Abstract
Summary
Bone mineral density (BMD) sometimes cannot be improved by long-term bisphosphonate (BP) therapy in osteoporosis (OP) patients with rheumatoid arthritis (RA). This study showed that BMD significantly increased after denosumab treatment in patients with long-term BP pre-treatment as much as in treatment-naïve patients. Thus, denosumab can be a strong OP treatment option for long-term BP pre-treated RA patients.
Introduction
The aim of this 24-month retrospective study was to evaluate differences in outcomes of denosumab with or without bisphosphonate (BP) pre-treatment in osteoporosis (OP) patients with rheumatoid arthritis (RA).
Methods
Patients were divided into those with (BP group, 26 cases) or without (denosumab group, 26 cases) BP pre-treatment. We measured serum BAP, TRACP-5b, and urinary NTX at baseline and every 3 months for 24 months. We also assessed bone mineral density (BMD) of the lumbar 1–4 vertebrae (L-BMD) and total hip BMD (H-BMD) at baseline and every 6 months for 24 months. MMP-3, DAS28-CRP, SDAI, and HAQ-DI were assessed at baseline and 24 months to evaluate RA state.
Results
In BP group, the percent changes of bone turnover markers decreased but were consistently higher compared with those in the denosumab group. There were significant differences of the percent changes in BAP at 9, 21, and 24 months; TRACP-5b at 9, 18, and 21 months; and urinary NTX at 3, 9, 12, 15, 18, and 21 months between the groups. The percent changes of L-BMD and H-BMD were significantly increased at 24 months in the BP pre-treated group (11.5 and 13.3%, respectively) and denosumab group (13.0 and 16.5%, respectively). There was a significant difference of the percent changes in H-BMD at 6 months between the groups. There was no significant difference in RA state between the groups.
Conclusions
Compared with BP group, denosumab group displayed significantly increased H-BMD at 6 months, while L-BMD and H-BMD were significantly increased for 24 months in both groups. Thus, regardless of BP pre-treatment, denosumab could be a good agent in OP with RA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis (OP) is a major complication of rheumatoid arthritis (RA) [1]. Gauri et al. have reported that approximately 30% RA patients are at increased risk of OP and the importance of bone mineral density (BMD) measurement to assess bone loss and fracture prevention [2]. Tada et al. have described that (1) the BMD was lower among patients with RA than non-RA controls and (2) use of bisphosphonate (BP) was a significant factor contributing to BMD increase, while use of biologic agents, reducing glucocorticoid (GC) dose, and control of disease activity were not significant factors for gain of BMD [3]. OP treatment has recently attracted attention, and several drugs have been developed. Among these, BPs, and denosumab, a fully human monoclonal antibody against RANKL, are the major OP agents. BP therapy is generally recognized as a first-line treatment of OP through the inhibition of osteoclast activity [4]. Also, denosumab strongly abrogates bone resorption, increases BMD, and prevents fragility fractures [5, 6]. Currently, there have been a few reports on the efficacy of BP and/or denosumab in OP with RA patients.
We have previously reported that calcium and vitamin D addition to denosumab represents an important treatment option with additive effects on the increase of H-BMD in OP with RA for 12 months. During the study period, no fracture or hypocalcemia occurred. Thus, denosumab plus calcium and vitamin D supplementation is a good therapeutic agent for OP patients with RA to improve BMD and bone turnover markers, and to prevent fractures. However, all of the patients had been pre-treated with BP prior to denosumab therapy in that study [7]. We have also previously reported that denosumab is a strong OP treatment option for BP-unresponsive OP patients in post-menopausal women [8]. Thus, we hypothesized that denosumab could improve BMD and prevent fracture in OP with RA, especially in long-term BP pre-treated cases.
Kinoshita et al. [9] recently compared the efficacies of denosumab and BPs for preventing secondary OP, and inflammation caused by excessive bone resorption in OP treatment-naive RA patients. They showed that neither treatment could suppress inflammation as measured by C-reactive protein (CRP) and matrix metalloproteinase-3 (MMP-3), RA disease activity as measured by disease activity score (DAS) 28-CRP and the simplified disease activity index (SDAI), or physical functional evaluation of RA as measured by the patient-reported health assessment questionnaire-disability index (HAQ-DI). However, denosumab significantly suppressed a marker of bone metabolism [9]. To date, there has been no report on the comparative data with or without BP pre-treatment in OP with RA after denosumab treatment.
