Abstract
Summary
This meta-analysis investigated the association of C677T polymorphism in MTHFR gene with bone mineral density (BMD) and fracture risk. The results suggested that C677T polymorphism was marginally associated with fracture risk. In addition, this polymorphism was modestly associated with BMD of lumbar spine, femoral neck, total hip, and total body, respectively.
Introduction
The methylenetetrahydrofolate reductase (MTHFR) gene has been implicated in the regulation of BMD and, thus, may serve as a potential risk factor for the development of fracture. However, results have been inconsistent. In this study, a meta-analysis was performed to clarify the association of C677T polymorphism in MTHFR gene with BMD and fracture risk.
Methods
Published literature from PubMed and EMBASE were searched for eligible publications. Pooled odds ratio (OR) or weighted mean difference (WMD) and 95% confidence interval (CI) were calculated using a fixed- or random-effects model.
Results
Twenty studies (3,525 cases and 17,909 controls) were included in this meta-analysis. The TT genotype of C677T polymorphism was marginally associated with an increased risk of fracture under recessive model (TT vs. TC + CC: OR = 1.23, 95% CI 1.04–1.47). Using this model, similar results were found among East Asians (OR = 1.40, 95% CI 1.07–1.83), female subpopulation (1.27, 95% CI 1.04–1.55), cohort studies (OR = 1.24, 95% CI 1.08–1.44), and subjects younger than aged 60 years (OR = 1.51, 95% CI 1.10–2.07). In addition, under homogeneous co-dominant model, there was a modest association of C677T polymorphism with BMD of lumbar spine (WMD = −0.017 g/cm2; 95%CI, −0.030−(−0.005) g/cm2), femoral neck (WMD = −0.010 g/cm2; 95% CI −0.017–(−0.003) g/cm2), total hip (WMD = −0.013 g/cm2, 95% CI −0.022–(−0.004) g/cm2), and total body (WMD = −0.020 g/cm2; 95% CI −0.027–(−0.013) g/cm2), respectively.
Conclusions
This meta-analysis suggested that C677T polymorphism was marginally associated with fracture risk. In addition, this polymorphism was modestly associated with BMD of lumbar spine, femoral neck, total hip, and total body, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis is a common complex disease, which is characterized by decrease in bone mineral density (BMD) and deterioration of skeletal microarchitecture, leading to increased bone fragility and fracture [1]. Although osteoporosis and fracture are influenced by many environmental factors, such as exercise and calcium intake [2], genetic factors also play important roles in the pathogenesis of fracture [3]. Evidence has suggested that about 30% of heritability for fracture can be attributed to the genetics [4]. In recent years, several candidate genes, including vitamin D receptor (VDR) [5], estrogen receptor [6], and collagen type 1α1 (COL1A1) [7], have been demonstrated to be involved in bone mineral homeostasis, bone remodeling, and bone matrix composition. The genome-wide association studies also identified many susceptibility loci, including GPR177, SPTBN1, LRP5, TNFRSF11B, RANKL, and OPG, which have been associated with BMD or fracture risk [8–11]. Although polymorphisms of those genes alone have limited capability to predict risk of individual, they provide insight into the genetic pathways underling osteoporosis and fracture [11].
The enzyme methylenetetrahydrofolate reductase (MTHFR) plays an important role in the removal of circulating homocysteine via the methionine cycle. The MTHFR gene is located on chromosome 1p36 within a linkage region for regulation of BMD [12]. Two functional polymorphisms (C677T and A1298C) have been identified, which both result in amino acid substitutions in the MTHFR protein. Two variants are both associated with higher plasma homocysteine levels, which could affect collagen maturation [13]. To date, C677T polymorphism has been the most studied one; therefore, in this meta-analysis, we only focus on this polymorphism. Jørgensen et al. [14] first reported that C677T polymorphism in MTHFR gene was associated with fracture risk in European postmenopausal women. Since then, a great number of studies regarding the association between C677T polymorphism and fracture have been published. However, the results have been inconsistent [15–26]. In addition, the association between C677T polymorphism and BMD has also been conflicting.
