Abstract
Genetic factors have been shown to be of great importance for the pathogenesis of bone diseases, such as fracture, osteoporosis (OP), and osteoarthritis (OA). However, published studies on the correlations of transforming growth factor-β1 (TGF-β1) gene polymorphisms with bone diseases have been hampered by small sample sizes or inconclusive findings. We hence aimed at examining the relationships between a single nucleotide polymorphism in the TGF-β1 gene (rs1982073 C>T) with bone fracture, OP, and OA risks in this meta-analysis. A systematic electronic search of literature was conducted to identify all published studies in English or Chinese on the association between the TGF-β1 gene and fracture, OP, or OA risks. Data were abstracted independently by two reviewers. To investigate the strength of this relationship, crude odds ratios with 95 % confidence intervals were used. An updated meta-analysis based on nine independent case–control studies were chosen (patients with fracture, OP, or OA = 1569; healthy controls = 1638). Results identified a higher frequency of rs1982073 C>T in patients with fracture, OP, or OA than in healthy controls. Ethnicity and genotyping method-stratified analysis under both models implied that the rs1982073 C>T polymorphism was positively correlated with the risk of fracture, OP, and OA among Asians under detection via the non-PCR-RFLP method. Disease-stratified results yielded that rs1982073 C>T may increase the risk of fracture, OP, and OA under the allele model, but was only significantly related to OP under the dominant model. According to the sample size-stratified analysis, subjects with the rs1982073 C>T polymorphism in the allele model were more likely to develop the three bone diseases in both the small and large sample size groups, and only in the large sample size under the dominant model. Our findings show that TGF-β1 rs1982073 C>T has a modest effect in increasing susceptibility to bone fracture, OP, and OA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bone fracture is one of the major public health problems in the world and can be classified into various types, such as foot and ankle fractures [1]. Of note, the morbidity and mortality of bone fracture are very high, resulting in financial burdens worldwide [2]. According to statistics, out of 10,000 people, approximately 78 to 94 might suffer from bone fracture [3]. It is reported that many factors, including age, physiology, body habitus, and traumatic injury, might be significantly related to the risk of bone fracture [4, 5]. Acting as another risk factor for bone fracture, osteoporosis (OP) is well known as a systemic skeletal disease, which negatively influences susceptibility to fractures and the fragility of bones [6]. Investigations have revealed the incidence of OP varies greatly across race, with a prevalence rate of 7 % among white men but 3 % among black men [7]. Compared with women, men with OP have higher morbidity and mortality rates [8]. Apart from bone fracture and OP, osteoarthritis (OA) is another common musculoskeletal disease and is one of the leading causes of disability among the elderly [9]. Historically, patients who are diagnosed with OA have chronic nociceptive pain, which results in disabilities and increased health care costs [10]. According to studies published on the prevalence of OA, out of 100 people aged 60 years or older, approximately 10 people have clinical problems that might be attributable to OA [11]. As for the etiology of OP and OA, they are deemed to be complex multifactorial diseases, both of which are caused by the interaction and correlation between environmental and genetic factors [12, 13]. Recently, numerous studies have shown that transforming growth factor-β, impacting cartilage maintenance and formation, might be protective against bone fracture, OP, and OA [14–16].
Transforming growth factor-β1 (TGF-β1), a potent cytokine and bone-derived factor, is the main member of the TGF-β super family, which also includes activins, inhibins, and bone morphogenetic proteins [17]. TGF-β family encodes large protein precursors with a N-terminal signal peptide of 20–30 amino acids and mature TGF-β molecule from pre-region released by proteolytic cleavage [18]. TGF-β1 is localized on chromosome 19q13.1–q13.3, consisting of seven exons and six large introns [19]. Secreted by platelets, macrophages, and other cell types, TGF-β1 exerts lots of physiological and pathological effects involving cell cycle, proliferation, differentiation, maturation, and apoptosis or immune activity [20]. Specifically speaking, TGF-β1 has a crucial role in osteoblast differentiation, assisting tissue regeneration and bone remodeling, while it also reversely acts on osteoclast growth, affecting bone resorption and recovery [21]. Up to now, large experimental studies have shown that genetic polymorphisms of TGF-β1 are found to be a predictive marker during the bone healing process [22]. Among these TGF-β1 genetic variations, it was found that TGF-β1 gene, single nucleotide polymorphism (SNPs), rs2278422, along with being overweight, may increase the risk of knee OA [23]. Additionally, previous studies among Japanese community-dwelling adolescents and postmenopausal women discovered that the TGF-β1 rs1982073 C>T polymorphism, a substitution of leucine (Leu) to proline (Pro), was associated with lower bone mineral density (BMD), suggesting higher risk of osteoporotic fracture [24, 25]. Moreover, observational studies verified that the TGF-β1 polymorphism rs1982073 is found to be located in the signal peptides, consequently leading to dysfunction of the signal peptide and blockage of intracellular signal traffic, indicating that the variation has an impact on the prevalence of vertebral fractures by affecting the signaling pathway and mediating cell apoptosis [26]. The existing findings support the possibility that the TGF-β1 polymorphism rs1982073 may be implicated in susceptibility to bone fracture, OP, and OA [27, 28]. Nevertheless, other results suggest that there is no relationship between the genetic polymorphisms of TGF-β1 and bone diseases [29, 30]. Accordingly, this meta-analysis synthesized data from available previous studies to explore the correlation between TGF-β1 gene polymorphism at rs1982073 with susceptibility to bone fracture, OP, and OA.