The aim of this 24-month retrospective study was to evaluate differences in the outcomes with or without BP pre-treatment in OP patients with RA.
Materials and methods
The inclusion criteria of this 24-month retrospective study were OP patients with low bilateral total hip BMD (H-BMD) and/or lumbar 1–4 BMD (L-BMD) (i.e., less than − 3.0 standard deviation (SD)) with RA. The exclusion criteria in this study were patients with chronic renal failure (estimated glomerular filtration rate [eGFR] < 40 ml/min/1.73 m2); bone metabolic disorders or diabetes mellitus, both of which affect OP; and fracture within 1 year prior to the study. The diagnosis of OP was made in accordance with the revised criteria established by the Japanese Society of Bone and Mineral Research [10]. The diagnosis and treatment of RA were conducted in accordance with the 2010 ACR/European League Against Rheumatism (EULAR) classification system [11].
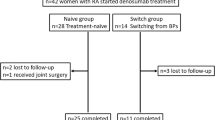
Fifty-two Japanese female OP patients having RA with low-to-moderate disease activity (2.6 < DAS28-CRP ≤ 5.1) were recruited at our institution between 2015 and 2017 and summarized in Table 1. The patients were retrospectively classified into the cases with BPs who had been taking them for 5 years or longer (BP group; 26 cases) or the treatment-naive group (denosumab group; 26 cases) matched on the basis of age, gender, body mass index (BMI), pre-treated BP period, disease duration of RA, and disease activity (Table 1). Alendronate, risedronate, and minodronate were adopted in various regimens as long-term BP pre-treatment. We did not examine the effects of individual BP drugs since they were routinely changed for patients exhibiting low responsiveness. In the denosumab group, all treatments were substituted from BPs to denosumab at the baseline. BP treatment was ceased prior to study commencement. Mean age was 69.8 ± 1.3 years in the BP pre-treated group and 70.6 ± 1.9 years in the denosumab group. Enrolled patients in the BP pre-treated group had received BP pre-treatment for an average duration of 15.3 ± 2.2 years. The average doses of methotrexate (MTX) and prednisolone (PSL) in the BP pre-treated group and denosumab groups were 7.7 ± 0.7 and 7.7 ± 1.0 mg/week and 5.8 ± 0.2 and 6.0 ± 1.0 mg/day, respectively (Table 1).
All serologic analyses were conducted just prior to denosumab commencement (baseline) and at 24 months of treatment using cryogenically stored samples by commercially available kits in accordance with each manufacturer’s instructions, including MMP-3 (Kyowa Pharma Chemicals, Toyama, Japan). We also examined changes in DAS28-CRP, SDAI, and HAQ-DI as indicators of RA status for all patients at the same time points. All data are expressed as the mean ± standard error (SE).
Each patient received denosumab (60 mg, s.c.) once every 6 months in both groups. We gave vitamin D supplementation tablets (762.5 mg of precipitated calcium carbonate, 200 IU of cholecalciferol, 59.2 mg of magnesium carbonate) twice daily to all of the patients after denosumab administration.
Serum bone alkaline phosphatase (BAP) was measured as bone formation marker using a chemiluminescent enzyme immunoassay. Serum tartrate-resistant acid phosphatase (TRACP)-5b and urinary N-terminal telopeptide of type I collagen (NTX) (Osteomark, Osteox International, Seattle, WA) were assessed as markers of bone resorption. TRACP-5b and urinary NTX were measured using ELISA. Serum levels of whole parathyroid hormone (PTH 1–84) were evaluated as bone turnover markers by immunoradiometric assays. Serum levels of 1,25(OH)2D3 were measured by immunoradiometric assays. After overnight fasting and omitting the first morning samples, serum and urine were collected between 8:30 a.m. and 11:00 a.m.. Serum samples were stored at − 80 °C until bone turnover marker assessment at the end of the study. Samples were collected before treatment administration, at 3, 6, 9, 12, 15, 18, 21, and 24 months after denosumab treatment.