One previous meta-analysis suggested null association between C677T polymorphism and fracture [20], and another meta-analysis indicated modest association of C677T polymorphism with BMD of both lumbar spine and total hip [27]. However, limited studies were included in both meta-analyses. Recently, several new papers are further available. Therefore, in this study, we performed an updated meta-analysis to clarify the association of C677T polymorphism in MTHFR gene with BMD and fracture risk across different populations.
Materials and methods
Literature and search strategy
PubMed and EMBASE were searched for eligible articles. The search strategy to identify all potential studies involved use of combinations of the following key words: “methylenetetrahydrofolate reductase” or “MTHFR”; and “variant” or “variation” or “polymorphism;” and “bone mineral density” or “BMD” or “fracture.” The reference lists of retrieved reviews and articles were hand-searched. The publication language was restricted to English. If more than one article was published using the same case series, only the study with largest sample size was selected. The literature search was updated on December 5, 2011.
Inclusion criteria and data extraction
Studies were included if they met the following three inclusion criteria: (1) using case–control or cohort design, (2) evaluating the association of C677T polymorphism with fracture risk or BMD, and (3) providing sufficient data for calculation of odds ratio (OR) or weighted mean difference (WMD) with 95% confidence interval (CI). For fracture phenotype, the following information was extracted from each study: (1) name of the first author, (2) year of publication, (3) country of origin, (4) ethnicity of the studied population, (5) study design, (6) sample size in cases and controls, (7) genotype distributions in cases and controls, (8) minor allele frequency in controls, and (9) p value for the test of Hardy–Weinberg equilibrium in controls. For BMD phenotype, the following information was extracted: (1) name of the first author, (2) year of publication, (3) country of origin, (4) ethnicity of the studied population, (5) study design, (6) type of BMD phenotype, (7) mean and standard deviation of BMD across three genotypes, and (8) sample size across three genotypes. Two authors independently assessed the articles for compliance with the inclusion criteria, and disagreement was followed by discussion until consensus was reached.
Statistical analysis
The association between C677T polymorphism and fracture risk was estimated by calculating a pooled OR and 95% CI under co-dominant model, dominant model, and recessive model, respectively. The association between C677T polymorphism and BMD was estimated by a pooled WMD under three genetic models above, respectively. The significance of the pooled OR or WMD was determined by a Z test (p < 0.05 was considered statistically significant). A Q test was performed to evaluate whether the variation was due to heterogeneity or due to chance. A random (DerSimonian–Laird method [28]) or fixed (Mantel–Haenszel method) effects model [29] was used to calculate the pooled OR or WMD in the presence (p < =0.10) or absence (p > 0.10) of heterogeneity, respectively. Begg’s funnel plot, a scatter plot of effect against a measure of study size, was generated as a visual aid to detect bias or systematic heterogeneity [30]. Publication bias was assessed by Egger’s test [31] (p < 0.05 was considered statistically significant). Subgroup analyses based on ethnicity (European vs. East Asian), type of fracture (hip fracture vs. vertebral fracture), sex (male vs. female), study design (case–control design vs. cohort design), and mean age of subjects (<60 years vs. ≥60 years) were performed. Sensitivity analysis was performed by removing one study at a time to evaluate the stability of the results. Data analyses were performed using STATA version 11 (StataCorp LP, College Station, TX, USA).
Results
Characteristics of the studies
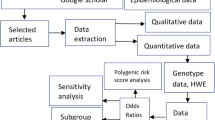
The literature search identified a total of 41 potentially relevant papers. Of these, 25 papers were excluded after reading the title or abstract because of obvious irrelevance to our study aim. In addition, one duplicated paper [32] and one paper [33], which did not provide sufficient data for the calculation of an OR and 95%CI, were further excluded. If more than one study was included in one paper or data were presented by sex, they were considered as separate study in our meta-analysis. Therefore, 20 studies for fracture risk [14–27] and 16 studies for BMD [15, 17–19, 22, 25, 26, 34–38] met the inclusion criteria and were included in the final meta-analysis. A flow chart summarizing the process of study inclusion is depicted in Fig. 1. For fracture, 15 studies were performed in Europeans, and five studies were performed in East Asians; ten studies were on vertebral fracture, and five studies were on hip fracture; 14 studies were performed in female, five studies were performed in male, and one study did not present the sex-specific data; ten studies used case–control design, and ten studies used cohort design (Supplementary Table 1). For BMD phenotypes, 14 studies were on BMD of lumbar spine, 13 studies were on BMD of femoral neck, six studies were on BMD of total hip, and seven studies were on BMD of total body (Supplementary Table 2).