Materials and methods
Data sources and keywords
To identify all pertinent papers that assessed the correlations of TGF-β1 rs1982073 C>T polymorphism with the susceptibility of fracture, OP, and OA, we comprehensively searched PubMed, Embase, Web of Science, Cochrane Library, CISCOM, CINAHL, Google Scholar, China BioMedicine (CBM), and China National Knowledge Infrastructure (CNKI) databases (last updated search in May 30, 2014). We utilized the following common keywords regarding the TGF-β1 gene, fracture, OP, and OA: (“Transforming Growth Factor beta1” or “Transforming Growth Factor beta-1” or “Transforming Growth Factor beta 1” or “TGF beta 1” or “TGF-beta-1” or “TGF-beta1” or “Transforming Growth Factor beta 1 Latency Associated Peptide” or “TGF-beta1 Latency-Associated Protein” or “TGF beta1 Latency Associated Protein”) for the exposure factors, and (“Fractures, Bone” or “Broken Bones” or “Fractures” or “Fracture” or “Broken Bone” or “Bone Fractures” or “Bone Fracture”), (“Osteoporosis, Postmenopausal” or “Osteoporosis” or “Juvenile OP” or “Osteoporoses” or “Age-Related Bone Loss” or “Age-Related Osteoporosis”), and (“Osteoarthritis, Spine” or “Osteoarthritis, Knee” or “Osteoarthritis, Hip” or “osteoarthritis” or “knee OA” or “spine OA” or “hip OA” or “spinal OA” or “lumbar OA” or “coxarthrosis”) for the outcome factors. No restriction was set on the language of the article. We also further scanned the bibliographies of relevant articles manually to identify additional relevant papers. When the enrolled papers supplied unclear data in their original publications, the first authors would be contacted and asked for clarifications.
Selection criteria
We searched for all human case–control studies providing genotypic data for TGF-β1 genetic polymorphisms, including subjects with fracture, OP, or OA, and reporting the adjusted odd ratios (ORs) and 95 % confidence intervals (CIs). We only included studies that supplied the sample number and sufficient information about TGF-β1 genetic variants, and excluded articles with incomplete, unavailable, or inappropriate clinicopathologic data or those regarding fracture, OP, or OA not confirmed by histopathologic examinations. OP is defined by the World Health Organization as a T score <−2.5 SD, and OA is diagnosed based on clinical and radiographic evaluation, or ascertained by total joint replacement [31, 32]. In addition, only studies involving more than 25 cases were enrolled. Furthermore, only those studies that conformed to Hardy–Weinberg equilibrium (HWE) in the control group were enrolled. However, when the extracted studies had more than 50 % of subjects overlapping, we merely enrolled the one whose population was the most comprehensive. At the same time, only the newest or most complete study was included when multiple studies were published by the same authors on the same study population.
Data extraction
In order to reduce bias and enhance credibility, two investigators independently extracted information from all included papers and arrived at a consensus on all the items through discussion and reexamination. The following relevant data were extracted from eligible studies: surname of first author, year of publication, source of publication, source of controls, study type, study design, sample size, age, sex, ethnicity and country of origin, disease type, genotyping method, source of controls, disease type, available genotype, genotype and mutation frequencies, and HWE evidence in controls. All authors approved the final determinant of the studies to be enrolled.
Quality assessment
To decide whether the study in question is of high quality, the two investigators used the Strengthening the Reporting of Observational studies in Epidemiology (STROBE) quality score systems to assess the studies independently [33]. The STROBE is comprised of 40 assessment items associated with quality appraisal, with scores ranging from 0 to 40. According to the STROBE scores, the included studies were classified into three levels: low quality (0–19), moderate quality (20–29), and high quality (30–40), respectively. Any discrepancies in assigned STROBE scores were resolved through discussion with a third reviewer.
