIN) and higher rate of RIS (as MIN showed stronger bone turnover inhibition than RIS in RA [43]), and lower rate of calcium and vitamin D supplementation (which may affect BMD increase as previously described [44]) than the BP-continue and switch-to-DMAb groups, which may affect the results. Since spinal X-rays were not routinely performed, subclinical vertebral fractures could not be monitored, and fracture risk comparisons should be assessed in a larger cohort.
In conclusion, both the switch-to-DMAb group and the switch-to-TPTD group showed significant increases in LS, TH, and FN BMD, and the switch-to-TPTD group showed a higher increase in the TBS compared to the BP-continue group at 18 months. Switching BPs to DMAb or TPTD in female RA may provide some useful osteoporosis treatment options.
References
Mori Y, Kuwahara Y, Chiba S, Kogre A, Baba K, Kamimura M, Itoi E (2017) Bone mineral density of postmenopausal women with rheumatoid arthritis depends on disease duration regardless of treatment. J Bone Miner Metab 35:52–57
Peel NF, Moore DJ, Barrington NA, Bax DE, Eastell R (1995) Risk of vertebral fracture and relationship to bone mineral density in steroid treated rheumatoid arthritis. Ann Rheum Dis 54:801–806
van Staa TP, Geusens P, Bijlsma JW, Leufkens HG, Cooper C (2006) Clinical assessment of the long-term risk of fracture in patients with rheumatoid arthritis. Arthritis Rheum 54:3104–3112
Wright NC, Lisse JR, Walitt BT, Eaton CB, Chen Z (2011) Arthritis increases the risk for fractures—results from the Women’s Health Initiative. J Rheumatol 38:1680–1688
Braun T, Schett G (2012) Pathways for bone loss in inflammatory disease. Curr Osteoporos Rep 10:101–108
Ebina K, Oshima K, Matsuda M, Fukuhara A, Maeda K, Kihara S, Hashimoto J, Ochi T, Banda NK, Yoshikawa H, Shimomura I (2009) Adenovirus-mediated gene transfer of adiponectin reduces the severity of collagen-induced arthritis in mice. Biochem Biophys Res Commun 378:186–191
Kaneshiro S, Ebina K, Shi K, Higuchi C, Hirao M, Okamoto M, Koizumi K, Morimoto T, Yoshikawa H, Hashimoto J (2014) IL-6 negatively regulates osteoblast differentiation through the SHP2/MEK2 and SHP2/Akt2 pathways in vitro. J Bone Miner Metab 32:378–392
Noguchi T, Ebina K, Hirao M, Kawase R, Ohama T, Yamashita S, Morimoto T, Koizumi K, Kitaguchi K, Matsuoka H, Kaneshiro S, Yoshikawa H (2015) Progranulin plays crucial roles in preserving bone mass by inhibiting TNF-alpha-induced osteoclastogenesis and promoting osteoblastic differentiation in mice. Biochem Biophys Res Commun 465:638–643
Rhee Y, Lee EY, Lezcano V, Ronda AC, Condon KW, Allen MR, Plotkin LI, Bellido T (2013) Resorption controls bone anabolism driven by parathyroid hormone (PTH) receptor signaling in osteocytes. J Biol Chem 288:29809–29820
Terpos E, Fragiadaki K, Konsta M, Bratengeier C, Papatheodorou A, Sfikakis PP (2011) Early effects of IL-6 receptor inhibition on bone homeostasis: a pilot study in women with rheumatoid arthritis. Clin Exp Rheumatol 29:921–925
Almeida M, Han L, Ambrogini E, Weinstein RS, Manolagas SC (2011) Glucocorticoids and tumor necrosis factor alpha increase oxidative stress and suppress Wnt protein signaling in osteoblasts. J Biol Chem 286:44326–44335
Kanis JA, Johansson H, Oden A, Johnell O, de Laet C, Melton Iii LJ, Tenenhouse A, Reeve J, Silman AJ, Pols HA, Eisman JA, McCloskey EV, Mellstrom D (2004) A meta-analysis of prior corticosteroid use and fracture risk. J Bone Miner Res 19:893–899
Van Staa TP, Leufkens HG, Abenhaim L, Zhang B, Cooper C (2000) Use of oral corticosteroids and risk of fractures. J Bone Miner Res 15:993–1000
Saag KG, Zanchetta JR, Devogelaer JP, Adler RA, Eastell R, See K, Krege JH, Krohn K, Warner MR (2009) Effects of teriparatide versus alendronate for treating glucocorticoid-induced osteoporosis: thirty-six-month results of a randomized, double-blind, controlled trial. Arthritis Rheum 60:3346–3355
Kendler DL, Roux C, Benhamou CL, Brown JP, Lillestol M, Siddhanti S, Man HS, San Martin J, Bone HG (2010) Effects of denosumab on bone mineral density and bone turnover in postmenopausal women transitioning from alendronate therapy. J Bone Miner Res 25:72–81