Fractures
A total of 3,525 cases and 17,909 controls were identified for the analysis on C677T polymorphism and fracture. The overall result showed that there was a marginally significant association between this polymorphism and fracture risk under homogeneous co-dominant model (TT vs. CC: OR = 1.23; 95% CI 1.00–1.51) and under recessive model (TT vs. TC + CC: OR = 1.23, 95% CI 1.04–1.47; Fig. 2). Subgroup analyses showed that the effect size was statistically significant among East Asians (TT vs. TC + CC: OR = 1.40, 95% CI 1.07–1.83), studies with vertebral fracture (TT vs. CC: OR = 1.46, 95% CI 1.07–1.98), female subpopulation (TT vs. TC + CC: 1.27, 95% CI 1.04–1.55), cohort studies (TT vs. CC: OR = 1.21, 95% CI 1.02–1.42; TT vs. TC + CC: OR = 1.24, 95% CI 1.08–1.44), and subjects younger than aged 60 years (TT vs. CC: OR = 1.68, 95% CI 1.17–2.40; TT vs. TC + CC: OR = 1.51, 95% CI 1.10–2.07), but not among Europeans, studies with hip fracture, male subpopulation, case–control studies, and subjects younger than 60 years under all genetic models (Table 1).
Sensitivity analysis was conducted by excluding each study at a time. The results confirmed the marginally significant association under recessive model, with OR with 95% CI ranging from 1.20 (1.01–1.43) to 1.27(1.06–1.51). However, the results were not robust under homogeneous co-dominant model, with OR with 95% CI ranging from 1.17 (0.96–1.43) to 1.26 (1.02–1.57).
BMD phenotypes
For BMD of lumbar spine, a total of 13,454 subjects were identified for the data analysis. C677T polymorphism was modestly associated with BMD of the lumbar spine from the L2 to L4 vertebrae (TT vs. CC: WMD = −0.017 g/cm2, 95% CI −0.030–(−0.005) g/cm2, Fig. 3a; TC vs. CC: WMD = −0.009 g/cm2, 95% CI −0.015–(−0.004) g/cm2; dominant model: WMD = −0.011 g/cm2, 95% CI −0.016–(−0.005) g/cm2). Similar results were found among each group by ethnicity, sex, and study design (Table 2).
For BMD of femoral neck, a total of 13,567 subjects were identified for the data analysis. There was a modest association between C677T polymorphism and BMD of femoral neck (TT vs. CC: WMD = −0.010 g/cm2, 95% CI −0.017–(−0.003) g/cm2, Fig. 3b). The results were similar when different sexes, ethnicities, and study designs were considered separately (Table 2).
For BMD of total hip and total body, a total of 6,356 and 5,652 individuals were identified for the data analysis, separately. There was a modest association between C677T polymorphism and BMD of total hip (TT vs. CC: WMD = −0.013 g/cm2, 95% CI −0.022–(−0.004) g/cm2, Fig. 3c; TT vs. TC + CC: WMD = −0.012 g/cm2, 95% CI −0.020–(−0.004) g/cm2) and BMD of total body (TT vs. CC: WMD = −0.020 g/cm2, 95% CI −0.027–(−0.013) g/cm2, Fig. 3d; TC vs. CC: WMD = −0.007 g/cm2, 95% CI −0.012–(−0.002) g/cm2; TT + TC vs. CC: WMD = −0.011 g/cm2, 95% CI −0.017–(−0.004) g/m2; TT vs. TC + CC: WMD = −0.015 g/m2, 95% CI −0.022–(−0.008) g/m2) (Table 2).