Statistical analysis
To calculate the effect size for each study, the summary ORs with 95 % CIs were used under the allele model [mutant (M) allele versus wild (W) allele] and dominant model (WM + MM versus WW) with the utilization of the Z test. In order to supply quantitative evidence of all selected studies and minimize the variance of the summary ORs with 95 % CIs, we conducted the current statistical meta-analyses by utilizing a random-effects model (DerSimonian and Laird method) or a fixed-effects model (Mantel–Haenszel method) of individual study results under the situation where data from independent studies could be combined. The random-effect model was applied when heterogeneity exists among studies, while the fixed-effect model was applied when there was no statistical heterogeneity. The subgroup meta-analyses were also conducted by ethnicity, disease type, genotyping method, and sample size to explore potential effect modification, and heterogeneity across the enrolled studies was evaluated by the Cochran’s Q-statistic (P < 0.05 was regarded as statistically significant) [34]. As a result of the low statistical power of the Cochran’s Q-statistic, the I 2 test (0 %, no heterogeneity; 100 %, maximal heterogeneity) was also conducted to reflect the possibility of heterogeneity between studies [35]. The one-way sensitivity analysis was performed to evaluate whether the results could have been affected significantly by excluding each study in our meta-analysis one by one to reflect the influence of the individual data set on the pooled ORs. The funnel plot was constructed to assess publication bias, which might affect the validity of the estimates. The symmetry of the funnel plot was further evaluated by Egger’s linear regression test [36]. All tests were two-sided and a P value of <0.05 was regarded as statistically significant. To make sure that the results are credible and accurate, two investigators inputted all information in the STATA software, version 12.0 (Stata Corp, College Station, TX, USA) separately and arrived at an agreement.
Results
Included studies
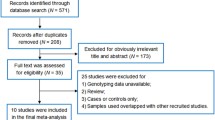
Our present meta-analysis hit a total of nine case–control papers that provided information on the correlation between TGF-β1 genetic variants and susceptibility to fracture, OP, or OA (two, four, and three articles, respectively) [26–30, 37–40]. Six studies were conducted in populations of Asian descent and three in populations of Caucasian descent, including 3207 subjects altogether (1569 patients with fracture, OP, or OA and 1638 healthy controls), which were published between 2000 and 2013. The characteristics and methodological quality of the extracted studies are presented in Table 1. The countries where the studies were performed include Turkey, Thailand, Croatia, Czech, China, Denmark, and Japan. The sources of controls in our present meta-analysis were all from population-based (PB) subjects. The genotyping methods detecting TGF-β1 genetic polymorphisms in this current meta-analysis include TaqMan assay (n = 1), polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) (n = 5), and allele-specific PCR (n = 3). The available SNP involved in our meta-analysis was rs1982073 C>T in the TGF-β1 gene. All included studies showed evidence of HWE (all P > 0.05). Additionally, as for the step of screening, a flow chart of the study selection process is displayed in Fig. 1. Initially, a total of 101 papers were selected from the nine databases through screening the title and key words. We then excluded duplicates (n = 1), letters, reviews or meta-analyses (n = 11), non-human studies (n = 15), and studies not related to our research topics (n = 21). The remaining studies (n = 53) were reviewed and an additional 41 studies were excluded for not being case–control or cohort studies (n = 12), not relevant to the TGF-β1 gene (n = 14), or not relevant to fracture, OP, or OA (n = 15). After the remaining 12 trials were further reviewed, nine papers were enrolled in the final analysis. During the final selection process, the major reason for exclusion was not supplying enough information (n = 3). All quality scores of the included studies were higher than 20 (moderate to high quality). From 2001 to 2014, the number of articles selected from those electronic databases is shown in Fig. 2.