Bone turnover markers and BMD were determined for each time point and comparisons were made between the groups using statistical analysis. BMD was measured using a dual-energy X-ray absorption fan-beam bone densitometer (Lunar Prodigy; GE Healthcare Bio-Sciences Corp., Piscataway, NJ, USA) at the L1–4 levels of the posteroanterior spine and bilateral total hips. H-BMD was calculated as the average BMD of the right and left hips. BMD was examined before treatment administration and at 6, 12, 18, and 24 months. Coefficients of variation for the lumbar spine and bilateral total hips were 1.0 and 0.6%, respectively [12].
In both groups, the percent changes of markers were determined at each time point using Bonferroni correction for multiple comparisons. Marker comparisons between the groups at each time point were performed by Welch’s t test. On the basis of an SD of 2–5% and a sample size of 26 women in the BP group and 26 women in the denosumab group, we calculated that the study had 80% power to detect at least a 3% difference in L-BMD values. P values of < 0.05 were considered to be statistically significant.
This investigation was approved by the Institutional Ethical Review Board of Shinshu University School of Medicine no. 2365), Japan, prior to its commencement. Written informed consent was obtained from all subjects. Study methods were carried out in accordance with the approved guidelines.
Results
Table 1 presents the patient characteristics prior to this study. Table 2 presents the value changes of key parameters at the study end point.
Serum-corrected calcium levels
The percent changes of serum calcium were not significant in either group during the observational period as compared with pre-treatment levels. There was no significant difference between the groups (Fig. 1a).
Serum whole PTH and 1,25(OH)2D3
The percent change of serum whole PTH tended to increase at 3 months in both groups, with no significant differences between them (Fig. 1b).
The percent change of serum 1,25(OH)2D3 increased at 3 months and then gradually decreased in both groups, with no significant differences between them (Fig. 1c).
Bone turnover markers
Serum BAP, TRACP-5b, and urinary NTX values in the BP pre-treated were significantly lower (P = 0.017, P = 0.0002, or P = 0.0003, respectively) than those in the denosumab group prior to denosumab therapy (Table 1 and Fig. 3). The percent changes of serum BAP, TRACP-5b, and urinary NTX were also evaluated (Fig. 2).
Percent changes of serum bone-specific alkaline phosphatase (BAP) (a), serum tartrate-resistant acid phosphatase (TRACP)-5b (b), and urinary cross-linked N-terminal telopeptides of type I collagen (NTX) (c) for the 24-month study period. Closed circles show the BP pre-treated group and closed triangles show the denosumab group. Single asterisk denotes significant differences (P < 0.05) at each time point compared with pretreatment in the BP pre-treated group or denosumab groups. Single hashtag shows significant differences (P < 0.05) between the groups at each time point
Bone formation markers
The percent changes of serum BAP were significantly decreased at 3 and 12 months in the BP pre-treated group, and at 9 to 24 months in the denosumab group, compared with pre-treatment levels. There were significant differences at 9, 21, and 24 months between them (Fig. 2a). The changes of serum BAP values were significantly decreased at 3, 9, 12, 15, and 21 months in the BP pre-treated group and significantly decreased at 3, 9, 12, 15, and 18 months in the denosumab group, compared with pre-treatment levels (Fig. 3a). There were significant differences at 0, 3, 6, 12, 15, and 18 months between them (Fig. 3a).
Value changes of serum bone-specific alkaline phosphatase (BAP) (a), serum tartrate-resistant acid phosphatase (TRACP)-5b (b), and urinary cross-linked N-terminal telopeptides of type I collagen (NTX) (c) for the 24-month study period. Closed circles show the BP pre-treated group and closed triangles show the denosumab group. Single asterisk denotes significant differences (P < 0.05), at each time point compared with pretreatment in the BP pre-treated group or denosumab groups. Single hashtag shows significant differences (P < 0.05), between the groups at each time point
Bone resorption markers
The percent changes of TRACP-5b were significantly decreased at 3, 9, and 15 months in the BP pre-treated group and 3, 9, 15, 18, and 21 months in the denosumab group, compared with pre-treatment levels. There were significant differences at 9, 18, and 21 months between the groups (Fig. 2b). The changes of serum TRACP-5b values were significantly decreased at every time point in the BP pre-treated group and significantly decreased at every time point except for 24 months in the denosumab group, compared with pre-treatment levels (Fig. 3b). There were significant differences at 0, 3, 6, 9, and 12 months between the groups (Fig. 3b).