Sensitivity analysis was conducted by excluding each study at a time. The results confirmed the modest association of C677T polymorphism with BMD of lumbar spine, femoral neck, total hip, and total body, respectively (data not shown).
Potential publication bias
Using the Egger’s test, no publication bias could be detected for the association of C677T polymorphism with fracture risk (TT vs. CC, p = 0.949; TC vs. CC, p = 0.631; TT + TC vs. CC, p = 0.541; TT vs. TC + CC, p = 0.666) and BMD phenotypes (BMD of lumbar spine: TT vs. CC, p = 0.896; TC vs. CC, p = 0.276; TT + TC vs. CC, p = 0.427; TT vs. TC + CC, p = 0.686; BMD of femoral neck: TT vs. CC, p = 0.366; TC vs. CC, p = 0.237; TT + TC vs. CC, p = 0.175; TT vs. TC + CC, p = 0.911; BMD of total hip, TT vs. CC: p = 0.175; TC vs. CC, p = 0.403; TT + TC vs. CC, p = 0.796; TT vs. TC + CC, p = 0.132; BMD of total body: TT vs. CC, p = 0.747; TC vs. CC, p = 0.841; TT + TC vs. CC, p = 0.770; TT vs. TC + CC, p = 0.977).
Discussion
Our study suggested that C677T polymorphism in MTHFR gene was marginally associated with fracture risk. In the subgroup analyses, significant associations were observed among East Asians, female subpopulation, cohort studies, and subjects younger than aged 60 years. The result was somewhat different from a previous meta-analysis, which suggested non-significant association between C677T polymorphism and fracture risk [20]. In addition, the present meta-analysis suggested the modest association of C677T polymorphism with BMD of lumbar spine, femoral neck, total hip, and total body, respectively, which was in agreement with the previous meta-analysis [27].
It is possible that the effect sizes of genetic factors predisposing to human diseases are different across various ethnic populations [39]. The frequency of C677T polymorphism TT genotype is much lower in Europeans than East Asians, according to the HapMap data. For example, the frequencies of CC, CT, and TT were about 59.3%, 33.9%, and 6.8%, respectively, in European population, while the frequencies of CC, CT, and TT were about 26.7%, 44.4%, and 28.9%, respectively in the Chinese population. Indeed, we observed a significant association between C677T polymorphism and fracture risk among East Asians but not Europeans. In addition, we also found a significant association in women rather than in men. It should be noted that nearly all women included in the present meta-analysis were postmenopausal. As is known, menopause is a critical period that presents a high bone turnover state. Moreover, some evidences have indicated that the plasma homocysteine level of a woman could increase even more when she reaches menopause, causing further worsening of bone quality and eventually increasing her risk of osteoporotic fractures [40]. A significant association was also observed among subjects younger than 60 years. However, the result should be interpreted with caution because of the limited sample size within this subgroup.
Recent meta-analyses have indicated that C677T polymorphism was associated with several chronic diseases, including hypertension [41], coronary heart disease [42], Alzheimer’s disease [43], migraine [44], stroke [45], and some cancers [46]. The mechanisms by which MTHFR gene affects these diseases, as well as BMD and fracture, remain unknown. Besides folate intake, homocysteine levels are also affected by the activity of the MTHFR, which converts 5,10-methylenetetrahydrofolate to 5-methyltetrahydrofolate, the primary circulatory form of folate, and directs the homeostasis between DNA synthesis and methylation [45]. Evidences have suggested that TT genotype of C677T polymorphism was associated with elevated levels of circulating homocysteine [47, 48], which could interfere with collagen synthesis, resulting in lower bone quality and increased fracture risk [13]. In addition, homocysteine may have a direct effect on bone by stimulating osteoclast formation and osteoclast activity [49]. Further function studies are required to investigate the effect of MTHFR gene on BMD and fracture.