Finkelstein JS, Wyland JJ, Lee H, Neer RM (2010) Effects of teriparatide, alendronate, or both in women with postmenopausal osteoporosis. J Clin Endocrinol Metab 95:1838–1845
Ebina K, Hashimoto J, Kashii M, Hirao M, Kaneshiro S, Noguchi T, Tsukamoto Y, Yoshikawa H (2016) The effects of switching daily teriparatide to oral bisphosphonates or denosumab in patients with primary osteoporosis. J Bone Miner Metab 35(1):91–98
Mok CC, Ho LY, Ma KM (2015) Switching of oral bisphosphonates to denosumab in chronic glucocorticoid users: a 12-month randomized controlled trial. Bone 75:222–228
Takeuchi T, Tanaka Y, Ishiguro N, Yamanaka H, Yoneda T, Ohira T, Okubo N, Genant HK, van der Heijde D (2016) Effect of denosumab on Japanese patients with rheumatoid arthritis: a dose-response study of AMG 162 (Denosumab) in patients with Rheumatoid arthritis on methotrexate to Validate inhibitory effect on bone Erosion (DRIVE)—a 12-month, multicentre, randomised, double-blind, placebo-controlled, phase II clinical trial. Ann Rheum Dis 75:983–990
Ebina K, Hashimoto J, Shi K, Kashii M, Hirao M, Yoshikawa H (2014) Comparison of the effect of 18-month daily teriparatide administration on patients with rheumatoid arthritis and postmenopausal osteoporosis patients. Osteoporos Int 25:2755–2765
Orimo H, Nakamura T, Hosoi T, Iki M, Uenishi K, Endo N, Ohta H, Shiraki M, Sugimoto T, Suzuki T, Soen S, Nishizawa Y, Hagino H, Fukunaga M, Fujiwara S (2012) Japanese 2011 guidelines for prevention and treatment of osteoporosis—executive summary. Arch Osteoporos 7:3–20
Nawata H, Soen S, Takayanagi R, Tanaka I, Takaoka K, Fukunaga M, Matsumoto T, Suzuki Y, Tanaka H, Fujiwara S, Miki T, Sagawa A, Nishizawa Y, Seino Y (2005) Guidelines on the management and treatment of glucocorticoid-induced osteoporosis of the Japanese Society for Bone and Mineral Research (2004). J Bone Miner Metab 23:105–109
Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS (1988) The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 31:315–324
Ebina K, Shi K, Hirao M, Kaneshiro S, Morimoto T, Koizumi K, Yoshikawa H, Hashimoto J (2013) Vitamin K2 administration is associated with decreased disease activity in patients with rheumatoid arthritis. Mod Rheumatol 23:1001–1007
Matsui T, Kuga Y, Kaneko A, Nishino J, Eto Y, Chiba N, Yasuda M, Saisho K, Shimada K, Tohma S (2007) Disease activity score 28 (DAS28) using C-reactive protein underestimates disease activity and overestimates EULAR response criteria compared with DAS28 using erythrocyte sedimentation rate in a large observational cohort of rheumatoid arthritis patients in Japan. Ann Rheum Dis 66:1221–1226
Ebina K, Hashimoto J, Shi K, Kashii M, Hirao M, Yoshikawa H (2015) Undercarboxylated osteocalcin may be an attractive marker of teriparatide treatment in RA patients: response to Mokuda. Osteoporos Int 26:1445
Hans D, Barthe N, Boutroy S, Pothuaud L, Winzenrieth R, Krieg MA (2011) Correlations between trabecular bone score, measured using anteroposterior dual-energy X-ray absorptiometry acquisition, and 3-dimensional parameters of bone microarchitecture: an experimental study on human cadaver vertebrae. J Clin Densitom 14:302–312
Boutroy S, Hans D, Sornay-Rendu E, Vilayphiou N, Winzenrieth R, Chapurlat R (2013) Trabecular bone score improves fracture risk prediction in non-osteoporotic women: the OFELY study. Osteoporos Int 24:77–85
Booth SL, Centi A, Smith SR, Gundberg C (2013) The role of osteocalcin in human glucose metabolism: marker or mediator? Nat Rev Endocrinol 9:43–55
Aloia J, Bojadzievski T, Yusupov E, Shahzad G, Pollack S, Mikhail M, Yeh J (2010) The relative influence of calcium intake and vitamin D status on serum parathyroid hormone and bone turnover biomarkers in a double-blind, placebo-controlled parallel group, longitudinal factorial design. J Clin Endocrinol Metab 95:3216–3224
Chaitou A, Boutroy S, Vilayphiou N, Varennes A, Richard M, Blaizot S, Munoz F, Delmas PD, Goudable J, Chapurlat R, Szulc P (2011) Association of bone microarchitecture with parathyroid hormone concentration and calcium intake in men: the STRAMBO study. Eur J Endocrinol 165:151–159
Aeberli D, Schett G (2013) Cortical remodeling during menopause, rheumatoid arthritis, glucocorticoid and bisphosphonate therapy. Arthritis Res Ther 15:208