The percent changes of urinary NTX were significantly decreased at 15 months in the BP pre-treated group and every time point in the denosumab group, compared with pre-treatment levels. There were significant differences at 3, 9, 12, 15, 18, and 21 months between the groups (Fig. 2c). The changes of urinary NTX values were significantly lower at 3, 9, 12, and 15 months in the BP pre-treated group, and at every time point except for 24 months in the denosumab group, compared with pre-treatment levels (Fig. 3c). There were significant differences at 0 and 12 months between them (Fig. 3c).
Bone mineral density
L-BMD and H-BMD
The percent changes of L-BMD increased steadily during the study period in the BP pre-treated group (11.5% increase at 24 months) and denosumab group (13.0% increase at 24 months). The percent changes of L-BMD significantly increased in both groups at every time point except for at 6 months in the BP pre-treated group, compared with pre-treatment levels. There were also no significant differences between the groups at any time point (Fig. 4a).
Percent changes in lumbar bone mineral density (L-BMD) (a) and bilateral total hip BMD (H-BMD) for the 24-month study period. Closed circles show the BP pre-treated group, and closed triangles show the denosumab group. Single asterisk denotes significant differences (P < 0.05), at each time point compared with pretreatment in the BP pre-treated group or denosumab groups. Single hashtag shows significant differences (P < 0.05) between the groups at each time point
The percent changes of H-BMD also rose steadily during the observational period in the BP pre-treated group (13.3% increase at 24 months) and denosumab group (16.5% increase at 24 months). H-BMD was significantly higher in the denosumab group at every time point and in the BP pre-treated group at 18 and 24 months, compared with pre-treatment levels. There was a significant difference between the groups at 6 months (P < 0.05) (Fig. 4b).
Indicators of RA state
MMP-3, DAS28-CRP, SDAI, and HAQ-DI
RA state before treatment was matched in both groups (Table 1). There were no significant differences in the percent changes of MMP-3, DAS28-CRP, SDAI, or HAQ-DI between the groups during follow-up (Tables 1 and 2).
No serious adverse events, such as hypocalcemia or fracture, were noted during the 24-month study period.
Discussion
We report for the first time comparative data with or without BP pre-treatment in OP patients with RA after denosumab therapy for 24 months. Both L-BMD and H-BMD significantly increased for 24 months, while the percent changes of bone turnover markers were more strongly suppressed in the denosumab group than those in the BP pre-treated group. No fracture or hypocalcemia occurred, and denosumab did not affect the RA state during the treatment period in both groups. Thus, regardless with or without BP pre-treatment, denosumab is a good agent to treat OP with RA.
We hypothesized that denosumab could improve BMD and prevent fracture in OP with RA, especially in long-term BP pre-treated cases that had been receiving BPs for 5 years or longer, since we have previously reported that denosumab improved L-BMD as well as H-BMD in BP-unresponsive cases who had been taking BPs for 2 years or longer in primary OP [8]. Moreover, in post-menopausal Japanese OP patients, compared with denosumab with BP therapy, denosumab without BP therapy more significantly increased the percent changes of L-BMD, and denosumab also significantly increased BMD in both groups for 36 months, compared with pre-treatment levels (Nakamura et al., unpublished data). This study showed that in the BP pre-treated group, L-BMD and H-BMD increased at 24 months (11.5 or 13.0%, respectively) and in the denosumab group, L-BMD and H-BMD increased at 24 months (13.3 or 16.5%, respectively), compared with those before treatment. BMD significantly and approximately equally increased for 24 months in both groups after therapy. In addition, no fracture occurred for 24 months during the study period. Thus, denosumab is a good therapeutic option to improve BMD and prevent fracture, not only in primary OP but also OP with RA. Even though there was no significant difference of L-BMD, there was a significant difference of H-BMD at 6 months between the groups. These findings suggest that denosumab could be more useful to improve H-BMD, and thereby preventing proximal femoral fracture, particularly at early phases of the treatment in OP with RA.