The current meta-analysis has some limitations. First, the present meta-analysis was based primarily on unadjusted effect estimates and 95% CIs, so the confounding factors might influence the effect estimates. Second, the effects of gene–gene/gene–environment (e.g., riboflavin and folate) interactions were not addressed in this meta-analysis. Besides C677T polymorphism, other genes, such as VDR, estrogen receptor, and COL1A1, may affect BMD and fracture and modulate the effect of C677T polymorphism on BMD and fracture [50]. In addition, environmental factors, such as diet, physical activity, smoking, and alcohol consumption, have been shown to influence BMD, osteoporosis, and fracture [51]. Therefore, these gene–environmental factors may act as modifiers that affect the association between C677T polymorphism and fracture. However, most included studies did not provided the related data, which impeded us for further analysis.
In summary, our meta-analysis suggested that C677T polymorphism was marginally associated with fracture risk. In addition, there was modest association between C677T polymorphism and BMD. We believe that our conclusions were credible since our meta-analyses had sufficient statistical power (using Quanto software http://hydra.usc.edu/gxe/, we calculated the power for the overall cohort (dominant model, 99%; recessive model, 98%), the European cohort (dominant model, 99%; recessive model, 75%), and the East Asian cohort (dominant model, 85%; recessive model, 63%). However, further studies with the consideration of gene–gene/gene–environment interactions are needed to investigate the role of the MTHFR gene polymorphisms in the regulation of BMD and the pathogenesis of fracture in the future.
References
Kanis JA, Melton LJ 3rd, Christiansen C, Johnston CC, Khaltaev N (1994) The diagnosis of osteoporosis. J Bone Miner Res 9:1137–1141
NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy (2001) Osteoporosis prevention, diagnosis, and therapy. JAMA 285:785–795
Hosoi T (2010) Genetic aspects of osteoporosis. J Bone Miner Metab 28:601–607
Michaelsson K, Melhus H, Ferm H, Ahlbom A, Pedersen NL (2005) Genetic liability to fractures in the elderly. Arch Intern Med 165:1825–1830
Ji GR, Yao M, Sun CY, Li ZH, Han Z (2010) BsmI, TaqI, ApaI and FokI polymorphisms in the vitamin D receptor (VDR) gene and risk of fracture in Caucasians: a meta-analysis. Bone 47:681–686
Lei MM, Yang TF, Tu ZQ, Liu L, Fang Y, Wang GL (2010) Oestrogen receptor-alpha polymorphism and risk of fracture: a meta-analysis of 13 studies including 1279 cases and 6069 controls. J Int Med Res 38:1575–1583
Jin H, Evangelou E, Ioannidis JP, Ralston SH (2011) Polymorphisms in the 5′ flank of COL1A1 gene and osteoporosis: meta-analysis of published studies. Osteoporos Int 22:911–921
Styrkarsdottir U, Halldorsson BV, Gretarsdottir S, Gudbjartsson DF, Walters GB, Ingvarsson T, Jonsdottir T, Saemundsdottir J, Center JR, Nguyen TV, Bagger Y, Gulcher JR, Eisman JA, Christiansen C, Sigurdsson G, Kong A, Thorsteinsdottir U, Stefansson K (2008) Multiple genetic loci for bone mineral density and fractures. N Engl J Med 358:2355–2365
Richards JB, Rivadeneira F, Inouye M, Pastinen TM, Soranzo N, Wilson SG, Andrew T, Falchi M, Gwilliam R, Ahmadi KR, Valdes AM, Arp P, Whittaker P, Verlaan DJ, Jhamai M, Kumanduri V, Moorhouse M, van Meurs JB, Hofman A, Pols HA, Hart D, Zhai G, Kato BS, Mullin BH, Zhang F, Deloukas P, Uitterlinden AG, Spector TD (2008) Bone mineral density, osteoporosis, and osteoporotic fractures: a genome-wide association study. Lancet 371:1505–1512