Association of TGFβ1 genetic polymorphisms with fracture, OP, or OA
As shown in Fig. 3 and Table 2, the major findings of the present meta-analysis revealed a higher frequency of the rs1982073 C>T genetic mutation in the TGF-β1 gene in patients with fracture, OP, or OA than in healthy controls (allele model—OR = 1.26, 95 % CI = 1.13–1.41, P < 0.001; dominant model—OR = 1.41, 95 % CI = 1.13–1.75, P = 0.002). Subgroup analysis based on ethnicity implies that the rs1982073 C>T genetic polymorphism in the TGF-β1 gene was positively correlated with the risk of fracture, OP, and OA in Asians (allele model—OR = 1.33, 95 % CI = 1.18–1.49, P < 0.001; dominant model—OR = 1.51, 95 % CI = 1.19–1.93, P = 0.001), but a similar correlation was not found in Caucasians (both P > 0.05) (as shown in Fig. 4). In addition, subgroup analyses by disease type revealed that the frequencies of the TGF-β1 rs1982073 C>T genetic polymorphism in the case groups were higher than those in the control groups in all fracture, OP, and OA subgroups under the allele model (all P < 0.05) (as seen in Fig. 4). However, the association of the TGF-β1 rs1982073 C>T genetic polymorphism with the occurrence of fracture, OP, and OA was observed by subgroup analyses based on disease type (Fig. 4) to be positive in OP patients under the dominant model (OR = 1.34, 95 % CI = 1.04–1.72, P = 0.026), but not in OA or fracture patients under the dominant model (both P > 0.05). Further subgroup analysis based on genotyping method implied that the rs1982073 C>T genetic polymorphism in the TGF-β1 gene was positively related to fracture, OP, and OA occurrence for both PCR-RFLP and non-PCR-RFLP methods under the allele model (PCR-RFLP, OR = 1.25, 95 % CI = 1.00–1.57, P = 0.048; non-PCR-RFLP, OR = 1.31, 95 % CI = 1.15–1.49, P < 0.001), as shown in Fig. 4. However, in Fig. 4, subgroup analysis by genotyping method revealed that the positive relationship between the TGF-β1 rs1982073 C>T genetic variant and fracture, OP, and OA susceptibility was only observed in the non-PCR-RFLP subgroup under the dominant model (OR = 1.53, 95 % CI = 1.21–1.93, P < 0.001) and not in the PCR-RFLP subgroup under the dominant model (P = 0.156). In addition, subgroup analysis by sample size under the allele model (Fig. 4) suggested that the TGF-β1 rs1982073 C>T mutation in fracture, OP, or OA patients occurred more frequently than in normal controls in both the small size subgroup (OR = 1.38, 95 % CI = 1.03–1.83, P = 0.028) and the large size subgroup (OR = 1.24, 95 % CI = 1.10–1.41, P = 0.001). However, the positive relationship between the TGF-β1 rs1982073 C>T variant and susceptibility of fracture, OP, and OA, in subgroup analysis by sample size under the dominant model (Fig. 4), was only detected in the large sample subgroup (OR = 1.36, 95 % CI = 1.11–1.67, P = 0.003) and not in the small sample subgroup (P = 0.214).
Sensitivity analysis and publication bias
A leave-one-out sensitivity analysis was carried out to evaluate whether the present meta-analysis is stable. Each study enrolled in our meta-analysis was evaluated one by one to reflect its effect on the significance of pooled SMDs (Table 3). The overall statistical significance did not change when any single study was omitted. Therefore, the current meta-analysis data is relatively stable and credible (Fig. 5). The graphical funnel plots of those nine studies for TGF-β1 rs1982073 C>T genetic variant are symmetrical for both the allele and dominant models, and Egger's test showed no publication bias (all P > 0.05) (Fig. 6).