Patients with RA are more susceptible for bone loss in comparison to normal age and gender-related subjects. Also, RA patients taking anti-rheumatic therapy (steroids and disease-modifying antirheumatic drugs) are at increased risk of bone loss. All these factors contribute to bone loss independent of each other [2]. Bone loss is thought to be mediated via the RANKL pathway, which is a key player in bone destruction in RA. RANKL has also been identified as an essential cytokine for the differentiation, function, formation, activation, and survival of osteoclasts [13]. Very recently, Mochizuki et al. reported that denosumab could suppress bone erosion in RA patients and concluded that it might be effective for OP and joint destruction in patients with RA [14].
There have been numerous reports that RA patients have high risk for OP and fracture [15]. Thus, appropriate OP treatment in RA patients is required to reduce fracture risk and bone loss. In this study, the patients in each group were retrospectively matched on the basis of age, gender, BMI, disease duration of RA and the disease activity, except for the BP pre-treatment therapy. The patients in the BP pre-treated group took 7.7 ± 0.7 mg methotrexate (MTX) per week and 5.8 ± 0.2 mg prednisolone (PSL) per day while the patients in the denosumab group did 7.7 ± 1.0 mg MTX per week and 6.0 ± 1.0 mg PSL per day on average, all of which showed no significant difference between the groups. In both groups, state (DAS28-CRP, SDAI, HAQ-DI, and MMP-3) was improved in neither group, which are consistent with the previous report [9]. These findings suggest that denosumab did not suppress RA activity in either BP pre-treated or treatment-naïve OP patients.
The efficacy and safety of denosumab therapy in patients with GC-induced OP (GIO) have yet to be established. Ishiguro et al. recently described that BMD was significantly improved by denosumab therapy in GIO subjects with pulmonary diseases [16], and Sawamura et al. reported that denosumab significantly ameliorated L-BMD as well as BMD of the femoral neck at 12 months and bone turnover markers at 12 months [17]. In their study, no serious adverse effects occurred in comparisons with patients having post-menopausal OP [16, 17]. Thus, denosumab represents a good agent for improving bone metabolism in GIO as well.
The main limitation of this investigation is its small sample size. A subsequent long-term observational period also will be needed to clarify if (1) BMD increases continuously by denosumab and to what extent fractures are prevented and (2) hypocalcemia or adverse effects will later occur.
Conclusions
This study is the first to demonstrate a direct comparison between the effects of denosumab in treatment-naïve (denosumab group) and long-term BP pre-treated (BP group) OP cases complicated with RA. Compared with BP group, denosumab group significantly increased H-BMD at 6 months in OP patients with RA, while L-BMD and H-BMD significantly increased for 24 months in both groups, compared with those before treatment. Thus, regardless of BP pre-treatment, denosumab is a good option to treat OP with RA.