Richards JB, Kavvoura FK, Rivadeneira F, Styrkársdóttir U, Estrada K, Halldórsson BV, Hsu YH, Zillikens MC, Wilson SG, Mullin BH, Amin N, Aulchenko YS, Cupples LA, Deloukas P, Demissie S, Hofman A, Kong A, Karasik D, van Meurs JB, Oostra BA, Pols HA, Sigurdsson G, Thorsteinsdottir U, Soranzo N, Williams FM, Zhou Y, Ralston SH, Thorleifsson G, van Duijn CM, Kiel DP, Stefansson K, Uitterlinden AG, Ioannidis JP, Spector TD, Genetic Factors for Osteoporosis Consortium (2009) Collaborative meta-analysis: associations of 150 candidate genes with osteoporosis and osteoporotic fracture. Ann Intern Med 151:528–537
Rivadeneira F, Styrkársdottir U, Estrada K, Halldórsson BV, Hsu YH, Richards JB, Zillikens MC, Kavvoura FK, Amin N, Aulchenko YS, Cupples LA, Deloukas P, Demissie S, Grundberg E, Hofman A, Kong A, Karasik D, van Meurs JB, Oostra B, Pastinen T, Pols HA, Sigurdsson G, Soranzo N, Thorleifsson G, Thorsteinsdottir U, Williams FM, Wilson SG, Zhou Y, Ralston SH, van Duijn CM, Spector T, Kiel DP, Stefansson K, Ioannidis JP, Uitterlinden AG, Genetic Factors for Osteoporosis (GEFOS) Consortium (2009) Twenty bone-mineral-density loci identified by large-scale meta-analysis of genome-wide association studies. Nat Genet 41:1199–1206
Devoto M, Specchia C, Li HH, Caminis J, Tenenhouse A, Rodriguez H, Spotila LD (2001) Variance component linkage analysis indicates a QTL for femoral neck bone mineral density on chromosome 1p36. Hum Mol Genet 10:2447–2452
Lubec B, Fang-Kircher S, Lubec T, Blom HJ, Boers GH (1996) Evidence for McKusick’s hypothesis of deficient collagen crosslinking in patients with homocystinuria. Biochim Biophys Acta 1315:159–162
Jørgensen HL, Madsen JS, Madsen B, Saleh MM, Abrahamsen B, Fenger M, Lauritzen JB (2002) Association of a common allelic polymorphism (C677T) in the methylene tetrahydrofolate reductase gene with a reduced risk of osteoporotic fractures. A case control study in Danish postmenopausal women. Calcif Tissue Int 71:386–392
Abrahamsen B, Madsen JS, Tofteng CL, Stilgren L, Bladbjerg EM, Kristensen SR, Brixen K, Mosekilde L (2003) A common methylenetetrahydrofolate reductase (C677T) polymorphism is associated with low bone mineral density and increased fracture incidence after menopause: longitudinal data from the Danish osteoporosis prevention study. J Bone Miner Res 18:723–729
Bathum L, von Bornemann HJ, Christiansen L, Madsen JS, Skytthe A, Christensen K (2004) Evidence for an association of methylene tetrahydrofolate reductase polymorphism C677T and an increased risk of fractures: results from a population-based Danish twin study. Osteoporos Int 15:659–664
Li M, Lau EM, Woo J (2004) Methylenetetrahydrofolate reductase polymorphism (MTHFR C677T) and bone mineral density in Chinese men and women. Bone 35:1369–1374
Villadsen MM, Bünger MH, Carstens M, Stenkjaer L, Langdahl BL (2005) Methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism is associated with osteoporotic vertebral fractures, but is a weak predictor of BMD. Osteoporos Int 16:411–416
Hong X, Hsu YH, Terwedow H, Tang G, Liu X, Jiang S, Xu X, Xu X (2007) Association of the methylenetetrahydrofolate reductase C677T polymorphism and fracture risk in Chinese postmenopausal women. Bone 40:737–742
Valero C, Alonso MA, Zarrabeitia MT, Viadero C, Hernández JL, Riancho JA (2007) MTHFR C677T polymorphism and osteoporotic fractures. Horm Metab Res 39:543–547
Gjesdal CG, Vollset SE, Ueland PM, Refsum H, Meyer HE, Tell GS (2007) Plasma homocysteine, folate, and vitamin B 12 and the risk of hip fracture: the hordaland homocysteine study. J Bone Miner Res 22:747–756