Discussion
A meta-analysis of the connection between the rs1982073 C>T polymorphism in the TGF-β1 gene and the susceptibility to bone diseases (fracture, OP, and OA) was established with the main results of our meta-analysis demonstrating an obvious connection. TGF-β1, belonging to the TGF-β super family, which includes TGF-β, inhibits activins, MIS, and BMPs, and plays an essential role in the regulation of tissue morphogenesis and repair through its function on the proliferation, differentiation, and apoptosis of cells and production of extracellular matrix [41]. The participation of TGF-β1 in gene transcription regulation starts with a cell surface heteromeric receptor complex and combines with the intracellular signal-transducing Smad, which could be activated and transferred to the nucleus sites [42]. The role of TGF-β1 in the progression of cancer could be double sided: on the one hand, TGF-β1 could induce cell growth arrest, which might induce the dormancy of cancer cells, beneficial at the cancer early stages; on the other hand, TGF-β1 could activate extracellular matrix components expression to affect the cancer cell microenvironment, thus promoting the metastasis and invasion of tumors [43, 44]. Besides, TGF-β1, also known as a major factor in the immune system, could influence the proliferation and immunomodulation of cells responsible for chronic inflammatory diseases due to their role in regenerative processes and immune response [45]. TGF-β1 is an abundant cytokine in the bone matrix that could influence the biology and physiology of bones by increasing the formation of bone through osteoprogenitor proliferation stimulation and osteoblast precursor expansion [21]. Functioning on the osteoclast–osteoblast coupling, TGF-β1 could remodel the bones by facilitating recruit osteoblast progenitors to bone resorption sites and by stimulating RANKL production, a critical factor for osteoclast differentiation [46]. The expression of TGF-β1 could be regulated by the TGF-β1 gene and thus the polymorphism of the TGF-β1 gene might have a close connection with bone diseases, such as fracture, OP, and OA. It has been reported the rs1982073 (T869C) polymorphism with the TC genotype had a higher fracture possibility and bone turnover rate in postmenopausal women because of the role of TGF-β1 in mediating formation and resorption of bones [47]. Additionally, the T869C polymorphism in the TGF-b1 gene, resulting in a protein substitution at the tenth amino acid from Leu to Pro, is connected with the bone mineral density in both postmenopausal women and adolescents, and susceptibility to OP, which may also be due to the role of TGF-β1 in controlling bone formation and resorption [48]. Furthermore, TGF-β1 gene polymorphism at rs1982073 might also be involved in OA due to the function of TGF-β1 in the integrity of cartilage which is found to be decreased in the cartilage of OA patients [23]. Thus, we could know that the rs1982073 polymorphism in the TGF-β1 gene could regulate the expression of TGF-β1, causing many kinds of bone diseases, including fracture, OP, and OA, due to the role of TGF-β1 in the formation and resorption of bone and the integrity of cartilage. This conclusion is also supported by other studies. Utennam et al. demonstrated that the CT and CC genotype of the rs1982073 polymorphism in the TGF-β1 gene was linked with lower expression of TGF-β1 in OP of Thai women [27]. Kolundzic et al. found that the TGF-β1 gene polymorphism in the rs1982073 and the C allele carriage phenomenon were associated with higher TGF-β1 circulation levels in adult OA [37].
Many other factors which might affect the connection between rs1982073 in TGF-β1 gene polymorphisms and susceptibility to bone diseases were taken into consideration via stratified analyses based on ethnicity, different kind of diseases, genotyping method, and the size of samples. Subgroup analysis on ethnicity showed effects in Caucasians but not in Asians, perhaps due to living environment and genetic background differences. As for different kinds of diseases, fracture and OA had an effect on the relationship in the dominant model but not in the allele model. In addition, the PCR-RFLP method influenced the relationship in the dominant model, while the non-PCR-RFLP showed no influence, which may be explained by detection deviation. At the time the small size of samples affected the relationship in the dominant model, the large size of samples did not affect the relationship, suggesting that a large sample size could be more objective. In brief, polymorphism of rs1982073 in the TGF-β1 gene was significantly associated with fracture, OP, and OA. This significant relationship suggests a role for rs1982073 polymorphisms as a potent marker for bone disease diagnosis.
Finally, our meta-analysis has several potential advantages. First, our research sheds light on the relation of the rs1982073 C>T polymorphism in the TGFβ1 gene with susceptibility to bone fracture, OP, and OA. Additionally, our exhaustive search for unpublished articles via additional electronic databases and manual searches enhances the power and persuasion of our conclusion. Moreover, all included literatures had acceptable moderate to high quality scores (quality scores were higher than 20). However, some limitations of this meta-analysis should also be acknowledged when interpreting the results. Firstly, only one single SNP (rs1982073 C>T) was included, though the relation of other SNPs to bone fracture, OP, and OA risk has also been studied. More importantly, the existence of selection bias was due to the lack of a screening process for papers published in languages other than English or Chinese. In addition, the crude division criteria of ethnic groups into “Caucasian”, “Asian”, or “African” may also lead to bias. Nearly all of the studies were performed in Asians and Caucasians, but to capture the full range of possible ethnic differences in TGFβ1 rs1982073 C>T polymorphisms, deeper investigations of different populations are warranted to clarify the present results. Another important concern should also be taken into consideration: different diseases have different risk factors and diverse sensitivities to them. In particular, we did not evaluate family history or the clinical implications of bone fracture, OP, or OA in our study since such data was not available for collection. Finally, the present sample size (only nine articles included in interpreting three different bone diseases) limits the power to identify the small influence of the TGFβ1 rs1982073 C>T polymorphism on bone fracture, OP, and OA.