Zhu TY, Griffith JF, Qin L, Hung VW, Fong TN, Au SK, Tang XL, Kwok AW, Leung PC, Li EK, Tam LS (2013) Structure and strength of the distal radius in female patients with rheumatoid arthritis: a case–control study. J Bone Miner Res 28:794–806
Zebaze RM, Libanati C, Austin M, Ghasem-Zadeh A, Hanley DA, Zanchetta JR, Thomas T, Boutroy S, Bogado CE, Bilezikian JP, Seeman E (2014) Differing effects of denosumab and alendronate on cortical and trabecular bone. Bone 59:173–179
Kostenuik PJ, Smith SY, Samadfam R, Jolette J, Zhou L, Ominsky MS (2015) Effects of denosumab, alendronate, or denosumab following alendronate on bone turnover, calcium homeostasis, bone mass and bone strength in ovariectomized cynomolgus monkeys. J Bone Miner Res 30:657–669
Ominsky MS, Libanati C, Niu QT, Boyce RW, Kostenuik PJ, Wagman RB, Baron R, Dempster DW (2015) Sustained modeling-based bone formation during adulthood in cynomolgus monkeys may contribute to continuous BMD gains with denosumab. J Bone Miner Res 30:1280–1289
Baron R, Hesse E (2012) Update on bone anabolics in osteoporosis treatment: rationale, current status, and perspectives. J Clin Endocrinol Metab 97:311–325
Drake MT, Srinivasan B, Modder UI, Peterson JM, McCready LK, Riggs BL, Dwyer D, Stolina M, Kostenuik P, Khosla S (2010) Effects of parathyroid hormone treatment on circulating sclerostin levels in postmenopausal women. J Clin Endocrinol Metab 95:5056–5062
Obermayer-Pietsch BM, Marin F, McCloskey EV, Hadji P, Farrerons J, Boonen S, Audran M, Barker C, Anastasilakis AD, Fraser WD, Nickelsen T (2008) Effects of two years of daily teriparatide treatment on BMD in postmenopausal women with severe osteoporosis with and without prior antiresorptive treatment. J Bone Miner Res 23:1591–1600
Di Gregorio S, Del Rio L, Rodriguez-Tolra J, Bonel E, Garcia M, Winzenrieth R (2015) Comparison between different bone treatments on areal bone mineral density (aBMD) and bone microarchitectural texture as assessed by the trabecular bone score (TBS). Bone 75:138–143
Krieg MA, Aubry-Rozier B, Hans D, Leslie WD (2013) Effects of anti-resorptive agents on trabecular bone score (TBS) in older women. Osteoporos Int 24:1073–1078
Koumakis E, Avouac J, Winzenrieth R, Toth E, Payet J, Kahan A, Allanore Y, Cormier C (2015) Trabecular bone score in female patients with systemic sclerosis: comparison with rheumatoid arthritis and influence of glucocorticoid exposure. J Rheumatol 42:228–235
Ebina K, Noguchi T, Hirao M, Hashimoto J, Kaneshiro S, Yukioka M, Yoshikawa H (2016) Effects of switching weekly alendronate or risedronate to monthly minodronate in patients with rheumatoid arthritis: a 12-month prospective study. Osteoporos Int 27:351–359
Joo NS, Dawson-Hughes B, Kim YS, Oh K, Yeum KJ (2013) Impact of calcium and vitamin D insufficiencies on serum parathyroid hormone and bone mineral density: analysis of the fourth and fifth Korea National Health and Nutrition Examination Survey (KNHANES IV-3, 2009 and KNHANES V-1, 2010). J Bone Miner Res 28:764–770
Acknowledgements
The authors would like to thank Dr. Masao Yukioka and Dr. Kenrin Shi for their excellent cooperation in conducting the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
K. Ebina, M. Hirao, J. Hashimoto, and H. Yoshikawa have received research grants from Astellas Pharma and Eisai Co. Ltd. K. Ebina, M. Hirao, K. Hagihara, and H. Yoshikawa have received research grants from Daiichi Sankyo. H. Yoshikawa has received a research grant from MSD. K. Hagihara has received a research grant from Eli Lily. K. Ebina has received payments for lectures from Daiichi Sankyo. M. Kashii, K. Kitaguchi, H. Matsuoka, T. Iwahashi, R. Chijimatsu declare that they have no conflicts of interest. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
About this article
Cite this article
Ebina, K., Hirao, M., Hashimoto, J. et al. Assessment of the effects of switching oral bisphosphonates to denosumab or daily teriparatide in patients with rheumatoid arthritis. J Bone Miner Metab 36, 478–487 (2018). https://doi.org/10.1007/s00774-017-0861-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-017-0861-4