References
Kvien TK, Haugeberg G, Uhlig T, Falch JA, Halse JI, Lems WF, Dijkmans BA, Woolf AD (2000) Data driven attempt to create a clinical algorithm for identification of women with rheumatoid arthritis at high risk of osteoporosis. Ann Rheum Dis 59:805–811
Gauri LA, Fatima Q, Diggi S, Khan A, Liyakat A, Ajay BR (2017) Study of bone mineral density (BMD) in patients with rheumatoid arthritis and its co-relation with severity of the disease. J Assoc Physicians India 65:26–30
Tada M, Inui K, Sugioka Y, Mamoto K, Okano T, Anno S, Koike T (2017) Use of bisphosphonate might be important to improve bone mineral density in patients with rheumatoid arthritis even under tight control: the TOMORROW study. Rheumatol Int 37:999–1005
Black DM, Bauer DC, Schwartz AV, Cummings SR, Rosen CJ (2012) Continuing bisphosphonate treatment for osteoporosis—for whom and for how long? N Engl J Med 366(22):2051–2053
McClung MR, Lewiecki EM, Cohen SB, Bolognese MA, Woodson GC, Moffett AH, Peacock M, Miller PD, Lederman SN, Chesnut CH, Lain D, Kivitz AJ, Holloway DL, Zhang C, Peterson MC, Bekker PJ, AMG 162 Bone Loss Study Group (2006) Denosumab in postmenopausal women with low bone mineral density. N Engl J Med 354:821–831
Silva I, Branco JC (2012) Denosumab: recent update in postmenopausal osteoporosis. Acta Reumatol Port 37:302–313 Review
Nakamura Y, Suzuki T, Yoshida T, Yamazaki H, Kato H (2017) Vitamin D and calcium are required during denosumab treatment in osteoporosis with rheumatoid Arthritis. Nutrients 9(5):E428 https://doi.org/10.3390/nu9050428
Kamimura M, Nakamura Y, Ikegami S, Uchiyama S, Kato H, Taguchi A (2017) Significant improvement of bone mineral density and bone turnover markers by denosumab therapy in bisphosphonate-unresponsive patients. Osteoporos Int 28:1757–1758
Kinoshita H, Miyakoshi N, Kashiwagura T, Kasukawa Y, Sugimura Y, Shimada Y (2017) Comparison of the efficacy of denosumab and bisphosphonates for treating secondary osteoporosis in patients with rheumatoid arthritis. Mod Rheumatol 27(4):582-586
Soen S (2014) New diagnostic criteria and guidelines on osteoporosis. Diagnostic criteria for primary osteoporosis : year 2012 revision. (article in Japanese). Clin Calcium 24:323–329
van der Linden MP, Knevel R, Huizinga TW, van der Helm-van Mil AH (2011) Classification of rheumatoid arthritis: comparison of the 1987 American College of Rheumatology criteria and the 2010 American College of Rheumatology/European League Against Rheumatism criteria. Arthritis Rheum 63:37–42
Nakamura Y, Suzuki T, Kamimura M, Ikegami S, Uchiyama S, Kato H (2017) Alfacalcidol increases the therapeutic efficacy of Ibandronate on bone mineral density in Japanese women with primary osteoporosis. Tohoku J Exp Med 241:319–326
Boyle WJ, Simonet WS, Lacey DL (2003) Osteoclast differentiation and activation. Nature 423(6937):337–342
Mochizuki T, Yano K, Ikari K, Kawakami K, Hiroshima R, Koenuma N, Ishibashi M, Momohara S (2017) Effects of denosumab treatment on bone mineral density and joint destruction in patients with rheumatoid arthritis. J Bone Miner Metab https://doi.org/10.1007/s00774-017-0848-1
Kim D, Cho SK, Choi CB, Jun JB, Kim TH, Lee HS, Lee J, Lee SS, Yoo DH, Yoo WH, Sung YK, Bae SC (2016) Incidence and risk factors of fractures in patients with rheumatoid arthritis: an Asian prospective cohort study. Rheumatol Int 36:1205–1214
Ishiguro S, Ito K, Nakagawa S, Hataji O, Sudo A (2017) The clinical benefits of denosumab for prophylaxis of steroid-induced osteoporosis in patients with pulmonary disease. Arch Osteoporos 12:44
Sawamura M, Komatsuda A, Togashi M, Wakui H, Takahashi N (2017) Effects of denosumab on bone metabolic markers and bone mineral density in patients treated with glucocorticoids. Intern Med 56:631–636
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This investigation was approved by the Institutional Ethical Review Board of Shinshu University School of Medicine no. 2365), Japan, prior to its commencement.
Conflict of interest
None.
Rights and permissions
About this article
Cite this article
Nakamura, Y., Suzuki, T. & Kato, H. Denosumab significantly improves bone mineral density with or without bisphosphonate pre-treatment in osteoporosis with rheumatoid arthritis. Arch Osteoporos 12, 80 (2017). https://doi.org/10.1007/s11657-017-0371-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11657-017-0371-y