In summary, we have identified that the TGFβ1 rs1982073 C>T variant may increase the susceptibility to bone fracture, OP, and OA among all our studied populations. SNP in TGFβ1 genes may act as a potential candidate biomarker for the screening, diagnosis, and treatment of bone fracture, OP, and OA. The results needed replication in other populations for confirmation and further well-designed research studies with larger sample sizes are needed to better understand the underlying mechanisms responsible for bone fracture, OP, and OA.
References
Shibuya N, Davis ML, Jupiter DC (2014) Epidemiology of foot and ankle fractures in the United States: an analysis of the national trauma data bank (2007 to 2011). J Foot Ankle Surg 53(5):606–608
Wang XF, Seeman E (2012) Epidemiology and structural basis of racial differences in fragility fractures in Chinese and Caucasians. Osteoporos Int 23(2):411–422
Lanza LL, McQuay LJ, Rothman KJ, Bone HG, Kaunitz AM, Harel Z, Ataher Q, Ross D, Arena PL, Wolter KD (2013) Use of depot medroxyprogesterone acetate contraception and incidence of bone fracture. Obstet Gynecol 121(3):593–600
Pressley JC, Kendig TD, Frencher SK, Barlow B, Quitel L, Waqar F (2011) Epidemiology of bone fracture across the age span in blacks and whites. J Trauma 71(5 Suppl 2):S541–S548
Henrotin Y (2011) Muscle: a source of progenitor cells for bone fracture healing. BMC Med 9:136
Kawate H, Takayanagi R (2011) Efficacy and safety of bazedoxifene for postmenopausal osteoporosis. Clin Interv Aging 6:151–160
Banu J (2013) Causes, consequences, and treatment of osteoporosis in men. Drug Des Devel Ther 7:849–860
Lambert JK, Zaidi M, Mechanick JI (2011) Male osteoporosis: epidemiology and the pathogenesis of aging bones. Curr Osteoporos Rep 9(4):229–236
Guillemin F, Rat AC, Mazieres B, Pouchot J, Fautrel B, Euller-Ziegler L, Fardellone P, Morvan J, Roux CH, Verrouil E, Saraux A, Coste J, Osteoarthritis G (2011) Prevalence of symptomatic hip and knee osteoarthritis: a two-phase population-based survey. Osteoarthritis Cartilage 19(11):1314–1322
Lluch Girbes E, Nijs J, Torres-Cueco R, Lopez Cubas C (2013) Pain treatment for patients with osteoarthritis and central sensitization. Phys Ther 93(6):842–851
Pereira D, Peleteiro B, Araujo J, Branco J, Santos RA, Ramos E (2011) The effect of osteoarthritis definition on prevalence and incidence estimates: a systematic review. Osteoarthritis Cartilage 19(11):1270–1285
Raje M, Botre C, Ashma R (2013) Genetic epidemiology of osteoporosis across four microsatellite markers near the VDR gene. Int J Mol Epidemiol Genet 4(2):101–108
Inanir A, Yigit S, Tural S, Cecen O, Yildirim E (2013) MTHFR gene C677T mutation and ACE gene I/D polymorphism in Turkish patients with osteoarthritis. Dis Markers 34(1):17–22
de Gorter DJ, van Dinther M, Korchynskyi O, ten Dijke P (2011) Biphasic effects of transforming growth factor beta on bone morphogenetic protein-induced osteoblast differentiation. J Bone Miner Res 26(6):1178–1187
Toti P, Sbordone C, Martuscelli R, Califano L, Ramaglia L, Sbordone L (2013) Gene clustering analysis in human osteoporosis disease and modifications of the jawbone. Arch Oral Biol 58(8):912–929
Remst DF, Blom AB, Vitters EL, Bank RA, van den Berg WB, Blaney Davidson EN, van der Kraan PM (2014) Gene expression analysis of murine and human osteoarthritis synovium reveals elevation of transforming growth factor beta-responsive genes in osteoarthritis-related fibrosis. Arthritis Rheumatol 66(3):647–656
Quan J, Elhousiny M, Johnson NW, Gao J (2013) Transforming growth factor-beta1 treatment of oral cancer induces epithelial-mesenchymal transition and promotes bone invasion via enhanced activity of osteoclasts. Clin Exp Metastasis 30(5):659–670
Brophy TM, Coller BS, Ahamed J (2013) Identification of the thiol isomerase-binding peptide, mastoparan, as a novel inhibitor of shear-induced transforming growth factor beta1 (TGF-beta1) activation. J Biol Chem 288(15):10628–10639