Yazdanpanah N, Uitterlinden AG, Zillikens MC, Jhamai M, Rivadeneira F, Hofman A, de Jonge R, Lindemans J, Pols HA, van Meurs JB (2008) Low dietary riboflavin but not folate predicts increased fracture risk in postmenopausal women homozygous for the MTHFR 677 T allele. J Bone Miner Res 23:86–94
Shiraki M, Urano T, Kuroda T, Saito M, Tanaka S, Miyao-Koshizuka M, Inoue S (2008) The synergistic effect of bone mineral density and methylenetetrahydrofolate reductase (MTHFR) polymorphism (C677T) on fractures. J Bone Miner Metab 26:595–602
Urano W, Furuya T, Inoue E, Taniguchi A, Urano T, Kotake S, Sekita C, Inoue S, Hara M, Momohara S, Kamatani N, Yamanaka H (2009) Associations between methotrexate treatment and methylenetetrahydrofolate reductase gene polymorphisms with incident fractures in Japanese female rheumatoid arthritis patients. J Bone Miner Metab 27:574–583
Zhu K, Beilby J, Dick IM, Devine A, Soós M, Prince RL (2009) The effects of homocysteine and MTHFR genotype on hip bone loss and fracture risk in elderly women. Osteoporos Int 20:1183–1191
Agueda L, Urreizti R, Bustamante M, Jurado S, Garcia-Giralt N, Díez-Pérez A, Nogués X, Mellibovsky L, Grinberg D, Balcells S (2010) Analysis of three functional polymorphisms in relation to osteoporosis phenotypes: replication in a Spanish cohort. Calcif Tissue Int 87:14–24
Riancho JA, Valero C, Zarrabeitia MT (2006) MTHFR polymorphism and bone mineral density: meta-analysis of published studies. Calcif Tissue Int 79:289–293
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188
Mantel N, Haenszel W (1959) Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 22:719–748
Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50:1088–1101
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634
Abrahamsen B, Madsen JS, Tofteng CL, Stilgren L, Bladbjerg EM, Kristensen SR, Brixen K, Mosekilde L (2005) Are effects of MTHFR (C677T) genotype on BMD confined to women with low folate and riboflavin intake? Analysis of food records from the Danish osteoporosis prevention study. Bone 36:577–583
Nissen N, Madsen JS, Bladbjerg EM, Beck Jensen JE, Jørgensen NR, Langdahl B, Abrahamsen B, Brixen K (2009) No association between hip geometry and four common polymorphisms associated with fracture: the Danish osteoporosis prevention study. Calcif Tissue Int 84:276–285
Miyao M, Morita H, Hosoi T, Kurihara H, Inoue S, Hoshino S, Shiraki M, Yazaki Y, Ouchi Y (2000) Association of methylenetetrahydrofolate reductase (MTHFR) polymorphism with bone mineral density in postmenopausal Japanese women. Calcif Tissue Int 66:190–194
McLean RR, Karasik D, Selhub J, Tucker KL, Ordovas JM, Russo GT, Cupples LA, Jacques PF, Kiel DP (2004) Association of a common polymorphism in the methylenetetrahydrofolate reductase (MTHFR) gene with bone phenotypes depends on plasma folate status. J Bone Miner Res 19:410–418
Golbahar J, Hamidi A, Aminzadeh MA, Omrani GR (2004) Association of plasma folate, plasma total homocysteine, but not methylenetetrahydrofolate reductase C667T polymorphism, with bone mineral density in postmenopausal Iranian women: a cross-sectional study. Bone 35:760–765
Macdonald HM, McGuigan FE, Fraser WD, New SA, Ralston SH, Reid DM (2004) Methylenetetrahydrofolate reductase polymorphism interacts with riboflavin intake to influence bone mineral density. Bone 35:957–964
Abrahamsen B, Jørgensen HL, Nielsen TL, Andersen M, Haug E, Schwarz P, Hagen C, Brixen K (2006) MTHFR c.677C>T polymorphism as an independent predictor of peak bone mass in Danish men–results from the Odense Androgen Study. Bone 38:215–219