Campos-Xavier B, Saraiva JM, Savarirayan R, Verloes A, Feingold J, Faivre L, Munnich A, Le Merrer M, Cormier-Daire V (2001) Phenotypic variability at the TGF-beta1 locus in Camurati–Engelmann disease. Hum Genet 109(6):653–658
Park J, Lee J, Kang W, Chang S, Shin EC, Choi C (2013) TGF-beta1 and hypoxia-dependent expression of MKP-1 leads tumor resistance to death receptor-mediated cell death. Cell Death Dis 4:e521
Ochiai H, Okada S, Saito A, Hoshi K, Yamashita H, Takato T, Azuma T (2012) Inhibition of insulin-like growth factor-1 (IGF-1) expression by prolonged transforming growth factor-beta1 (TGF-beta1) administration suppresses osteoblast differentiation. J Biol Chem 287(27):22654–22661
Kaiser G, Thomas A, Kottstorfer J, Kecht M, Sarahrudi K (2012) Is the expression of Transforming Growth Factor-Beta1 after fracture of long bones solely influenced by the healing process? Int Orthop 36(10):2173–2179
Muthuri SG, Doherty S, Zhang W, Maciewicz RA, Muir KR, Doherty M (2013) Gene–environment interaction between body mass index and transforming growth factor beta 1 (TGFbeta1) gene in knee and hip osteoarthritis. Arthritis Res Ther 15(2):R52
Mori S, Fuku N, Chiba Y, Tokimura F, Hosoi T, Kimbara Y, Tamura Y, Araki A, Tanaka M, Ito H (2010) Cooperative effect of serum 25-hydroxyvitamin D concentration and a polymorphism of transforming growth factor-beta1 gene on the prevalence of vertebral fractures in postmenopausal osteoporosis. J Bone Miner Metab 28(4):446–450
Wildemann B, Schmidmaier G, Ordel S, Stange R, Haas NP, Raschke M (2003) Cell proliferation and differentiation during fracture healing are influenced by locally applied IGF-I and TGF-beta1: comparison of two proliferation markers, PCNA and BrdU. J Biomed Mater Res B Appl Biomater 65(1):150–156
Yamada Y, Miyauchi A, Takagi Y, Nakauchi K, Miki N, Mizuno M, Harada A (2000) Association of a polymorphism of the transforming growth factor beta-1 gene with prevalent vertebral fractures in Japanese women. Am J Med 109(3):244–247
Utennam D, Tungtrongchitr A, Phonrat B, Tungtrongchitr R, Preutthipan S (2012) Association of T869C gene polymorphism of transforming growth factor-beta1 with low protein levels and anthropometric indices in osteopenia/osteoporosis postmenopausal Thai women. Genet Mol Res 11(1):87–99
Yamada Y, Miyauchi A, Takagi Y, Tanaka M, Mizuno M, Harada A (2001) Association of the C-509–>T polymorphism, alone of in combination with the T869–>C polymorphism, of the transforming growth factor-beta1 gene with bone mineral density and genetic susceptibility to osteoporosis in Japanese women. J Mol Med (Berl) 79(2–3):149–156
Hubacek JA, Weichetova M, Bohuslavova R, Skodova Z, Stepan JJ, Adamkova V (2006) No associations between genetic polymorphisms of TGF-beta, PAI-1, and COL1A1, and bone mineral density in Caucasian females. Endocr Regul 40(4):107–112
Tural S, Alayli G, Kara N, Tander B, Bilgici A, Kuru O (2013) Association between osteoporosis and polymorphisms of the IL-10 and TGF-beta genes in Turkish postmenopausal women. Hum Immunol 74(9):1179–1183
El Maghraoui A, Borderie D, Cherruau B, Edouard R, Dougados M, Roux C (1999) Osteoporosis, body composition, and bone turnover in ankylosing spondylitis. J Rheumatol 26(10):2205–2209
Xing D, Ma XL, Ma JX, Xu WG, Wang J, Yang Y, Chen Y, Ma BY, Zhu SW (2013) Association between aspartic acid repeat polymorphism of the asporin gene and susceptibility to knee osteoarthritis: a genetic meta-analysis. Osteoarthritis Cartilage 21(11):1700–1706
da Costa AG, Gago MF, Garrett C (2011) Cerebrospinal fluid biomarkers for the early diagnosis of Parkinson’s disease. Acta Med Port 24(Suppl 4):761–768
Jackson D, White IR, Riley RD (2012) Quantifying the impact of between-study heterogeneity in multivariate meta-analyses. Stat Med 31(29):3805–3820
Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L (2006) Comparison of two methods to detect publication bias in meta-analysis. JAMA 295(6):676–680
Zintzaras E, Ioannidis JP (2005) HEGESMA: genome search meta-analysis and heterogeneity testing. Bioinformatics 21(18):3672–3673
Kolundzic R, Trkulja V, Mikolaucic M, Kolundzic MJ, Pavelic SK, Pavelic K (2011) Association of interleukin-6 and transforming growth factor-beta1 gene polymorphisms with developmental hip dysplasia and severe adult hip osteoarthritis: a preliminary study. Cytokine 54(2):125–128
Langdahl BL, Carstens M, Stenkjaer L, Eriksen EF (2003) Polymorphisms in the transforming growth factor beta 1 gene and osteoporosis. Bone 32(3):297–310
Lau EM, Wong SY, Li M, Ma CH, Lim PL, Woo J (2004) Osteoporosis and transforming growth factor-beta-1 gene polymorphism in Chinese men and women. J Bone Miner Metab 22(2):148–152
Yamada Y, Okuizumi H, Miyauchi A, Takagi Y, Ikeda K, Harada A (2000) Association of transforming growth factor beta1 genotype with spinal osteophytosis in Japanese women. Arthritis Rheum 43(2):452–460
Andonegui G, Ni A, Leger C, Kelly MM, Wong JF, Jalloul A, Winston BW (2012) Sequential expression of IGF-IB followed by active TGF-beta1 induces synergistic pulmonary fibroproliferation in vivo. Am J Physiol Lung Cell Mol Physiol 303(9):L788–L798
Chen G, Ghosh P, O'Farrell T, Munk R, Rezanka LJ, Sasaki CY, Longo DL (2012) Transforming growth factor beta1 (TGF-beta1) suppresses growth of B-cell lymphoma cells by p14(ARF)-dependent regulation of mutant p53. J Biol Chem 287(27):23184–23195
Imai K, Minamiya Y, Goto A, Nanjo H, Saito H, Motoyama S, Sato Y, Kudo S, Takashima S, Kawaharada Y, Kurihara N, Orino K, Ogawa J (2013) Bronchioloalveolar invasion in non-small cell lung cancer is associated with expression of transforming growth factor-beta1. World J Surg Oncol 11:113
Zingariello M, Martelli F, Ciaffoni F, Masiello F, Ghinassi B, D'Amore E, Massa M, Barosi G, Sancillo L, Li X, Goldberg JD, Rana RA, Migliaccio AR (2013) Characterization of the TGF-beta1 signaling abnormalities in the Gata1low mouse model of myelofibrosis. Blood 121(17):3345–3363
Liberek A, Kmiec Z, Kartanowicz D, Wierzbicki PM, Stanislawowski M, Kaszubowska L, Luczak G, Gora-Gebka M, Landowski P, Szlagatys-Sidorkiewicz A, Liberek T, Kaminska B, Jakobkiewicz-Banecka J, Wegrzyn G (2013) The mRNA level of the transforming growth factor beta1 gene, but not the amount of the gene product, can be considered as a potential prognostic parameter in inflammatory bowel diseases in children. Int J Colorectal Dis 28(2):165–172
Mukherjee A, Larson EA, Carlos AS, Belknap JK, Rotwein P, Klein RF (2012) Congenic mice provide in vivo evidence for a genetic locus that modulates intrinsic transforming growth factor beta1-mediated signaling and bone acquisition. J Bone Miner Res 27(6):1345–1356
Lau HH, Ho AY, Luk KD, Kung AW (2004) Transforming growth factor-beta1 gene polymorphisms and bone turnover, bone mineral density and fracture risk in southern Chinese women. Calcif Tissue Int 74(6):516–521
Yamada Y (2000) Association of a Leu(10)–>Pro polymorphism of the transforming growth factor-beta1 with genetic susceptibility to osteoporosis and spinal osteoarthritis. Mech Ageing Dev 116(2–3):113–123
Acknowledgments
This study was supported by Jiangsu Clinical Science and Technology Program (BL2012002). We would like to acknowledge the helpful comments on this paper received from our reviewers.
Conflicts of interest
We declare that we have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Yu Cong and Jiang-Ying Ru are both considered as co-first authors.
Rights and permissions
About this article
Cite this article
Cong, Y., Ru, JY., Bao, NR. et al. A single nucleotide polymorphism in the TGF-β1 gene (rs1982073 C>T) may contribute to increased risks of bone fracture, osteoporosis, and osteoarthritis: a meta-analysis. Clin Rheumatol 35, 973–985 (2016). https://doi.org/10.1007/s10067-014-2840-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-014-2840-7