Pan Z, Trikalinos TA, Kavvoura FK, Lau J, Ioannidis JP (2005) Local literature bias in genetic epidemiology: an empirical evaluation of the Chinese literature. PLoS Med 2:e334
Hak AE, Polderman KH, Westendorp IC, Jakobs C, Hofman A, Witteman JC, Stehouwer CD (2000) Increased plasma homocysteine after menopause. Atherosclerosis 149:163–168
Qian X, Lu Z, Tan M, Liu H, Lu D (2007) A meta-analysis of association between C677T polymorphism in the methylenetetrahydrofolate reductase gene and hypertension. Eur J Hum Genet 15:1239–1245
Klerk M, Verhoef P, Clarke R, Blom HJ, Kok FJ, Schouten EG, MTHFR Studies Collaboration Group (2002) MTHFR 677C-->T polymorphism and risk of coronary heart disease: a meta-analysis. JAMA 288:2023–2031
Zhang MY, Miao L, Li YS, Hu GY (2010) Meta-analysis of the methylenetetrahydrofolate reductase C677T polymorphism and susceptibility to Alzheimer’s disease. Neurosci Res 68:142–150
Samaan Z, Gaysina D, Cohen-Woods S, Craddock N, Jones L, Korszun A, Owen M, Mente A, McGuffin P, Farmer A (2011) Methylenetetrahydrofolate reductase gene variant (MTHFR C677T) and migraine: a case control study and meta-analysis. BMC Neurol 11:66
Banerjee I, Gupta V, Ganesh S (2007) Association of gene polymorphism with genetic susceptibility to stroke in Asian populations: a meta-analysis. J Hum Genet 52:205–219
Zacho J, Yazdanyar S, Bojesen SE, Tybjærg-Hansen A, Nordestgaard BG (2011) Hyperhomocysteinemia, methylenetetrahydrofolate reductase c.677C>T polymorphism and risk of cancer: cross-sectional and prospective studies and meta-analyses of 75,000 cases and 93,000 controls. Int J Cancer 128:644–652
Passaro A, Vanini A, Calzoni F, Alberti L, Zamboni PF, Fellin R, Solini A (2001) Plasma homocysteine, methylenetetrahydrofolate reductase mutation and carotid damage in elderly healthy women. Atherosclerosis 157:175–180
Dekou V, Whincup P, Papacosta O, Ebrahim S, Lennon L, Ueland PM, Refsum H, Humphries SE, Gudnason V (2001) The effect of the C677T and A1298C polymorphisms in the methylenetetrahydrofolate reductase gene on homocysteine levels in elderly men and women from the British regional heart study. Atherosclerosis 154:659–666
Koh JM, Lee YS, Kim YS, Kim DJ, Kim HH, Park JY, Lee KU, Kim GS (2006) Homocysteine enhances bone resorption by stimulation of osteoclast formation and activity through increased intracellular ROS generation. J Bone Miner Res 21:1003–1011
Li WF, Hou SX, Yu B, Li MM, Férec C, Chen JM (2010) Genetics of osteoporosis: accelerating pace in gene identification and validation. Hum Genet 127:249–285
Samelson EJ, Hannan MT (2006) Epidemiology of osteoporosis. Curr Rheumatol Rep 8:76–83
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
H. Wang and C. Liu contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Table 1
Characteristics of the studies included in the meta-analysis of the association between C667T polymorphism in the MTHFR gene and fracture risk (DOC 79 kb)
Supplementary Table 2
Characteristics of studies included in meta-analysis of the association between C667T polymorphism in the MTHFR gene and bone material density (DOC 60 kb)
Rights and permissions
About this article
Cite this article
Wang, H., Liu, C. Association of MTHFR C667T polymorphism with bone mineral density and fracture risk: an updated meta-analysis. Osteoporos Int 23, 2625–2634 (2012). https://doi.org/10.1007/s00198-011-1885-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-011-1885-6